Five-Coordinate Zinc(II) Complex: Synthesis, Characterization, Molecular Structure, and Antibacterial Activities of Bis-[(E)-2-hydroxy-N′-{1-(4-methoxyphenyl)ethylidene}benzohydrazido]dimethylsulfoxidezinc(II) Complex
Abstract
The titled Zn(II) complex was synthesized by reacting the compound (E)-2-hydroxy-N′-{1-(4-methoxyphenyl)ethylidene}benzohydrazide with zinc(II) acetate dihydrate in alkaline DMSO and ethanol solution under reflux condition for 28 hours. The resulting solid was filtered and recrystallized from the mixture of ethanol and DMSO. The hydrazone Schiff base and its Zn(II) complex were characterized using 1H, 13C NMR, FTIR, UV-Vis spectroscopy, and single crystal X-ray diffraction analysis. Meanwhile, their antibacterial activities were examined using disc diffusion method. The spectral studies showed that the hydrazone Schiff base underwent keto-enol tautomerization, forming a bidentate ligand (N,O) towards Zn(II) ion. Surprisingly, on top of the two hydrazone Schiff base molecules which coordinated to the Zn metal center, an additional DMSO molecule was found attached to the Zn metal center in the crystal data, resulting in a 5-coordinate distorted trigonal bipyramidal Zn(II) complex. Both hydrazone Schiff base and its Zn(II) complexes were found to exhibit low antibacterial activity even when the concentrations were increased to 800 ppm.
1. Introduction
Hydrazone Schiff base plays an important role in inorganic chemistry, as it can easily form stable complexes with most transition metal ions due to its ability to form keto-enol tautomerism (Figure 1) [1–4]. In coordination chemistry, the hydrazone Schiff base ligand normally presents in enol conformation in order to bind with the metal center through the nitrogen atom from imine moiety [5–7] and oxygen from hydroxyl group [8, 9].
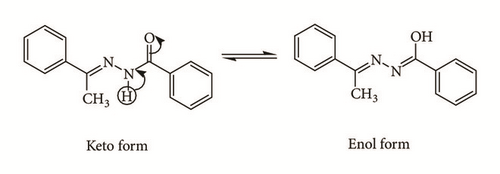
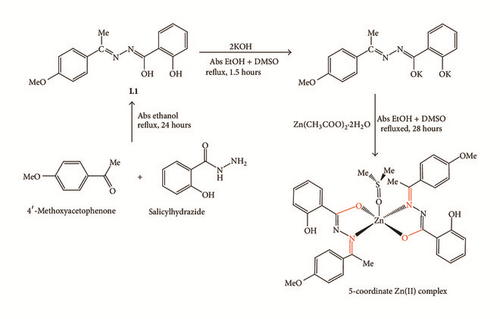
Complexation with Zn(II) metal center usually results in complexes with 4-coordination number and a tetrahedral geometry. This is due to the fact that Zn is a late transition metal with full d valence electrons, which means that a stable 18-electron complex can be formed through 4-coordination number with its ligands. Due to this reason, 5-coordinate Zn complex is considered unusual and expected to be unstable. Nevertheless, 5-coordinate Zn complexes were previously reported [10–14]. Similar to 4-coordinate Zn complexes, the common oxidation state for Zn ion in 5-coordinate Zn complexes is +2; however, the one reported by Song et al. is in 0 oxidation state [13]. In this paper, the synthesis (Scheme 1) and spectroscopic and crystallographic data of a 5-coordinate trigonal bipyramid bis-[(E)-2-hydroxy-N′-{1-(4-methoxyphenyl)ethylidene}benzohydrazido] dimethylsulfoxidezinc(II) complex are reported. In addition, the antibacterial activities of both hydrazone Schiff base L1 and Zn(II) complex have also been examined.
2. Experimental
2.1. Equipment or Instrumentation
The IR spectra of L1 and the Zn(II) complex were recorded in KBr disks by using Thermo Scientific/Nicolet iS10 8001PC FTIR Spectrometer with the wavelength range from 400 to 4000 cm−1. Jasco/V-630 UV-visible spectrophotometer was used to record the electronic absorption spectra with DCM solvent. JEOL ECA-500 NMR spectrometer with 500 MHz was used to record all the 1H and 13C NMR spectra. 1H-NMR chemical shift was reported relative to TMS and referenced via residual proton NMR resonance of the appropriate deuterated solvent (DMSO-d6: 2.50 ppm; CD2Cl2: 5.32 ppm). Stuart SMP3 Melting Point was used to measure the melting point of L1 and the Zn(II) complexes. The elemental analysis was conducted by using CHN Analyzer, Thermo-Flash EA 1112 series at the temperature up to 900°C, and vanadium pentoxide was used as oxidizer to prevent inhibition caused by sulphur.
X-ray measurements for the Zn complex were performed on a Bruker SMART diffractometer equipped with a graphite monochromated Mo Ka (λ = 0.71073) radiation source and a CCD detector. The frame integration was performed using the program SAINT [15]. The structure was solved by direct method provided by the program package SHELXTL-97 and refined a full matrix least square against F2 for all data [16]. All nonhydrogen atoms were refined anisotropically. All hydrogen atoms were introduced at idealized positions and were allowed to refine isotopically. Crystallographic data for Zn(II) complexes has been deposited in the Cambridge Crystallographic Data Centre CCDC number 1508885. The data are available free of charge via https://www.ccdc.cam.ac.uk/data_request/cif (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; +44 1223 336033; e-mail: [email protected]).
2.2. Antibacterial Study of the Compounds
The antibacterial study was carried out on hydrazone Schiff base L1 and its Zn(II) complex using disc diffusion method [17]. One colony of Bacillus cereus ATCC 33019 from a streak plate was inoculated in 20 mL of LB broth. After 16 hours of incubation, the optical density of the inoculums was measured and further diluted to achieve McFarland standard of 0.5. Using sterile cotton swab, agar plate was uniformly swabbed with diluted inoculums of the bacteria. After that, sterile filter papers (6 mm) which were impregnated with different concentrations (i.e., 200, 400, and 800 ppm using DCM as the solvent) of L1 and its Zn(II) complex were placed on the agar. The inhibitory zones in millimeters were measured after 24 hours of incubation. Similar procedures were conducted in another set for Escherichia coli ATCC 35150. All the antibacterial assays were performed in triplicate.
2.3. Synthesis
2.3.1. Preparation of (E)-2-Hydroxy-N′-(1-(4-methoxyphenyl)ethylidene)benzohydrazide (L1) [18]
Salicylhydrazide (1.522 g, 10 mmol) was dissolved in 25 mL of ethanol and added to a round bottom flask with constant stirring. An ethanolic solution of 4′-methoxyacetophenone (1.501 g, 10 mmol) was then added dropwise. The mixture was refluxed for 24 hours followed by cooling to room temperature. The precipitate which appeared as white crystals was filtered, washed with ethanol, and dried in vacuo over silica gel. Yield: 2.14 g, 75.4%. M.P.: 210.2–215.7°C. Anal. Calcd for C16H16N2O3: C, 67.09; H, 5.45; N.8.78; Found: C, 67.59; H, 5.67; N, 9.85%. IR (KBr disk, cm−1): 3435 (s), 3270 (s), 1605 (s), 982 (m). 1H NMR (DMSO-d6, δ): 11.81 (s, 1H, N-H), 11.28 (s, 1H, Ar-OH), 7.99 (d, 1H, J = 8 Hz, Ar-H), 7.82 (d, 2H, J = 8 Hz, Ar-H), 7.42 (t, 1H, J = 8 Hz, Ar-H), 6.97 (m, 4H, Ar-H), 3.81 (s, 3H, OCH3), 2.30 (s, 3H, H3C-C=N). 13C NMR (DMSO-d6, δ): 162.08, 160.40, 156.66, 152.35, 113.76, 116.89, 117.84, 119.64, 127.98, 130.30, 130.46, 133.29, 152.35, 55.24, 13.78. UV-Vis [DCM, λmax]: 320 nm.
2.3.2. Preparation and Crystallization of Bis-[(E)-2-hydroxy-N′-{1-(4-methoxyphenyl)ethylidene}benzohydrazido]dimethylsulfoxidezinc(II) Complex
The compound (E)-2-hydroxy-N′-(1-(4-methoxyphenyl)ethylidene)benzohydrazide (0.142 g, 0.5 mmol) was dissolved in 5 mL of DMSO in a round bottom flask with constant stirring. Potassium hydroxide, KOH (0.056 g, 1 mmol), was dissolved in 10 mL of absolute ethanol and added to the flask dropwise. The resulting mixture was refluxed for 1.5 hours, followed by dropwise addition of zinc(II) acetate dihydrate (0.11 g, 0.5 mmol) solution in 5 mL DMSO. The mixture turned from yellow to golden yellow color. After being refluxed for 28 hours, the solution was cooled to room temperature followed by filtration and recrystallization from the mixture of DMSO and absolute ethanol. A yellow crystal was obtained after one week, which was then filtered and washed with DMSO. Yield: 0.17 g, 92.9%. M.P.: 265.9–272.2°C. Anal. Calcd. for Zn (C34H36N4O7S): C, 57.51; H, 5.11; N.7.89; Found: C, 57.78; H, 5.39; N, 8.39%. IR (KBr disk, cm−1): 3443 (s), 1606 (s), 1098 (m), 992 (w), 669 (m). 1H NMR (CD2Cl2, δ): 8.12 (d, 1H, J = 6 Hz, Ar-H), 7.40 (t, 1H, J = 6 Hz, Ar-H), 7.24 (d, 2H, J = 9 Hz, Ar-H), 6.95 (d & t, 2H, J = 7 Hz, Ar-H), 6.71 (d, 2H, J = 9 Hz, Ar-H), 3.62 (s, OCH3, 3H), 2.53 (s, 3H, SCH3), 2.19 (s, 3H, H3C-C=N). 13C NMR (CD2Cl2, δ): 172.33, 164.78, 162.19, 160.56, 133.10, 130.39, 129.82, 128.40, 118.85, 117.33, 117.07, 114.36, 54.02, 41.41, 18.62. UV-Vis [DCM, λmax]: 345 nm.
3. Result and Discussion
3.1. Synthesis and Characterization
Hydrazone Schiff base L1 was successfully synthesized through condensation reaction between salicylhydrazide and 4′-methoxyacetophenone under reflux for 24 hours at 75°C. The color remained unchanged even after the addition of hot ethanolic salicylhydrazide into ethanolic solution of 4′-methoxyacetophenone with stirring and heating. A white color precipitate started to form after 12 hours with the yield of 75%. However, L1 was not able to be analyzed using GC-MS due to low solubility in DCM. Despite low solubility in DCM, the molecular structure of L1 has been reported by Qiu and coworkers in 2006 [18]. The ligand, L1, was then reacted with zinc(II) acetate under open air reflux condition for 28 hours. Upon completion of the reaction, the dark yellow solution was filtered, and the Zn(II) complex appeared as tiny pale yellow crystal after a week.
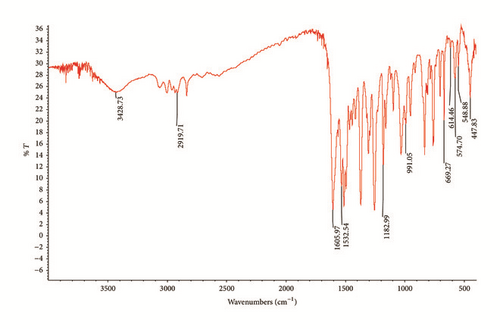
Based on the IR spectra of L1 (see Fig. S1 in Supplementary Material available online at https://doi.org/10.1155/2017/7520640) and Zn(II) complex (Figure 2), the absence of the v(N-H) and v(C=O) peak in the IR spectrum of Zn(II) complex indicates enolization of keto group in L1, which coordinated to the Zn metal center through enolate oxygen. This is supported by the appearance of new band attributed to v(C-O) at 1098 cm−1 after the complexation reaction [19]. However, the changes of v(C=N) chemical shift from 1605 to 1606 cm−1 in the IR spectra were rather insignificant after the complexation. This result is, indeed, parallel to the finding from Tay et al., [5] where the IR frequency of C=N was also shifted by only 2 cm−1 from 1621 cm−1 to 1619 cm−1 after their bis-2′-hydroxy Schiff base compound bound to Zn(II) metal center.
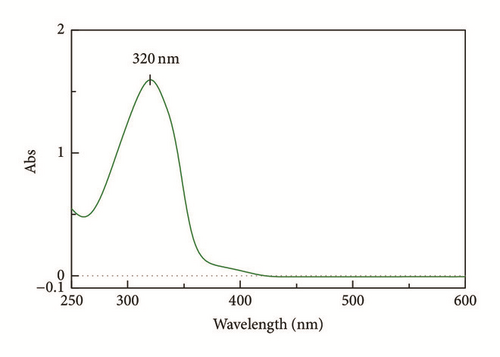
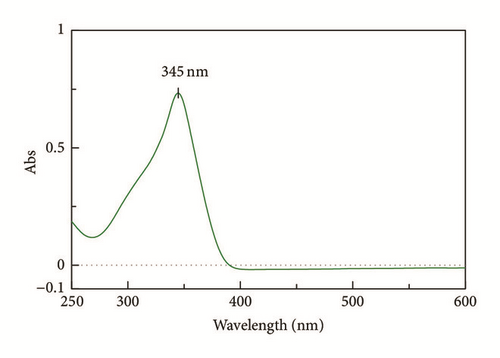
In fact, the IR data is supported by the UV-Vis result (Figure 3) where the n → π ∗ transition in C=N bond shifted from 320 nm to 345 nm after binding to the Zn metal center. The bathochromic shift is due to the backbonding from Zn to the C=N bond in L1 and subsequently weakened the bond energy of C=N.
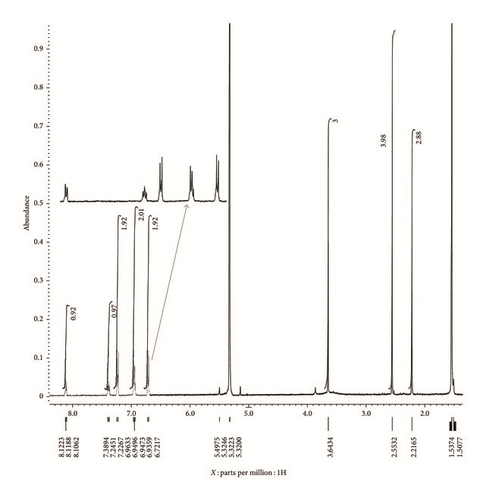
The 1H NMR spectrum of Zn(II) complex (Figure 4) also shows some differences compared to L1 (see Fig. S2). A broad signal at 11.81 ppm and a singlet present at 11.28 ppm in the 1H NMR spectrum of L1 are assigned to the N-H of azomethine and phenolic proton in L1, respectively. These two NMR resonances indicate that L1 is of keto form. This is also supported by the IR spectrum of L1 with the presence of v(N-H) and v(C=O) at 3270 and 1604 cm−1, respectively. The two NMR resonances at 11.81 and 11.28 ppm disappeared after complexation reaction, indicating that the structure of L1 changed from keto to enol conformation and the O atom from phenolic group has bound to the Zn metal center. On top of these, a new peak at 2.55 ppm was found in the 1H NMR spectrum of Zn(II) complex. Based on the integration, this singlet is the combination of the protons from dimethyl sulfoxide (DMSO) and enolic proton after forming the complex. The presence of DMSO is due to the solvent used during the recrystallization process, and the crystal data also confirms that a DMSO molecule is bound to Zn metal center.
The crystallographic molecular structure of bis-[(E)-2-hydroxy-N′-{1-(4-methoxyphenyl) ethylidene}benzohydrazido]dimethylsulfoxidezinc(II) complex is shown in Figure 5. The molecular structure is shown in different angles [Figures 5(a)–5(d)] for better clarity to show each coordination to Zn metal center. The crystal data is presented in Table 1 and selected bond lengths as well as bond angles are shown in Table 2. The complex crystallizes in monoclinic system and the molecule is believed to exhibit a distorted trigonal bipyramidal geometry. The molecular formula of the Zn(II) complex conforms to the C, H, and N% which were found from the elemental analysis.
| Compound | Zn(II) complex |
|---|---|
| Crystal system | Monoclinic |
| a (Å) | 24.5646 (10) |
| b (Å) | 8.7981 (3) |
| c (Å) | 15.7993 (6) |
| α(Å) | 90.00 |
| β(Å) | 96.6410 (3) |
| γ(Å) | 90.00 |
| Volume (Å 3) | 3391.7 (2) |
| Z | 8 |
| Density (Mg/m3) | 1.726 |
| Absorption coefficient (mm−1) | 1.482 |
| Θ range for data collection | 2.46–24.20 |
| Reflections collected | 3924 |
| Independent reflections | 2565 |
| Data/restraints/parameter | 3924/0/231 |
| Final R indices | R1 = 0.0913 |
| wR2 = 0.25 | |
| R indices (all data) | R1 = 0.1263 |
| wR2 = 0.2739 |
| Bond lengths | Å | Bond angles | (°) |
|---|---|---|---|
| Zn1-N1 | 2.209 | O1-Zn1-N1 | 102.35 |
| Zn1-O1 | 1.974 | O1-Zn-O1 | 111.0 |
| Zn1-O1AA | 2.049 | N1-Zn-N1 | 179.8 |
| C9-N1 | 1.291 | ||
| N1-N2 | 1.405 | ||
| N2-C1AA | 1.317 | ||
| C1AA-O1 | 1.276 |
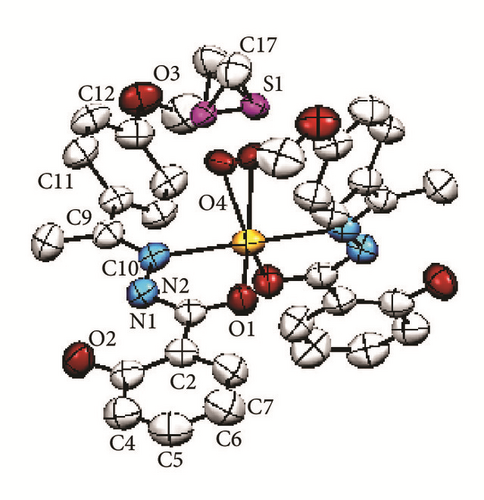
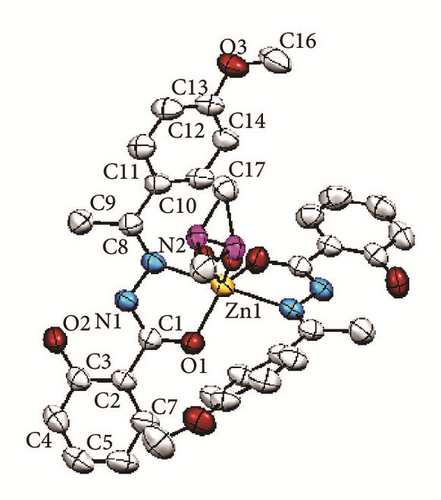
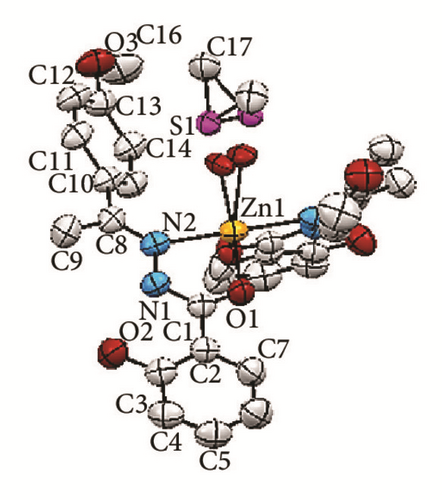
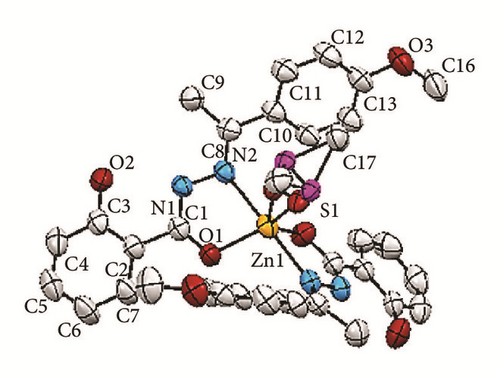
The bond distance for Zn1-N1 is 2.209 Å whereas the bond length for Zn1-O1 is 1.974 Å. The longer bond length in Zn-N compared to Zn-O indicates that the Zn-N bond is weaker than Zn-O. This is due to the stronger trans-effect of C=N bond compared to that of the C-O bond. The reason for that is because C=N contains π-bond which plays an important role in trans-effect. Besides the two L1 chelating ligands, a disordered DMSO molecule [Figures 5(a) and 5(c)] was found to coordinate to the Zn metal center via oxygen atom. The bond distance Zn-O4 is 2.049 Å. Meanwhile the interatomic distance for C8-N2, N1-N2, N1-C1, and C1-O1 in the azomethine group (-C=N-N=C-O) moiety is 1.294, 1.403, 1.317, and 1.278 Å, respectively. The longer bond lengths of C8-N2 and N1-C1 than the normal C=N bond (1.279 Å) and the shorter bond length for N1-N2 than the previously reported data (1.420 Å) [20] suggested the presence of a conjugation system along C=N-N=C moiety. This indicates that the N1-N2 bond became stronger, in parallel with the data obtained in the IR in which the v(N-N) underwent positive shift in the IR spectrum of the complex. Moreover, the bond length for C1-O1 is shorter than the normal C-O bond length (1.5 Å) due to keto-enol tautomerization [2, 3].
The dihedral angle between O1-Zn1-N1 planes of two L1 chelating ligands is 102.35°. The bond angles for O1-Zn-O1 and N1-Zn-N1 are 111.0° and 179.8°, respectively. The bond angle for the O1-Zn-O1 deviates from the ideal bond angle for square planar (180°). This can be explained by the presence of the disordered DMSO. The bond angles and the X-ray structure observed suggested that the chelating ligands which coordinate to the zinc metal ion are perpendicular to each other, parallel to the description of square planar.
3.2. Antibacterial Screening
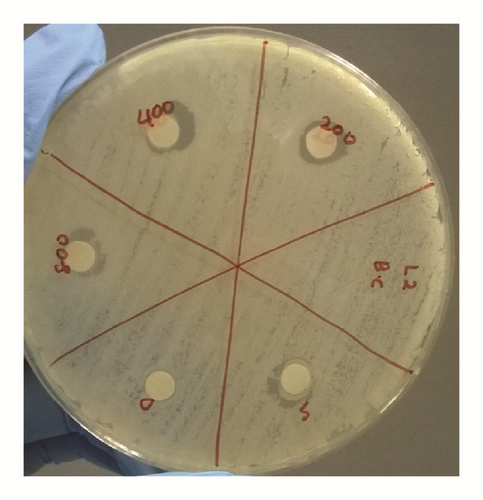
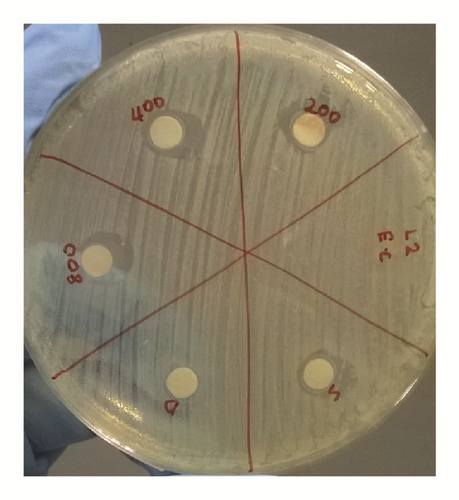
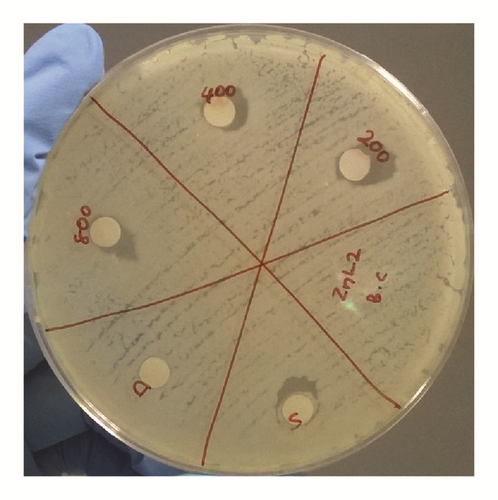
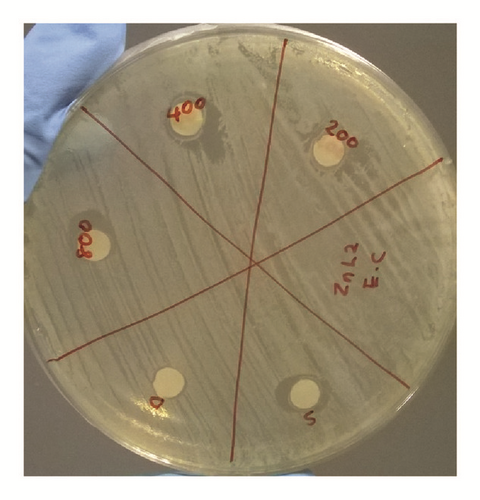
The antibacterial activity of hydrazone Schiff base L1 and its Zn(II) complex was examined using disc diffusion method and the results were tabulated in Table 3 and Figure 6. The results show that both L1 and its Zn(II) complex are considered nontoxic to gram-positive (Bacillus cereus) and gram-negative (Escherichia coli) bacteria as there is no significant difference in the inhibition areas with increasing concentration of both L1 and its Zn(II) complex; even the concentration was increased up to 800 ppm. The possible reason to this could be the absence of lipophilic group such as long carbon chains in both structures. Birnie and coworkers [21] reported the use of long carbon chains substituents, such as C8H17 and C10H21, in enhancing the antibacterial activity.
| Conc. (ppm) in DCM | Bacillus cereus ATCC 33019 | Escherichia coli ATCC 35150 | ||
|---|---|---|---|---|
| Diameter of inhibition zone, mm (average of triplicates) | ||||
| L1 | Zn(II) complex | L1 | Zn(II) complex | |
| 0 | 10.0 | 10.0 | 10.0 | 10.0 |
| 200 | 9.5 | 9.5 | 11.0 | 9.5 |
| 400 | 10.0 | 9.0 | 12.0 | 11.5 |
| 800 | 10.0 | 10.0 | 12.0 | 10.0 |
4. Conclusion
The synthesis and molecular structure of 5-coordinated bis-[(E)-2-hydroxy-N′-{1-(4-methoxyphenyl)ethylidene}benzohydrazido]dimethylsulfoxidezinc(II) complex were reported. The molecular structure and formula of the Zn(II) complex are in agreement with the results from CHN elemental analysis and 1H NMR spectroscopy. In addition, the bathochromic shift of C=N wavelength in absorption spectra indicates the binding of nitrogen atom from C=N to Zn(II) metal center. From the result of antibacterial study, both L1 and its Zn(II) complex are considered nontoxic to both gram-positive and gram-negative bacteria.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
The authors are grateful to the research funding from Malaysian Ministry of Higher Education under Research Acculturation Grant Scheme (RAGS) with no. RAGS/ST01(1)/1038/2013(05).




