Surfactant Incorporated Co Nanoparticles Polymer Composites with Uniform Dispersion and Double Percolation
Abstract
Series of Cobalt nanoparticles incorporated polymethylmethacrylate composites in the presence and absence of dodecyl-benzene-sulphonic acid (DBSA-CoNPs/PMMA and CoNPs/PMMA, resp.) were synthesized by solution mixing methodology. UV-visible and FTIR techniques were used to confirm the formation of nanocomposite. UV-visible spectra of the composites showed the incorporation of filler particles in the polymer matrix. On the other hand, FTIR spectra indicated the physical interaction between the two phases of the composite. Moreover, the electrical nature of the composites was studied by plotting graphs between electrical conductivity (measured using LCR meter at 100 kHz) and contents of the filler particles as introduced in the polymer matrix. An increase in electrical conductivity was first observed with increasing filler concentration up to the critical percolation threshold value (0.5% for DBSA-CoNPs/PMMA and 1% for CoNPs/PMMA), which then dropped upon further increments in the filler content. However, at higher concentrations, a second jump in the conductivity was observed in case of DBSA-CoNPs/PMMA composites.
1. Introduction
Nowadays, extensive research work has been conducted in the field of nanotechnology and nanoscience because of their versatile applications. [1]. Nanoscience is an emerging field and its impact is expected to be spread all over the world. An important aspect is that its sphere is not limited to the materials and its application but expands to life sciences as well [2]. Keeping in view high flexibility in polymers and ease in their processing, other properties of polymers were targeted to boost by doping of metal nanoparticles (NPs) such as cobalt, nickel, silver, and gold NPs [3, 4]. Some polymers are semiconductors such as polyaniline (PANI) and some are nonconductors like poly-methyl methacrylate (PMMA). After the discovery of conductive polymers like poly-acetylene conductor because of conjugated π bonds, these polymers are major key point for development of nanotechnology in various fields of science [5, 6]. Composite nanomaterials exhibit certain collegial properties between polymer and NPs, making them dormant candidates for application in diverse fields serving in catalysis, sensors, displays, data storage materials, polymer films, and so forth [7, 8]. A major issue related to composite formation is that problems occur during the incorporation of filler in the matrix. Common concern in this regard is nonhomogenous dispersion of the particles or agglomerate formation of the filler entities. By doping of some additives or modification of the filler to develop the interaction with matrix, aggregation of nanoparticles can be prevented, and it is very necessary for its homogenous dispersion [9–11]. Homogeneous dispersion of the filler in the polymer matrix is an essential requirement to impart the electrical and mechanical features of the fillers to the polymer composite [12]. Low value of electrical percolation is the index of good dispersion [13]. It is also observed that shape of the percolation curve and value of percolation threshold depend strongly upon different factors like temperature, pressure, aspect ratio of fillers, additives used, and method of preparation [11, 14–16].
In the present work dodecyl-benzene-sulphonic acid (DBSA) was used for the synthesis of PMMA based composites along the CoNPs to attain their homogeneous and uniform dispersion to get the maximum out of it. For this study PMMA was selected as matrix because of its unique characteristics [5, 17]. It is low-cost polymer with extraordinary environmental stability and ability to form thin layers with good mechanical strength. It is believed that DBSA as surfactant will play its role in the better dispersion of CoNPs in PMMA so that characteristics of the CoNPs can be imparted in PMMA more effectively [18–20]. This will help in reducing the chances of agglomerate formation of CoNPs. Synthesized composites have been characterized by UV-visible and FTIR spectroscopy. Electrical character of the PMMA based CoNPs composites prepared with DBSA (DBSA-CoNPs/PMMA) and without the incorporation of DBSA (CoNPs/PMMA) was studied by using conductivity data measured by LCR meter.
2. Experimental Work
2.1. Reagents and Instruments
All the chemicals were used as purchased without further processing and purification except solvent. UV-Visible double beam spectrophotometer (PG Instruments Model: UVD-T90+) and Fourier Transform Infrared (FTIR) Spectroscopy (Agilent FT-IR model: CARY-630: range 4000–650 cm−1) were used to characterize the synthesis of the composites. LCR meter (Precision LCR meter E4980AL, 20 Hz–1 MHz) was used to measure the conductance of the composites.
2.2. Synthesis of CoNPs
A dark blue solution was formed after dissolving cobalt salt, CoCl2·6H2O, in ethanol and then the mixture of hydrazine hydrate (N2H4·H2O) and sodium hydroxide (NaOH) was put in this dark blue solution at room temperature. After about one minute, gray solid particles appear as a result of quick reduction of Co ions. The suspended gray particles can be precipitated by placing a magnet under the container. After completely washing with distilled water, ethanol, absolute ethanol to remove hydrazine, sodium, and chlorine ion, the sample was kept in absolute ethanol to avoid oxidation.
2.3. Synthesis of CoNPs/PMMA Composite
For the synthesis of CoNPs/PMMA composites, 0.025 g CoNPs were dispersed in 50 mL of chloroform containing 1.76 g of PMMA followed by stirring of 40 minutes. This mixture was ultrasonicated for 1 hour to obtain a uniform dispersion of CoNPs. Thin polymer film was casted by pouring the solution into a Petri-dish and allowing the chloroform to evaporate over several hours. The same experiment was performed with varying concentrations of CoNPs and PMMA to obtain composites with different compositions.
2.4. Synthesis of DBSA-CoNPs/PMMA Composite
In this method, DBSA was used additionally as surfactant. 1 g of DBSA was stirred in 200 mL of distilled water for 15 minutes. Then 1 g of CoNPs was added in the above solution and stirred for 50 minutes. The product was subjected to ultrasonic treatment for 50 minutes. The suspension was covered with perforated aluminium foil and kept for 14 hours. The resultant product was dried at 60°C for 14 hours after filtration and wash.
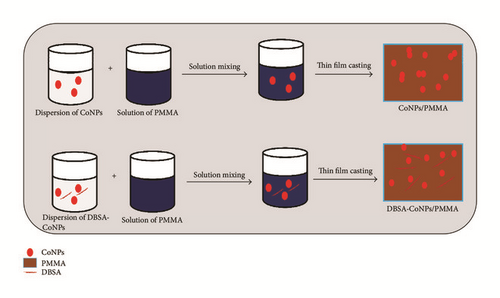
Afterwards, 0.05 g of the above product was dispersed in 50 mL chloroform containing 1.76 g of PMMA along constant stirring of 40 minutes. Then this mixture was ultrasonicated for one hour to obtain a uniform dispersion of DBSA-CoNPs. Thin films were formed by pouring the solutions into a Petri-dish and allowing the chloroform to evaporate for several hours. The same experiment was performed with varying concentrations of DBSA-CoNPs nanoparticles and PMMA to obtain composites with different compositions. Whole experimental scheme adopted in this research work is best explained by graphical presentation given in Figure 1.
3. Results and Discussion
3.1. UV-Vis Spectroscopy
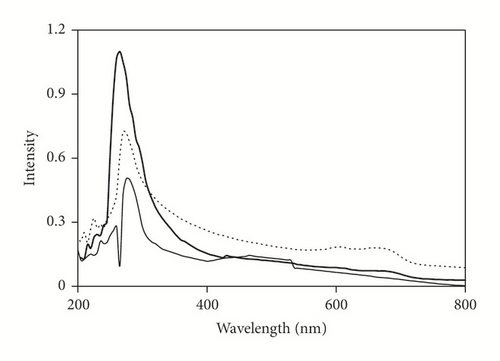
UV-Vis spectra of pure PMMA, CoNPs/PMMA, and DBSA-CoNPs/PMMA composites are given in Figure 2. The spectrum of pure PMMA shows maximum absorbance around 260 nm, which is ascribed to characteristics absorption curve of PMMA. With addition of CoNPs with and without DBSA, in the PMMA matrix, the absorption is increased showing incorporation of CoNPs in the structure of PMMA. This increase in the absorption intensity was more pronounced in the case when DBSA was added as an additive which clearly indicated that DBSA helped in better incorporation of CoNPs [4, 19, 21]. It can be noted that shift in the position of maximum absorption is not significant.
3.2. FTIR Spectroscopy
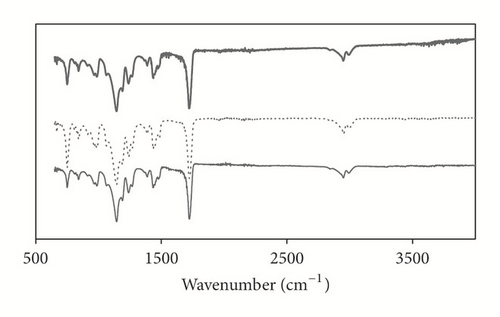
FTIR spectra of pure PMMA, CoNPs/PMMA, and DBSA-CoNPs/PMMA composites are shown in Figure 3. The FTIR spectrum of PMMA shows characteristic peaks of various functional groups present in it. Peak at 2950 cm−1 corresponds to C-H stretching vibrations and a peak corresponding to C=O appears at 1721 cm−1. Strong bands present at 1449 and 1437 cm−1 originate from the O-CH3 bending vibrations. Peak at 1146 cm−1 corresponds to the C-O in PMMA structure while bending vibrations of C-H exhibited absorption at 843, 914, and 986 cm−1 and peak at 749 cm−1 appeared due to polymer chains vibrations. On comparing FTIR spectrum of PMMA with those of its composites no significant difference in peak patterns was observed. This indicates no chemical reaction of filler and DBSA with matrix took place. All the chemical functional groups were intact since incorporation of the filler in matrix is physical.
3.3. Electrical Character
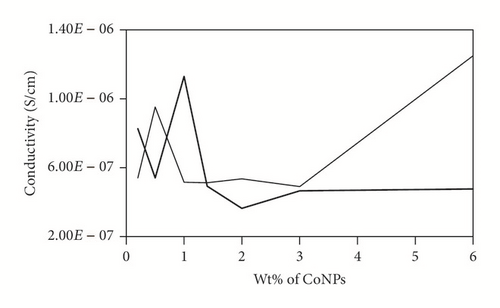
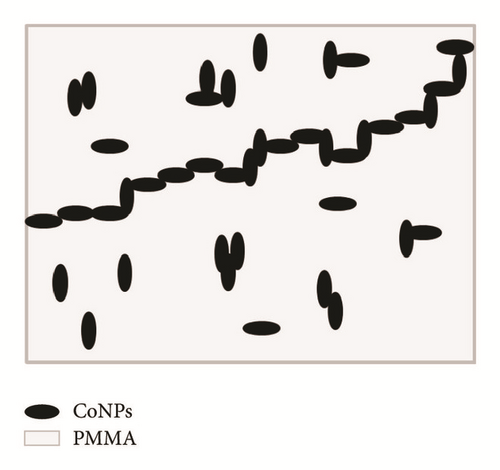
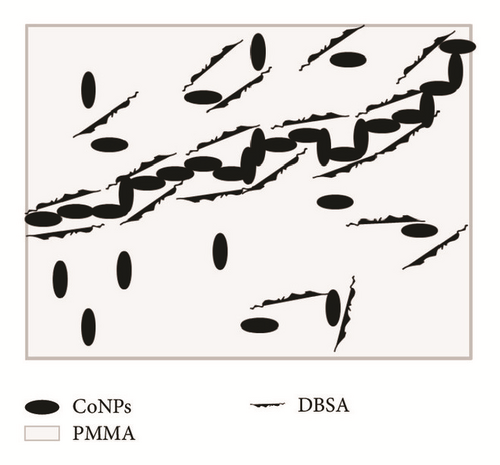
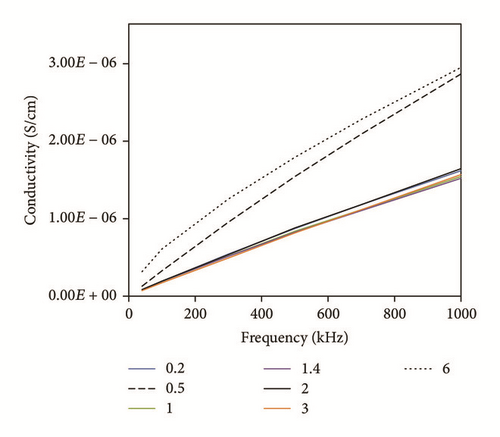
To investigate the electrical character of the series of the DBSA-CoNPs/PMMA and CoNPs/PMMA composites, their conductance has been measured using LCR meter. The trend of electrical conductivity as a function of filler contents has been portrayed in Figure 4. Critical percolation threshold is minimum concentration at which a network of fillers is just developed which is responsible for the flow of current. Upon formation of the network, current, which is stored due to micro size capacitors consisting of conductor, filler, and insulator matrix, flows away. This results in sudden rise in the value of the observed conductivity [22, 23]. This high level of observed conductivity lasts until network exists. It is often observed that, soon after attaining the maximum value of the current, further increase in the contents of fillers causes reagglomeration of the fillers which results in a decreased value of conductivity. Percolation phenomena occur in both types of the composites and can be seen in Figure 4. Value of the critical threshold for percolation can be determined with the help of (1) and (2) by using data of conductivity and dielectric constant, respectively, as a function of contents of filler [14]. Percolation behavior is quite different for both types of the composites. Value of the percolation threshold, calculated using data of conductivity, is lower for DBSA-CoNPs/PMMA composites as compared to CoNPs/PMMA composites, while value of the maximum conductivity is higher for CoNPs/PMMA composites than for DBSA-CoNPs/PMMA. Value of critical percolation threshold by using (1) is around 0.5 weight percent for DBSA-CoNPs/PMMA while that for CoNPs/PMMA is around 1 weight percentage of CoNPs. Values of percolation threshold for both the systems improved from previously reported percolation values for polymer composite incorporated conducting materials as filler [24]. On the parallel side a relatively high maximum value in the conductivity was observed for CoNPs/PMMA composites. Low value of percolation for DBSA-CoNPs/PMMA as compared to CoNPs/PMMA indicated that DBSA helped in network formation by reducing the chances of reagglomeration of the filler particles being a surfactant. The value of maximum conductivity was also reduced, due to the nonconducting nature of the surfactant which interrupted the electrical conductivity. This phenomenon of percolation can be well understood with the aid of Figures 5(a) and 5(b).


4. Conclusion
Series of PMMA based CoNPs polymer composites with DBSA and without DBSA have been prepared. Agglomeration of the filler in the polymer matrix is a big dilemma and shows encumbrance to attain the homogeneous and uniform dispersion of the filler particles. Addition of the DBSA additive, along the CoNPs, played an important role in uniform dispersion of the fillers in the matrix and achieving low percolation value as compared to that of series of composites where no such additive was added. DBSA also helped in establishing second conductivity network built of agglomerated CoNPs. That network was responsible for second jump in conductivity which is relatively high as compared to first jump.
Conflicts of Interest
The authors declare no conflicts of interest regarding the research project.
Authors’ Contributions
Tajamal Hussain and Aneela Nawaz contributed equally to this work.
Acknowledgments
University of the Punjab, Lahore, is highly acknowledged for research grant to carry out this work.