Preparation and Photocatalytic Activity of TiO2/Fine Char for Removal of Rhodamine B
Abstract
TiO2/fine char (FC) photocatalyst was prepared via sol-gel method with tetrabutyl titanate as the precursor and FC as the carrier. The structural property of TiO2/FC photocatalyst was investigated by X-ray diffraction (XRD) and scanning electron microscopy (SEM), and the photocatalytic activity of TiO2/FC was evaluated by photocatalytic degradation of rhodamine B (RhB) aqueous solution under UV light irradiation. The results showed that TiO2 was successfully coated on the surface of FC, and the TiO2/FC photocatalyst had better photocatalytic efficiency and stability for degradation of RhB under UV light illumination as compared to that of the pure TiO2 and FC. The study provided a novel way for the application of FC to the photocatalytic degradation of organic wastes.
1. Introduction
With the development of the industrialization process, more and more industrial waste water has been discharged and waste water treatment has become an urgent problem to be solved [1, 2]. In recent years, photocatalytic oxidation technology for environmental pollution control is becoming a hot spot of environmental science research. TiO2, a photocatalytic semiconductor, has been widely applied in the field of photocatalysis due to its many advantages such as low cost, relatively high efficiency, nontoxicity, and good chemical stability. Although TiO2 possesses high photocatalytic efficiency, it is always powder or particle, which may easily aggregate. In addition, the efficient recovery of TiO2 from treated water is still a challenge [3–7]. Immobilizing TiO2 on a porous solid surface, such as zeolites or activated carbon, can increase the efficiency of TiO2 and solve the problem of hard recovery [8–10].
FC is an industrial by-product derived from U-GAS gasifier; if not disposed properly, it may cause environmental pollution. Although it is a solid waste, it has many merits such as porosity, high surface area, and lightweight property, making it a potential application in wastewater and air treatment [11, 12]. In view of the merits of FC, it might be used as a carrier to immobilize TiO2 onto its surface to prepare photocatalyst, which can show a strong adsorption and photocatalytic degradation towards target pollutants. To the best of our knowledge, it was rarely reported that FC was employed as a photocatalyst.
In this work, TiO2/FC photocatalyst was prepared via sol-gel method with tetrabutyl titanate as the precursor and FC as the carrier. The as-prepared photocatalyst was characterized and its photodegradation efficiency was evaluated by photocatalytic degradation of RhB aqueous solution under UV light irradiation. The photocatalyst shows a high photodegradation efficiency. This work not only provided a novel way for the application of FC but also developed a low cost material for the degradation of organic wastes.
2. Experimental
2.1. Preparation of FC Carrier
FC was obtained from the U-GAS gasifier. 20 g of the FC was mixed with 200 mL distilled water under magnetic stirring for 6 hours to remove the soluble compounds. The disposed FC was dried at 105°C for 12 hours and then calcined in muffle furnace at 500°C for 3 hours in air.
2.2. Preparation of TiO2/FC Photocatalyst
Sol-gel method was utilized to prepare the TiO2/FC composite photocatalyst with tetrabutyl titanate as the precursor and FC as the carrier. Firstly, the tetrabutyl titanate (10 mL) was added into the solution containing glacial acetic acid (5 mL) and absolute ethyl alcohol (30 mL) drop by drop under the vigorous stirring. After stirring for 30 min, FC (10 g) was introduced with continuous stirring for 30 min. Subsequently, the mixing solution of distilled water (4 mL) and absolute ethyl alcohol (6 mL) was slowly dripped into the aforementioned mixture under rapid stirring. Thus, the TiO2/FC gel was obtained. The gel was aged at room temperature for 24 h, followed by drying at 70°C for 24 h. After calcination in air at 500°C for 2 h, the TiO2/FC photocatalyst was obtained, and the amount of TiO2 in TiO2/FC catalyst was 19%. In contrast, pure TiO2 nanoparticles were also prepared by the same procedure without the addition of FC.
2.3. Characterization
XRD was performed on a Rigaku X-ray diffractometer with Cu-Ka radiation (Bruker D8 ADVANCE diffractometer, λ = 1.5406 Å), over the 2θ scanning angle range 10 to 80°. The crystallite size of TiO2 was calculated by using the Scherrer formula, d = kλ/(βcosθ) [13], where d is the crystallite size, k is the shape factor (0.9), λ is the X-ray wavelength (1.5406 Å), β is the line broadening at half of the maximum intensity, and θ is the diffraction angle. SEM (JSM 6700F, Japan) was used to investigate the surface morphology at an accelerating voltage of 5 kv. The surface area (SBET) was calculated from the nitrogen adsorption isotherms obtained at 77 K using a NOVA 2000e apparatus.
2.4. Photocatalytic Experiment
3. Results and Discussions
3.1. Material Characterization
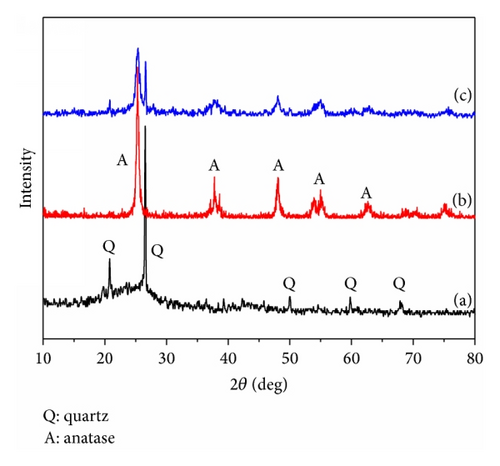
The composition and crystal structure of TiO2/FC photocatalyst were identified by XRD, and for a comparison, the XRD patterns of FC and TiO2 were also analyzed. As seen in Figure 1, the diffraction peaks at 2θ = 25.4°, 37.8°, 48°, 55°, and 62.6° can be perfectly indexed to those XRD patterns of anatase TiO2 nanoparticles [14]. Compared with diffraction peaks of pure FC, the peak intensity of FC in TiO2/FC was obviously weakened and only two relatively narrow diffraction peaks can be observed at 2θ = 20.8° and 26.5°. XRD analysis strongly confirmed the existence of anatase TiO2 on FC. The crystallite size of pure TiO2 was calculated to be about 31 nm and the crystallite size of TiO2 on the TiO2/FC was 12 nm, indicating that TiO2 particle size was significantly decreased after its loading onto FC, which will be favorable for its improvement of photocatalytic activity and efficiency. The BET measurement indicated that the specific surface area of TiO2, FC, and TiO2/FC reached 3.235, 28.544, and 39.113 m2/g, respectively, which further suggested that the TiO2 loading on FC was good for the enhanced photoactivity.
The morphology of FC, TiO2, and TiO2/FC was characterized by SEM. As seen in Figure 2(a), FC contained coarse, angular, and irregular particles. For the operational temperature range of U-GAS gasifier is 850~1000°C and FC cannot enter into the liquid state, giving rise to irregular particle shapes. The poor dispersion and obvious agglomeration were observed in the SEM image of TiO2 (Figure 2(b)). It could be seen from Figure 2(c) that the porous TiO2 coating was uniformly modified onto the surface of FC, indicating a successful immobilization of TiO2. Such results were consistent with the XRD analysis.



3.2. Photocatalytic Activity Test
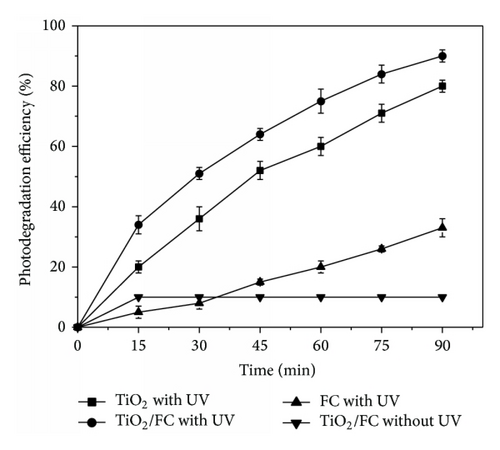
The photocatalytic activity of TiO2/FC was evaluated by monitoring the degradation efficiency of RhB in aqueous solution, which was also compared with other photocatalysts, for example, TiO2 and FC. As shown in Figure 3, the degradation efficiency of RhB was low in the dark and reached a ready value after 15 min, indicating that it was difficult for the degradation of RhB in the absence of light. Under UV irradiation, the degradation efficiency of RhB was significantly enhanced and increased with the increase of time. At a given time, the photodegradation efficiency of RhB on TiO2/FC was obviously higher than that on pure TiO2 and FC. After UV irradiation for 90 min, the photodegradation efficiency of RhB on TiO2/FC, TiO2, and FC reached 90%, 80%, and 33%, respectively. The high photocatalytic activity of TiO2/FC may be understood from two aspects. On one hand, the TiO2 modified onto FC possesses a better dispersibility and the number of photocatalytic activity sites will be increased, causing an enhanced photodegradation of RhB molecules. On the other hand, FC is strong adsorbent and can provide a high-density environment for the photocatalytic reaction, speeding up the efficiency of photocatalytic degradation [15].
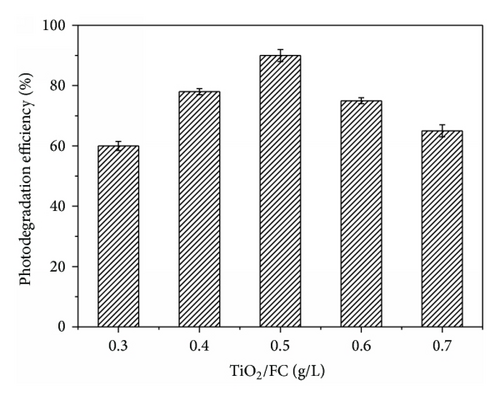
Photocatalytic degradation of RhB depended on the amount of the catalyst used. Figure 4 shows the photodegradation efficiency of RhB when the concentration of TiO2/FC was varied from 0.3 to 0.7 g/L. The photodegradation efficiency of RhB increased with an increase in TiO2/FC concentration up to 0.5 g/L, and after that a further increase in catalyst concentration led to a slow decrease in the photodegradation efficiency of RhB. Such phenomena can be understood from two aspects. On one hand, the increase of TiO2/FC concentration will increase the number of photoactive sites and also the number of the RhB molecules absorbed. And a further increase of the catalyst concentration beyond 0.5 g/L may cause light scattering and screening effects, which will hinder the penetration of light and reduce the specific activity of the catalyst. On the other hand, at high catalyst concentration, it is difficult to maintain the homogeneous suspension due to particle agglomeration, which may also reduce the catalytic activity. So the photodegradation efficiency of RhB decreases gradually. In the present study, the optimum concentration of TiO2/FC is found to be 0.5 g/L for the degradation of RhB.
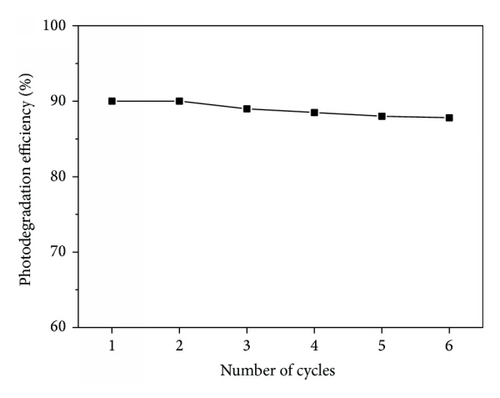
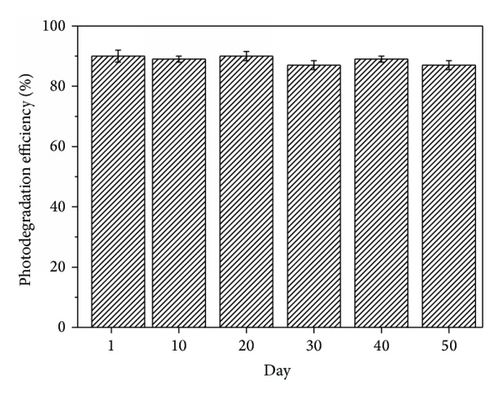
The reusability of the photocatalyst is of great importance in the photodegradation of organic contaminants. To evaluate the reusability of TiO2/FC, the photodegradation experiment of RhB was performed repeatedly for six times under the same experimental condition. As seen in Figure 5, the photodegradation efficiency of RhB showed a slight change and remained as high as 97.6% after 6 cycles. This indicates that TiO2/FC can be reusable with meager loss in activity during the photocatalytic oxidation of RhB molecules. The long-term storage stability was also investigated and the obtained results were shown in Figure 6. After storage for 50 days, the photodegradation efficiency of RhB on TiO2/FC decreased only 3.3% compared to the initial value. The results indicate the satisfactory reproducibility and stability of TiO2/FC towards RhB degradation.
4. Conclusions
The TiO2/FC photocatalyst was successfully obtained by the sol-gel method. The as-prepared photocatalyst had better photocatalytic efficiency and stability for degradation of RhB under UV light illumination as compared to that of the pure TiO2 and FC. FC is believed to enhance the dispersibility of TiO2, improving the photocatalytic degradation performance of nanometer TiO2. The photocatalyst had a potential application to purify polluted water, and it also provided a new way for FC utilization.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 51202059), the Research Foundation for Youth Scholars of Higher Education of Henan Province (no. 2013GGJS-047), the Key Foundation of Henan Educational Committee (no. 14A430014), and Foundation for Distinguished Young Scientists of Henan Polytechnic University (J2014-05) and the Opening Project of Henan Key Discipline Open Laboratory of Mining Engineering Materials (no. MEM13-4).




