Crystallization Behavior and Thermal Analysis of CoFeB Thin Films
Abstract
We examined two targets containing Co40Fe40B20 and Co60Fe20B20. We deposited Co40Fe40B20 and Co60Fe20B20 monolayer thin films of various thicknesses on glass substrates through DC magnetron sputtering; the thicknesses ranged from 25 to 200 Å. The thermal properties of the Co40Fe40B20 and Co60Fe20B20 thin films were determined using a differential scanning calorimeter (DSC). The thermal properties included the glass transition temperature (Tg), onset crystallization temperature (Tx), and glass-forming ability, which were determined according to these values. Using the Kissinger formula revealed that the activation energy of the Co60Fe20B20 with a thickness of 75 Å is the highest, implying that crystallization was the lowest and the Co60Fe20B20 film showed anticrystallization properties. However, the energy of 75 Å Co40Fe40B20 thin films was the lowest, which is opposite to that of Co60Fe20B20. This observation can be reasonably explained based on the concentration of Co or Fe. Therefore, a thickness of 75 Å is critical.
1. Introduction
In recent years, amorphous CoFeB has been found to have characteristics that can be exploited in scientific research and engineering applications, such as magnetic recording media, magnetoresistance random access memory (MRAM), and gauge sensors. CoFeB thin films with thicknesses in the range 10–50 Å are used in magnetoresistance (MR) devices, and annealed CoFeB thin films exhibit perpendicular or in-plane anisotropy and B diffusion [1–4]. CoFeB thin films demonstrate excellent magnetic and electrical properties because of their amorphous structure and high spin polarization. An as-deposited ferromagnetic CoFeB thin film is typically inserted into a spin-valve structure to form a free/pinned layer of magnetic tunneling junction (MTJ). The formation of the junction leads to increased tunneling magnetoresistance (TMR) and the development of ferromagnetism (FM)/antiferromagnetism (AFM) exchange-biasing anisotropy, which makes the structure suitable for both magnetoresistance random access memory and gauge sensor applications. The increased TMR and exchange-biasing anisotropy are caused by the mean free path of spin being shorter in amorphous materials compared with that of crystalline materials [5–11]. The concentration ratio of Co affects the stability of the amorphous state [12].
In this study, we investigated the crystalline behavior of amorphous CoFeB thin films through thermal analysis. The thermal characteristics and glass-forming ability (GFA) index of amorphous CoFeB thin films are worthy of research. The structure of CoFeB films was determined using X-ray diffraction (XRD) patterns. Nonisothermal differential scanning calorimetry (DSC; TA Instruments DSC 2920) was used to determine thermal properties at a heating rate of 20 K/min. These properties included the glass transition temperature (Tg), onset crystallization temperature (Tx), and liquid temperature (Tl). Crucial related parameters were also measured to estimate the thermal performance, including the range of temperatures at which the film was in the supercooled region (ΔTx = Tg − Tx) and the GFA index, which is obtained using γ = Tx/(Tg + Tl) and γm = (2Tx − Tg)/Tl. To evaluate the resistance to crystallization, the Kissinger formula was used to calculate the activation energy (Q) of crystallization. Thinner Co40Fe40B20 and Co60Fe20B20 films show a higher resistance to crystallization, a wider range of supercooled liquid temperatures, and a higher GFA index because their atomic arrangement is random. This experiment was conducted to improve the atomic crystalline behavior of transformed Co40Fe40B20 and Co60Fe20B20 films.
2. Experimental Details
CoFeB thin films were sputtered on a glass substrate by using DC magnetron sputtering at room temperature (RT) to obtain films with thicknesses ranging from 25 to 200 Å. The base chamber pressure exceeded 2 × 10−6 Torr, and the Ar working pressure was 5 × 10−3 Torr. The atomic compositions of the two CoFeB targets were 60 at % Co, 20 at % Fe, and 20 at % B and 40 at % Co, 40 at % Fe, and 20 at % B. XRD with a CuKα1 line (Philips X’pert) was used to determine the amorphous structure. Thermal performance was investigated using nonisothermal DSC at a heating rate of 20 K/min. Heating rates in the range 10–40 K/min were applied in nonisothermal DSC analysis to determine the crystallization behavior. Several temperatures between Tg and Tx were applied in isothermal DSC analysis to examine the crystallization kinetics. DSC measurement provides qualitative and quantitative data in endothermic (heat absorbing) and exothermic (heat releasing) processes; nonisothermal and isothermal heating methods can be used to obtain information on changes in physical and/or chemical properties.
3. Results and Discussion
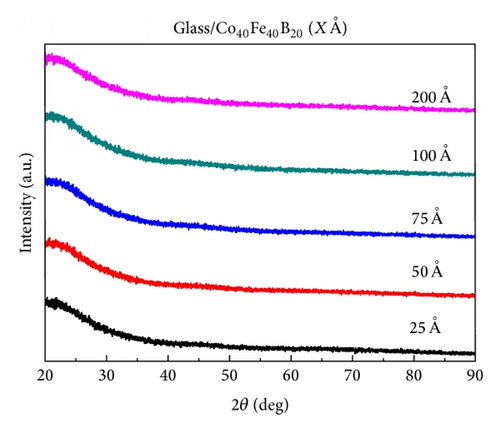
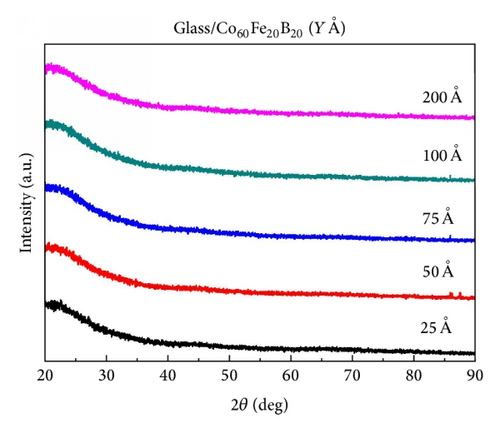
Figures 1(a) and 1(b) present the XRD patterns of Co40Fe40B20 and Co60Fe20B20 thin films in the 2θ range 20°–90°. This XRD result indicated that the XRD patterns of Co40Fe40B20 and Co60Fe20B20 thin films with thicknesses in the range 25–200 Å were amorphous. Thermal analysis was conducted to examine this phenomenon further (Figures 2(a) and 2(b)).
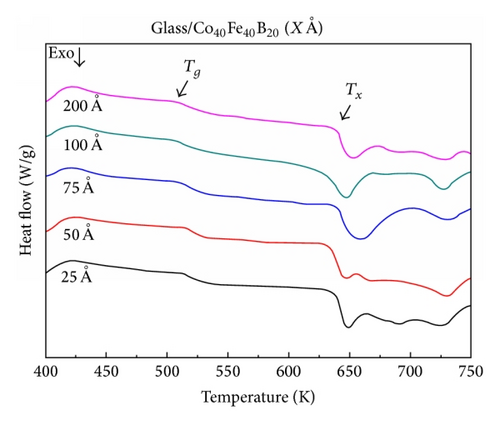
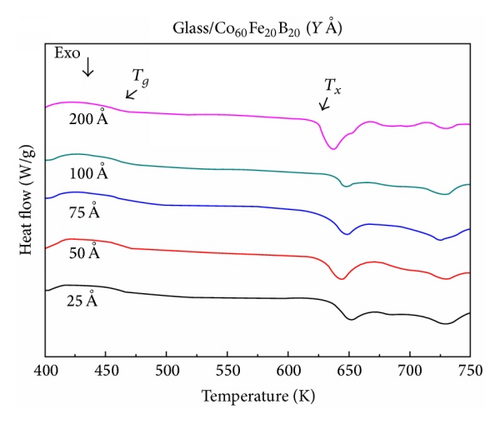
Figures 2(a) and 2(b) show that the amorphous Co40Fe40B20 and Co60Fe20B20 thin films undergo a change from an amorphous state to a crystalline state through an exothermic crystallization reaction and exhibit a supercooled liquid region in DSC measurements when heated at 40 K/min. Figure 2 shows that the two CoFeB thin films have a clear glass transition temperature and undergo an exothermic crystallization reaction in the supercooled liquid region.
The corresponding thermal properties of Co40Fe40B20 thin films are shown in Table 1(a), and those of the Co60Fe20B20 thin films are listed in Table 1(b). The evaluated parameters were used to calculate the supercooled liquid region (ΔTx = Tg − Tx), GFA index, and glass transition temperature (Trg = Tg/Tl, γ = Tx/(Tg + Tl)) and γm = (2Tx − Tg)/Tl [13–15]. The γ and γm values of the GFA index indicated that the Co60Fe20B20 thin film with a thickness of 75 Å demonstrated the most effective GFA and showed a high supercooled liquid region (146 K) and high GFA index (γ = 0.350 and γm = 0.586). The high ΔTx suggests that the film has high thermal stability. However, Co40Fe40B20 yielded opposite results for the same film thickness. These results suggested that, for CoFeB alloy films, a thickness of 75 Å is critical.
| Co40Fe40B20 thickness (Å) | Tg (K) | Tx (K) | ΔTx (K) | Tg/Tl | γ | γm | Q (kJ/mol) |
|---|---|---|---|---|---|---|---|
| 25 | 532 | 635 | 103 | 0.371 | 0.323 | 0.515 | 64.18 |
| 50 | 513 | 682 | 169 | 0.358 | 0.351 | 0.594 | 55.01 |
| 75 | 499 | 612 | 113 | 0.348 | 0.317 | 0.507 | 45.78 |
| 100 | 507 | 622 | 115 | 0.354 | 0.321 | 0.515 | 48.31 |
| 200 | 504 | 647 | 143 | 0.352 | 0.334 | 0.551 | 54.44 |
| Co60Fe20B20 thickness (Å) | Tg (K) | Tx (K) | ΔTx (K) | Tg/Tl | γ | γm | Q (kJ/mol) |
|---|---|---|---|---|---|---|---|
| 25 | 546 | 679 | 133 | 0.381 | 0.343 | 0.567 | 55.18 |
| 50 | 549 | 673 | 124 | 0.383 | 0.340 | 0.557 | 75.82 |
| 75 | 546 | 692 | 146 | 0.381 | 0.350 | 0.586 | 83.44 |
| 100 | 553 | 657 | 104 | 0.386 | 0.331 | 0.532 | 80.20 |
| 200 | 551 | 691 | 140 | 0.385 | 0.349 | 0.581 | 74.46 |
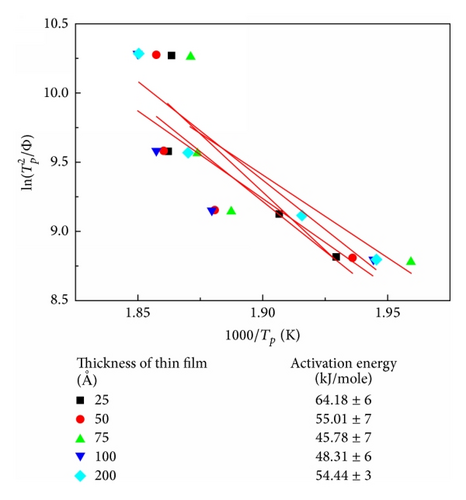
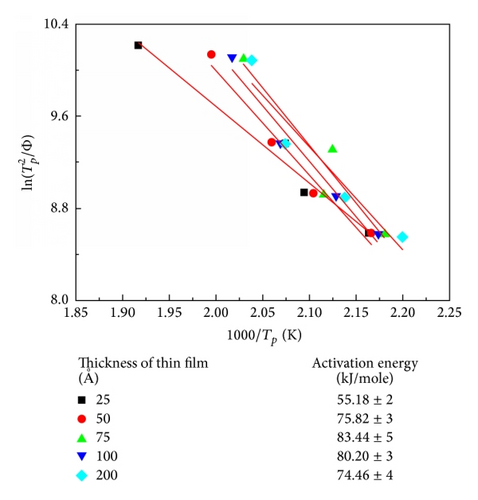
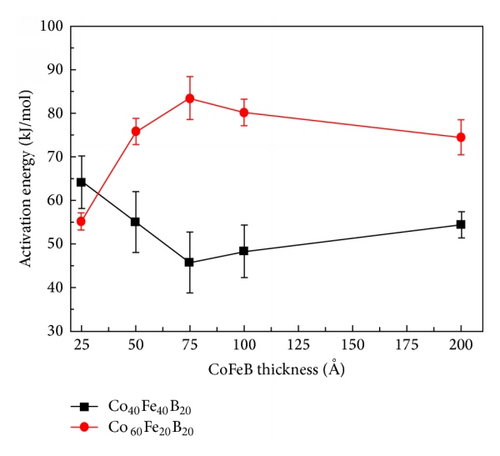
The Kissinger formula was used to calculate the dependence of the Q of crystallization on the thickness of the Co40Fe40B20 and Co60Fe20B20 films, as shown in Figure 4. The two curves exhibit a cross feature and share a critical thickness of 75 Å. These activation energies yielded a critical thickness of 75 Å for amorphous Co40Fe40B20 and Co60Fe20B20 thin films. For the amorphous Co40Fe40B20 film thickness of 75 Å, the lowest activation energy was approximately 45.78 kJ/mol. By contrast, at the same film thickness, the highest activation energy of Co60Fe20B20 was approximately 83.44 kJ/mol. This result was consistent with the calculated DSC results. A higher activation energy corresponds with a higher resistance to crystallization. The thermal performance of the Co60Fe20B20 thin film was higher than that of the Co40Fe40B20 thin film with various Co and Fe concentrations [18–20].
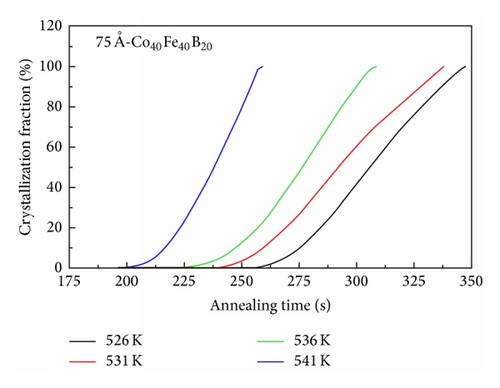
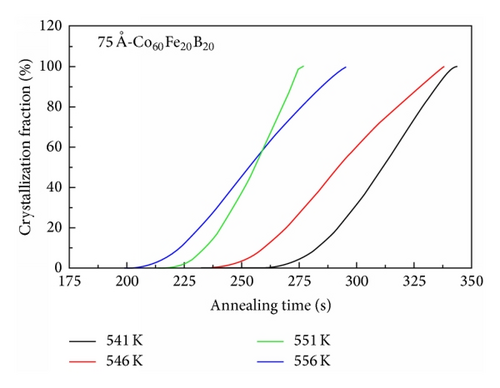
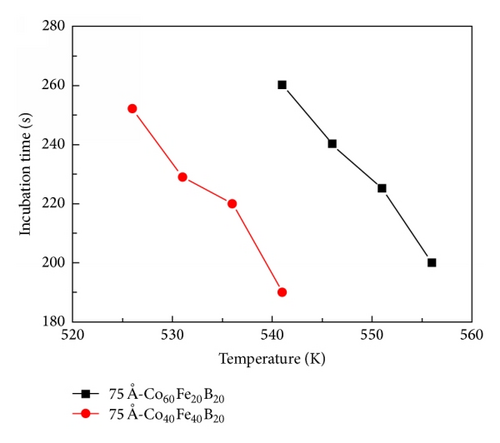
The incubation time of the 75 Å-thick Co60Fe20B20 thin film as a function of the isothermal annealing temperature revealed a longer time than that of the Co40Fe40B20 thin film, as shown in Figure 6. This demonstrates that the thermal stability of Co60Fe20B20 films is higher than that of the Co40Fe40B20 films in isothermal analysis. According to results of DSC and Kissinger formula, the 75 Å-thick is a critical and important point in Co60Fe20B20 and Co40Fe40B20 thin films. The 75 Å-thick Co60Fe20B20 thin film has the highest activation energy. In contrast, the 75 Å-thick Co40Fe40B20 thin film has the lowest activation energy. Figures 5 and 6 investigated the crystallization fraction and incubation time to prove that the thermal stability of Co60Fe20B20 thin film is stronger than Co40Fe40B20 thin film.
4. Conclusions
In summary, the thermal performance, crystallization behavior, calculated anticrystallization, and structure of Co40Fe40B20 and Co60Fe20B20 thin films were investigated using DSC, XRD, and the Kissinger fitting method. The GFA index, defined using γ and γm, increased as the thickness decreased. Moreover, the Kissinger fitting indicated that the critical thickness of the CoFeB thin film was 75 Å. The performance of Co60Fe20B20 thin films is more suitable for amorphous magnetic thin-film applications because of a high GFA index, Q, and ΔTx. A critical result is that amorphous CoFeB thin films can be used in the magnetic recording industry and crystalline applications because of their crystalline behavior. Finally, based on the nonisothermal and isothermal analyses, the thermal stability and incubation time of Co60Fe20B20 films were more favorable than those of Co40Fe40B20 films.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This work was supported by the Ministry of Science and Technology, under Grant no. MOST103-2112-M-224-002. The authors also would like to acknowledge the help on XRD analysis by the Micro and Nano Analysis Laboratory of I-Shou University.




