Co3O4 Electrode Prepared by Using Metal-Organic Framework as a Host for Supercapacitors
Abstract
Co3O4 nanoparticles were prepared from cobalt nitrate that was accommodated in the pores of a metal-organic framework (MOF) ZIF-8 (Zn(MeIM)2, MeIM = 2-methylimidazole) by using a simple liquid-phase method. Analysis by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) showed that the obtained Co3O4 was composed of separate nanoparticles with a mean size of 30 nm. The obtained Co3O4 nanoparticles exhibited superior electrochemical property. Co3O4 electrode exhibited a maximum specific capacitance of 189.1 F g−1 at the specific current of 0.2 A g−1. Meanwhile, the Co3O4 electrode possessed the high specific capacitance retention ratio at the current density ranging from 0.2 to 1.0 A g−1, thereby indicating that Co3O4 electrode suited high-rate charge/discharge.
1. Introduction
Supercapacitor has gained great interests from many researchers with fast charging rate, high power density, and long service life, which is widely applied to portable apparatus, data memory storage system, electric vehicle power supply, and emergency back-up power supply [1–3]. In particular, it has great advantage in the electric vehicle. The United States, Japan, Russia, and so forth have successively invested plenty of manpower and material to research and develop it. The supercapacitor electrode mainly includes three kinds of materials: carbon materials, transition metal oxides, and conductive polymers [4–6]. Co3O4 belongs to cheap metallic oxide in all transition metal oxides, which has been frequently used as the supercapacitor electrode for many years due to good electrochemical capacitance property thereof. However, Co3O4 nanoparticles have sufficiently small particle size; thereby its activity is greatly enhanced as electrode material, and the specific capacitance is effectively increased [7–9]. Currently, some methods of preparing the Co3O4 nanoparticles have been reported in a lot of literatures, and many Co3O4 nanoparticles with the small particle size have been introduced [10–12]. However, it is urgent to prepare Co3O4 nanoparticles with smaller particle size and better dispersion with continuous technology innovation and production demand [12].
Metal-organic frameworks (MOFs) have been widely noticed by people due to open pores, steady pore structures, excellent heat stability, and high ordered crystalline state as new porous materials. They become a new trend to the research fields, such as gas separation and preservation, sensors, drug delivery, and heterogeneous catalysis [13–16]. Bhakta et al. demonstrated that MOFs could be employed as effective hosts for nanoscale metal hydride such as NaAlH4 recently [17]. Fischer et al. were the first to show that MOFs could be used as hosts for transition metal oxides nanoclusters (e.g., TiO2 and ZnO) by gas-phase infiltration with organometallic precursors [18, 19]. The ZIF-8 framework (Zn(MeIM)2, MeIM = 2-methylimidazole), one of the representative MOFs, holds an intersecting three-dimensional channel system, a large pore size (11.6 Å in diameter), and high thermal stability (in N2) as well as good chemical resistance to water and organic solvents [20–23], which may be an appropriate host for preparing Co3O4 nanoparticles by using a solution-based method to introduce the cobalt precursor.
Herein, we report the example of adopting ZIF-8 as host for preparing Co3O4 nanoparticles by a facile liquid-phase method. We show that the directness of cobalt nitrate accommodated in the pores of a ZIF-8 yields Co3O4 nanoparticles after removing the ZIFs. For comparison, we simultaneously analyze the method for preparing Co3O4 nanoparticles by using thermal decomposition via ZIF-67 and compare the structures and the electrochemical property.
2. Experimental
2.1. Synthesis
ZIF-67 (Co(MeIM)2, MeIM = 2-methylimidazole) was prepared according to the reported procedures [24]. Co3O4 nanoparticles were synthesized by heating ZIF-67 at a temperature of 600°C for 5 hours with a heating rate of 2°C/min in air (the prepared Co3O4 nanoparticles were denoted as Co3O4-TH).
ZIF-8 was prepared according to a previously published procedure [21]. Then, the Co3O4 nanoparticles were prepared by soaking cobalt nitrate hexahydrate into the ZIF-8 pore, including the following steps: weighting 0.5 g of ZIF-8, decentralizing them into 10 mL of absolute ethyl alcohol where 0.6 g Co(NO3)2·6H2O is dissolved, stirring for 2 hours by a magnetic stirrer at the ambient temperature, washing the prepared powder three or four times by ethyl alcohol and deionized water, heating up the prepared compound to 600°C at the heating rate of 2°C/min and keeping for 5 hours, decentralizing the prepared powder into NH4Cl (5 M)-NH3·H2O (2.5 M) aqueous solution to remove ZnO decomposed by ZIF-8, centrifugally cleaning and collecting prepared black Co3O4, and then placing them into an oven at 100°C to dry for 12 hours (the prepared Co3O4 nanoparticles were denoted as Co3O4-ZIF).
The crystal structure of Co3O4 was studied by X-ray diffraction (Rigaku TTR-III). The morphology was analyzed by using a scanning electron microscope (SEM, JSM-6700F) and a transmission electron microscope (TEM, JEOL JEM-1200EX). Thermal gravimetric analysis (TGA) measurements were performed on a Netzsch STA 409 thermoanalyzer. The BET surface area measurements were performed with N2 adsorption/desorption isotherms at 77 K on a NOVA 2200e analyzer.
2.2. Electrochemical Performance Measurements
The working electrodes were prepared by mixing 80 wt.% active substance (the prepared Co3O4), 10 wt.% carbon black, and 10 wt.% polytetrafluoroethylene with N-methyl-2-pyrrolidone; subsequently, the mixture was pressed onto a treated nickel foam which served as a current collector under a pressure of 10 MPa and dried at 70°C for 12 h. All electrochemical measurements were performed by an electrochemical workstation in a three-electrode system, with Ag/AgCl as the reference electrode, the Pt electrode as counter electrode, and the Co3O4 film electrode as the working electrode. The electrolyte used in this study was 1 M KOH solution.
3. Experimental Results and Discussion
3.1. Characterization of Precursors ZIF-67 and ZIF-8
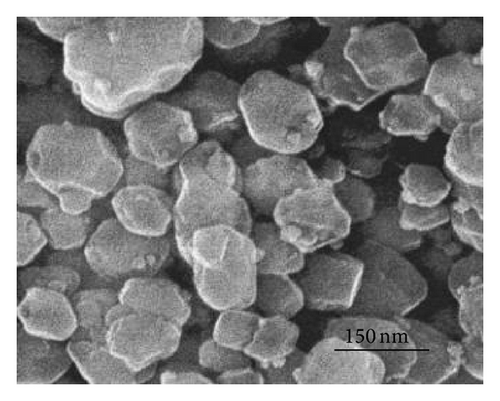
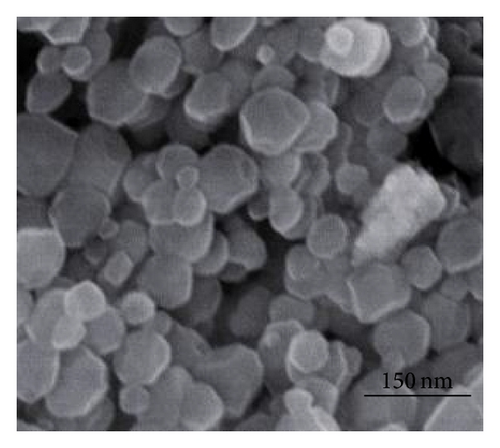
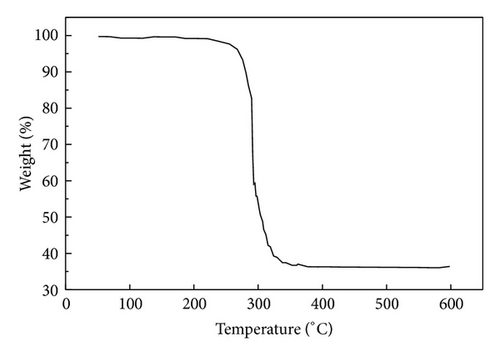
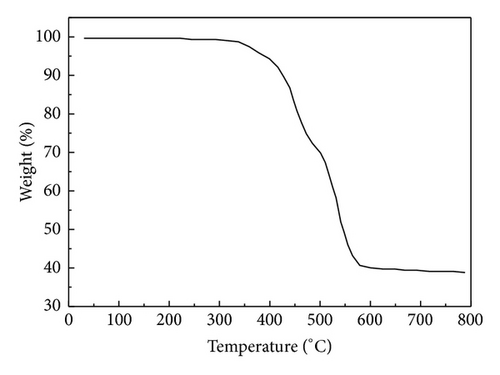
Figure 1 shows the SEM images of precursors ZIF-8 and ZIF-67. SEM pictures reveal that the particles are nanocrystals in a polyhedral shape. The average diameter of ZIF-67 is about 90 nm. It can be clearly seen from the panoramic view (Figure 1(b)) that ZIF-8 sample contains uniform nanoparticles at 50 nm in diameter. The particle size of the ZIF-8 sample is much smaller than that of ZIF-67. Figure 2 gives the TGA curves in air of the precursors. As shown in Figure 2(a), the weight loss of ZIF-67 begins at about 250°C, indicating the onset of oxidation. While thermal decomposition of ZIF-8 begins at 350°C and there is a loss of 58% by weight, the thermal stability of ZIF-67 was slightly lower than that of ZIF-8.
3.2. X-Ray Diffraction Analysis
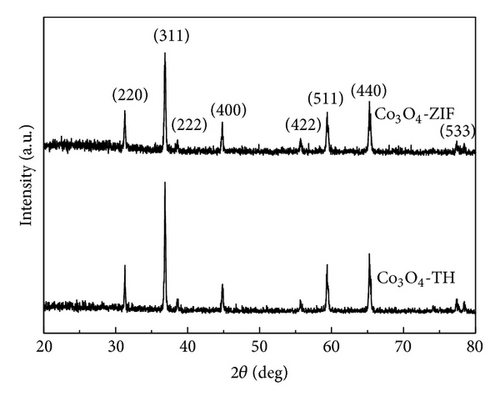
Figure 3 shows XRD chromatograms of Co3O4-TH and Co3O4-ZIF. It is obvious that the positions of the characteristic peaks in the two products are consistent. The peaks in curves of Co3O4-TH and Co3O4-ZIF are assigned to Co3O4 (JCPDS number 43-1003). No other peaks are observed in the XRD patterns. This suggests that the metal-organic framework does not exist after calcining, and there is no evidence of residual ZnO after washing. The average crystallite size calculated from the (311) peak using the Debye–Scherrer equation was 96 nm and 32 nm for Co3O4-TH and Co3O4-ZIF, respectively.
3.3. Morphology
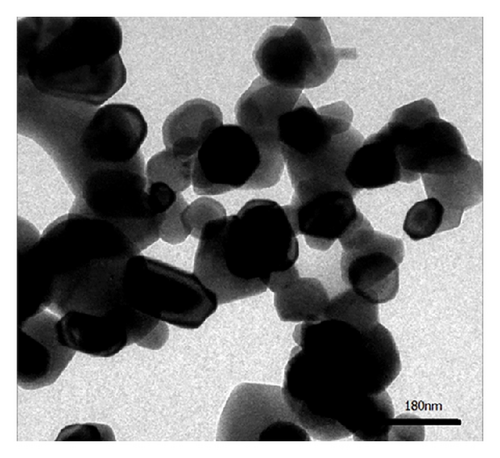
TEM is employed in Figure 4 in order to observe the morphology and microstructure of the Co3O4 prepared by different methods. Figure 4(a) clearly implies that Co3O4 prepared through thermal decomposition of ZIF-67 is sphere-like product in large size. From Figure 4(a), it was apparent that the nanoparticles are seriously aggregated. Meanwhile, the particles are sphere-like structures with an average size about 100 nm. Jiang et al. reported the preparation of Co3O4 by converting cobalt oxide subunits in a Co-MOF (Co3(NDC)3, NDC = 2,6-naphthalene dicarboxylate) by pyrolysis in air. The prepared Co3O4 was agglomerated with an average size around 250 nm [25]. It is basically consistent with our experimental results.
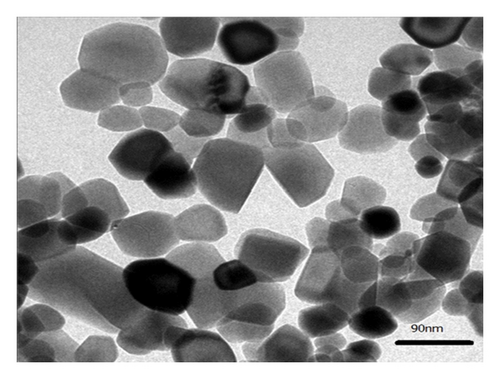
It can be clearly seen from the panoramic view (Figure 4(b)) that the sample contains uniform and weak agglomerated Co3O4 nanoparticles with 30 nm in diameter. We know that the particle size of the Co3O4-ZIF sample is much smaller than that of Co3O4-TH. This suggests that the ZIF-8 framework may play an important role in preventing the migration and aggregation of cobalt precursor in the pores during the formation of Co3O4 upon heating.
3.4. Electrochemical Capacitor Property
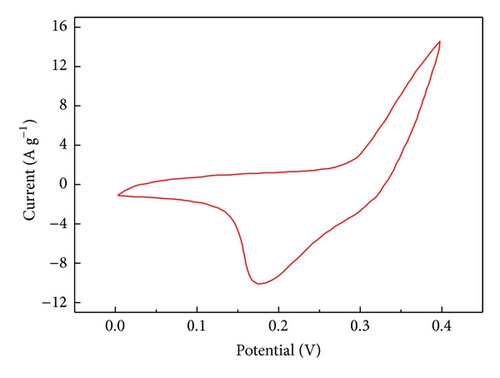
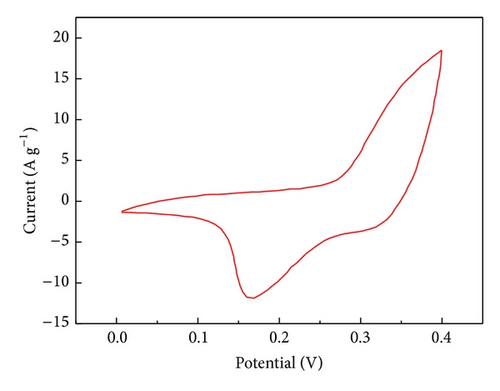
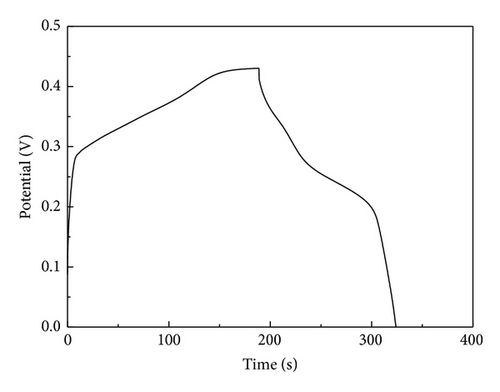
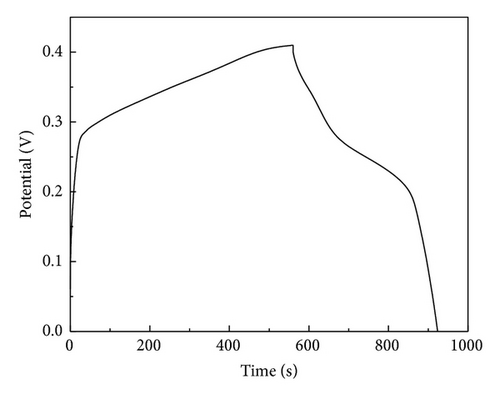
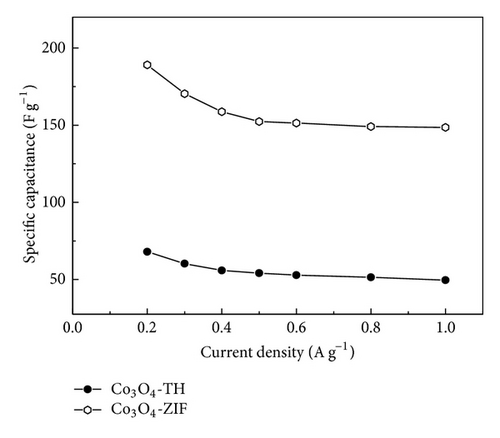
The specific capacitance of Co3O4-ZIF is higher than that of Co3O4-TH. It can be proved from results of TEM that the particle size of Co3O4-ZIF is about 30 nm, while that of Co3O4-TH is about 100 nm. We can see that the latter is much larger than the former in the particle size. At the same time, the Co3O4-TH nanoparticles are seriously aggregated, and the aggregation of particles may decrease the surface area (the BET surface areas were calculated to be 11.32 m2/g and 50.12 m2/g for Co3O4-TH and Co3O4-ZIF, resp.). Based on this point, the former can fully contact with the electrolyte to greatly improve the specific capacitance. We can see that Co3O4 prepared by taking the ZIFs as the host can effectively control particle size and agglomeration and can effectively improve the specific capacitance.
4. Summary
The particle size of Co3O4-TH prepared through thermal decomposition of ZIF-67 as a precursor is relatively large, about 100 nm; meanwhile the Co3O4-TH particle shows agglomeration; the particle size of Co3O4-ZIF prepared by taking ZIF-8 as the host is generally small, about 30 nm, and the Co3O4-ZIF particle does not show agglomeration. From electrochemical property test results of two materials, the specific capacitance of Co3O4-ZIF is obviously greater than that of Co3O4-TH. The specific capacitance of the former reaches 189.1 F g−1 at the current density of 0.2 A g−1. This suggests that the ZIF-8 framework may play an important role in preventing the migration and aggregation of cobalt precursor in the pores during the formation of Co3O4 upon heating. The Co3O4 can fully contact with the electrolyte, greatly improving the specific capacitance.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (2012QNA05), the National Natural Science Foundation of China (Project no. 51304198), and the Natural Science Foundation of Jiangsu Province (Project no. BK2014028-08).




