Anharmonic Spectroscopic Investigation of Tellurophene and Its Perdeuterated Isotopomer: Application of Second-Order Perturbation Theory
Abstract
Vibrational spectra of tellurophene and of its perdeuterated isotopomer were computed using the DFT-B3LYP functional with the LANL2DZ(d,p) basis set. The frequencies of fundamental and overtone transitions were obtained in vacuum under the harmonic approximation and anharmonic second-order perturbation theory (PT2). On the whole the anharmonic corrections reduce the harmonic wavenumber values, in many cases better reproducing the observed fundamental frequencies. The largest anharmonic effects are found for the C–H and C–D stretching vibrations, characterized by relatively high anharmonic coupling constants (up to ca. 120 cm−1). For the C–H/C–D stretches, the harmonic H→D isotopic frequency red-shifts overestimate the observed data by 47–63 cm−1 (5.9–8.1%), whereas the PT2 computations exhibit significantly better performances, predicting the experimental data within 1–19 cm−1 (0.1–2.4%).
1. Introduction
Tellurophene is a five-membered heterocycle (C4H4Te, Figure 1) homologue of the furan molecule. Tellurophene-based compounds have received great attention for the development and fabrication of promising polymeric conductors [1, 2] and nonlinear optical materials [3–8]. The experimental structure of C4H4Te is available from microwave measurements [9], whereas the infrared and Raman spectra of C4H4Te and of its perdeuterated isotopomer (C4D4Te) were recorded in various phases [10–14]. On the theoretical side, the vibrational spectra of C4H4Te were previously calculated in vacuum under the harmonic approximation by using Hartree-Fock [7] and Density Functional Theory (DFT) computations [15]. However, as well-known in the literature, the harmonic treatment often overestimates experimental wavenumbers of fundamentals and overtones, in particular, of the highest-energy spectral regions [16]. To partially circumvent this deficiency, harmonic frequencies can be corrected through scaling procedures [17, 18] or direct anharmonic calculations [19–21]. Anharmonic terms are usually calculated by means of variational [19] or perturbative [20, 21] treatments. As established in the literature [22], the perturbative methods are less accurate than the variational ones. Nevertheless, many recent results attest satisfactory performances of the perturbative methodologies, especially for the prediction of anharmonic contributions to fundamentals and overtones of cyclic compounds [22–30].
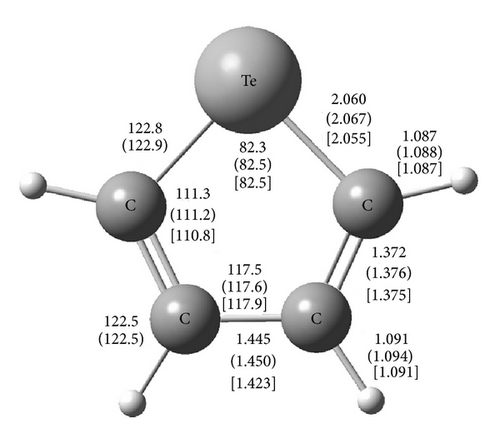
In this work we investigate the effects of the anharmonic corrections on the vibrational wavenumbers of fundamental and overtone transitions of C4H4Te and C4D4Te. The anharmonic terms were predicted in the gas phase using the second-order perturbation theory (PT2) as described in detail by Barone [20]. The calculations were performed using the hybrid three-parameters B3LYP DFT method [31, 32] with the LANL2DZ(d,p) basis set [33, 34]. Anharmonic PT2-DFT spectra have been previously reported for the congeners furan [22, 25], thiophene [22], and more recently selenophene [30]. To the best of our knowledge, anharmonic theoretical wavenumbers of fundamental and overtone transitions of tellurophene and its perdeuterated isotopomer have been computed here for the first time.
2. Computational Details
3. Result and Discussion
Figure 1 displays the bond lengths and angles of C4H4Te calculated at the B3LYP/LANL2DZ(d,p) level. In addition, the figure reports the available experimental data obtained by microwave measurements [9]. The B3LYP/LANL2DZ(d,p) geometry is in good agreement with the observed one, especially for the bond lengths C–Te (within 0.005 Å), C=C (within 0.003 Å), and C–H (the calculated and experimental values are identical), as well as for the bond angles (within 0.2–0.5°). Figure 1 also presents the vibrationally averaged geometries (rz structure) calculated by the vibration-rotation interaction constant values [20]. In line with previous studies on cyclic compounds [29, 30], the vibrational averaging corrections lengthen the bond lengths of C4H4Te by 0.001–0.003 Å, whereas the bond angles vary within 0.2° (C–Te–C).
The experimental infrared and Raman spectra of C4H4Te and C4D4Te were obtained in vapour, liquid, CCl4 solution, and solid phase [10–14]. Theoretically, some calculations on the C4H4Te isotopomer were previously performed using the harmonic treatment [7, 15]. In Tables 1 and 2 we present the B3LYP/LANL2DZ(d,p) harmonic (ω) and anharmonic wavenumbers (ν), infrared intensities (IIR) and Raman activities (ARaman) of C4H4Te and C4D4Te, together with the available observed data for comparison [12]. The assignments of the vibrations were performed using normal modes as displacements in redundant internal coordinates (in the GAUSSIAN 09, the option: Freq = IntModes) and also through the graphical program Chemcraft [36]. The title compounds belong to the C2v symmetry point group with the 21 normal modes categorized as 8A1 + 3A2 + 7B1 + 3B2. All the modes with the exception of the A2 vibrations are infrared active. It is worth noting that, the current assignments of the transitions reasonably agree with those previously determined by experimental [12] and computational [7, 15] studies. The complete sets of experimental fundamentals of both the investigated isotopomers are available from measurements in liquid phase [12], whereas three C–H stretching transitions (modes numbers 1, 2, and 13) of C4H4Te were also detected in gas [11].
| Descriptiona | Calc.b | Exp.c | |||||
|---|---|---|---|---|---|---|---|
| IIR | ARaman | ω | ν | ν | |||
| A1 | 1 | νC–H | 3 | 317 | 3237 | 3089 | 3084 (3106) |
| 2 | νC–H | 12 | 259 | 3191 | 3052 | 3045 (3064) | |
| 3 | νC=C + νC–C + δC–H | 25 | 54 | 1465 | 1430 | 1432 | |
| 4 | νring + δC–H | 12 | 3 | 1341 | 1311 | 1316 | |
| 5 | δC–H | 2 | 5 | 1103 | 1083 | 1079 | |
| 6 | Ring breathing + δC–H | 1 | 22 | 1010 | 986 | 984 | |
| 7 | δring | 18 | 22 | 702 | 692 | 687 | |
| 8 | δC–Te–C | 0 | 9 | 385 | 380 | 380 | |
| A2 | 9 | γC–H | 0 | 1 | 911 | 894 | 912 |
| 10 | γC–H | 0 | 0 | 684 | 663 | 690 | |
| 11 | τring | 0 | 0 | 512 | 503 | 507 | |
| B1 | 12 | νC–H | 3 | 2 | 3234 | 3085 | 3084 |
| 13 | νC–H | 4 | 118 | 3176 | 3037 | 3030 (3047) | |
| 14 | νC=C + δC–H | 3 | 0 | 1548 | 1516 | 1516 | |
| 15 | δC–H | 31 | 3 | 1255 | 1220 | 1246 | |
| 16 | δC–H | 0 | 4 | 1099 | 1077 | 1079 | |
| 17 | δring | 3 | 0 | 805 | 794 | 797 | |
| 18 | νC–Te | 2 | 8 | 557 | 547 | 552 | |
| B2 | 19 | γC–H | 0 | 0 | 876 | 859 | 884 |
| 20 | γC–H | 132 | 0 | 683 | 667 | 674 | |
| 21 | τring | 5 | 0 | 358 | 355 | 354 | |
| rms deviationd | 66 | 11 | |||||
| rms deviatione | 144 | 6 | |||||
| rms deviationf | 21 | 12 | |||||
- aν: stretching; δ: in-plane bending; τ: torsion; γ: out-of-plane bending.
- bCalculations were carried out in vacuum at the B3LYP/LANL2DZ(d,p) level.
- cLiquid phase [12]. The values in parentheses refer to the gas phase [11].
- dAll vibrational modes.
- eνC–H modes.
- fAll vibrational modes excluding νC–H modes.
| Descriptiona | Calc.b | Exp.c | |||||
|---|---|---|---|---|---|---|---|
| IIR | ARaman | ω | ν | ν | |||
| A1 | 1 | νC–D | 2 | 143 | 2400 | 2294 | 2308 |
| 2 | νC–D | 3 | 104 | 2355 | 2270 | 2262 | |
| 3 | νC=C + νC–C + δC–D | 31 | 57 | 1443 | 1409 | 1405 | |
| 4 | νring + δC–D | 11 | 7 | 1208 | 1169 | 1180 | |
| 5 | δC–D | 2 | 23 | 839 | 828 | 824 | |
| 6 | Ring breathing + δC–D | 0 | 10 | 782 | 770 | 768 | |
| 7 | δring | 14 | 12 | 640 | 632 | 630 | |
| 8 | δC–Te–C | 0 | 9 | 374 | 371 | 370 | |
| A2 | 9 | γC–D | 0 | 1 | 753 | 742 | 741 |
| 10 | γC–D | 0 | 0 | 533 | 520 | 548 | |
| 11 | τring | 0 | 0 | 438 | 432 | 437 | |
| B1 | 12 | νC–D | 1 | 4 | 2395 | 2313 | 2308 |
| 13 | νC–D | 1 | 55 | 2339 | 2249 | 2240 | |
| 14 | νC=C + δC–D | 7 | 0 | 1493 | 1458 | 1455 | |
| 15 | δC–D | 12 | 2 | 1002 | 986 | 995 | |
| 16 | δC–D | 2 | 4 | 853 | 840 | 811 | |
| 17 | δring | 1 | 0 | 706 | 697 | 704 | |
| 18 | νC–Te | 3 | 7 | 524 | 517 | 522 | |
| B2 | 19 | γC–D | 0 | 0 | 683 | 673 | 696 |
| 20 | γC–D | 77 | 0 | 506 | 497 | 502 | |
| 21 | τring | 3 | 0 | 329 | 325 | 326 | |
| rms deviationd | 43 | 12 | |||||
| rms deviatione | 91 | 10 | |||||
| rms deviationf | 18 | 12 | |||||
- aν: stretching; δ: in-plane bending; τ: torsion; γ: out-of-plane bending.
- bCalculations were carried out in vacuum at the B3LYP/LANL2DZ(d,p) level.
- cLiquid phase [12].
- dAll vibrational modes.
- eνC–H modes.
- fAll vibrational modes excluding νC–H modes.
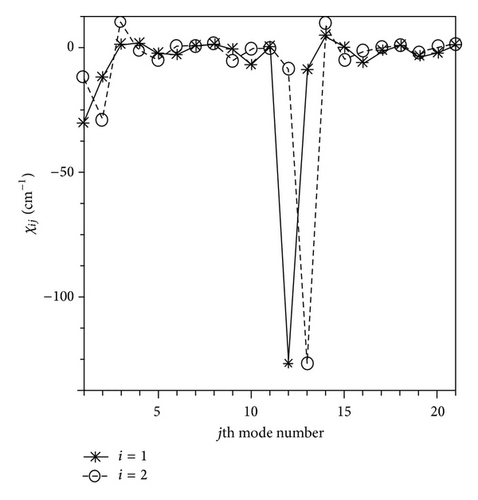
As can be appreciated from the data reported in Tables 1 and 2, the largest anharmonic contributions are found for the νC–H and νC–D transitions (modes. numbers 1, 2, 12, and 13), decreasing the harmonic frequency values of C4H4Te by 140–150 cm−1 (ca. 4%) and of C4D4Te 80–110 cm−1 (ca. 3-4%), in agreement with previous PT2 computations on other cyclic compounds [22–30]. As a consequence, the agreement between the experimental and calculated wavenumbers for the νC–H and νC–D stretches is significantly improved by the anharmonic treatment (within 1–14 cm−1, 0.03–0.61%). Note that when we consider the gas phase experimental νC–H fundamentals of C4H4Te (modes numbers 1, 2, and 13, Table 1), the experimental/calculation deviations are slightly augmented (up to 17 cm−1). To elucidate the origin of the above large anharmonic effects, as a case test, we analysed the contributions of the anharmonic constant values (χi,j) for the νC–H modes numbers 1 and 2 of C4H4Te (Figure 2). The most significant χi,j corrections are produced by the diagonal term (χi,i) as well as by the coupling with the remaining C–H stretching modes. In the specific case of the modes numbers 1 and 2, the largest anharmonic coupling are χ1,12 and χ2,13, which are predicted to be ca. −120 cm−1 and recover about 80% of the total anharmonic corrections (ω − ν, (3)). Other nonnegligible anharmonic contributions, although less substantial than χ1,12 and χ2,13, are given by the χ1,1, χ2,2 (ca. −30 cm−1 for both the couplings), and χ1,2 (−12 cm−1) terms.
For the νC–H/νC–D modes, we evaluated the H→D isotopic wavenumber downward shifts (ΔνH/D = νC–H − νC–D) obtained by the harmonic and anharmonic calculations. The deviations of the calculated ΔνH/D data from the observed values are reported in Figure 3, together with the percentage errors. The harmonic approximation overestimates the experimental shifts by 47–63 cm−1 (5.9–8.1%), whereas the anharmonic computations, with the exception of mode number 1, underestimate the isotopic shifts showing smaller errors (1–19 cm−1, 0.1–2.4%). The results are particularly excellent for the mode number 2.
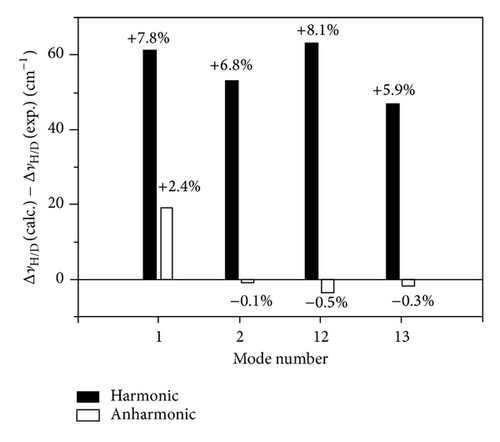
Table 3 collects the frequencies of the overtone bands of title compounds obtained using the harmonic and anharmonic approaches, together with the available experimental data which are only limited to eleven (C4H4Te) and five (C4D4Te) vibrational modes. Confirming the above results of the fundamentals, the greatest anharmonic corrections for the overtones occur for the νC–H (νC–D) transitions, which reduce the harmonic values by ca. 5-6% (4-5%).
| Symm. | Mode no.a | C4H4Te | C4D4Te | ||||
|---|---|---|---|---|---|---|---|
| Calc.b | Exp.c | Calc.b | Exp.c | ||||
| ω | ν | ν | ω | ν | ν | ||
| A1 | 1 | 6474 | 6144 | 4801 | 4575 | ||
| 2 | 6382 | 6047 | 4711 | 4516 | |||
| 3 | 2931 | 2848 | 2851 | 2886 | 2810 | ||
| 4 | 2683 | 2617 | 2627 | 2415 | 2343 | ||
| 5 | 2205 | 2167 | 2160 | 1678 | 1652 | ||
| 6 | 2019 | 1968 | 1966 | 1563 | 1541 | ||
| 7 | 1404 | 1382 | 1279 | 1263 | 1254 | ||
| 8 | 769 | 759 | 758 | 748 | 740 | ||
| A2 | 9 | 1823 | 1788 | 1822 | 1505 | 1485 | |
| 10 | 1369 | 1322 | 1385 | 1067 | 1039 | ||
| 11 | 1024 | 1009 | 1014 | 877 | 864 | 872 | |
| B1 | 12 | 6469 | 6083 | 4789 | 4598 | ||
| 13 | 6351 | 6013 | 4679 | 4478 | |||
| 14 | 3096 | 3019 | 3015 | 2986 | 2921 | 2916 | |
| 15 | 2510 | 2451 | 2004 | 1969 | |||
| 16 | 2199 | 2154 | 1706 | 1680 | |||
| 17 | 1611 | 1588 | 1412 | 1393 | |||
| 18 | 1114 | 1093 | 1111 | 1049 | 1031 | 1049 | |
| B2 | 19 | 1753 | 1717 | 1768 | 1367 | 1347 | 1383 |
| 20 | 1367 | 1331 | 1013 | 993 | |||
| 21 | 716 | 709 | 657 | 650 | |||
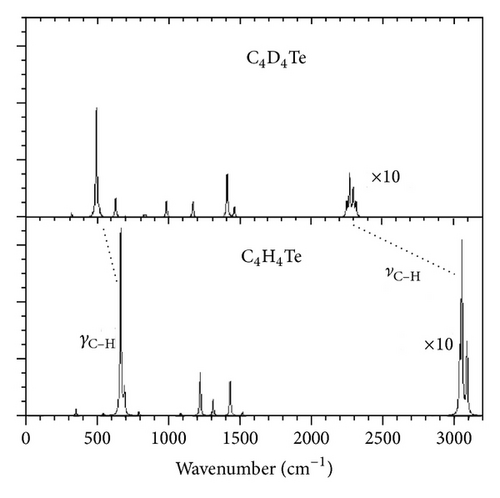
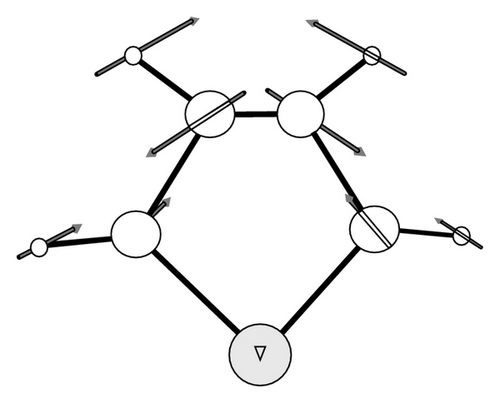
In Figures 4 and 5 we plot the infrared and Raman spectra obtained by the anharmonic calculations using pure Lorentzian band-shapes with a full width at half maximum of 10 cm−1. The C–H and C–D stretching transitions are placed in the highest-wavenumber regions of the vibrations spectra. The lowest-energy region of the infrared spectra of C4H4Te and C4D4Te is mainly characterized by an isolated and strong absorption located at 667 cm−1 (IIR = 132 km/mol) and 497 cm−1 (IIR = 77 km/mol), respectively, to be compared with the experimental values of 674 cm−1 (−1%) and 502 cm−1 (−1%), respectively. The vibrational analysis ascribes this transition (mode number 20) to a pure out-of-plane C–H (and C–D) bending deformation, γC–H (γC–D). A graphical representation of the atomic displacement vectors involved in this vibration is depicted in Figure 6(a).
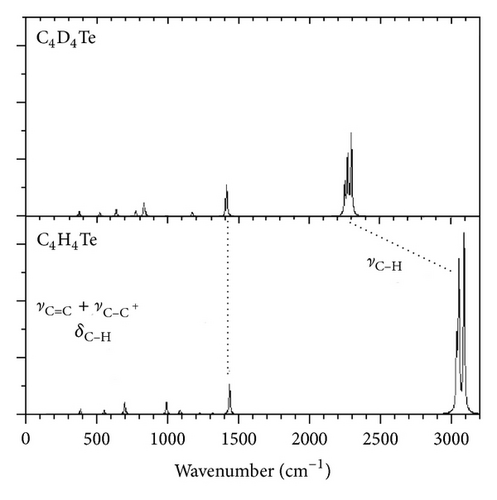

The most active transitions in the Raman spectra of C4H4Te and C4D4Te involve the C–H and C–D stretches (Tables 1 and 2). From the present calculations, an almost isolated and relatively intense peak (ARaman ~ 50 Å4/amu) located near 1400 cm−1 appears in the Raman spectra of both tellurophene and its perdeuterated isotopomer. This transition (mode number 3) also visible in the infrared spectra is assigned to the C=C + C–C bonds stretchings with the nonnegligible contribution from the in-plane C–H (C–D) bending motion (Figure 6(b)). The present harmonic computations place this band at 1465 cm−1 (C4H4Te) and 1443 cm−1 (C4D4Te), overestimating the observed wavenumbers of 1432 cm−1 (+2.3%) and 1405 cm−1 (+2.7%), respectively. Note that the corresponding PT2 anharmonic frequencies here calculated at 1430 cm−1 (C4H4Te) and 1409 cm−1 (C4D4Te), give a noticeably better agreement with experiment (within 0.1–0.3%).
4. Conclusions
In this work we computed harmonic and PT2 anharmonic vibrational frequencies of fundamentals and overtone bands of C4H4Te and C4D4Te. The computations were performed in vacuum at the B3LYP/LANL2DZ(d,p) level of theory. The infrared and Raman spectral profiles were simulated using Lorentzian band-shapes and the most informative transitions were discussed. In general, the calculated harmonic frequencies deviate from the observed ones, with rms deviations of 66 and 43 cm−1 for C4H4Te and C4D4Te, respectively. The introduction of the PT2 anharmonic contributions in several cases significantly reduces the calculation/experiment discrepancy, giving rms deviations of 11 cm−1 (C4H4Te) and 12 cm−1 (C4D4Te). A vibrational analysis reveals that the anharmonic corrections are especially conspicuous for the νC–H and νC–D transitions, decreasing the harmonic wavenumber values, respectively, by 140–150 cm−1 (ca. 4%) and 80–110 cm−1 (ca. 3-4%), and noticeably improving the agreement with the experimental data. As a consequence, in comparison to the harmonic approximation, the observed H→D isotopic frequency red-shifts for the C–H and C–D stretches are better reproduced by the PT2 scheme with deviation up to 1–19 cm−1 (0.1–2.4%). Important anharmonic effects (ca. 35 cm−1, 2.3%) are also found for the mode number 3 (νC=C + νC–C + δC–H, νC=C + νC–C + δC–D), characterized by a relatively intense peak in the Raman spectra. In line with the recent literature, the present results on tellurophene isotopomers confirm that the anharmonic PT2-DFT approach can be employed to accurately predict the infrared and Raman spectra of heterocyclic compounds.
Conflict of Interests
The author declares that there is no conflict of interests regarding the publication of this paper.




