Molecular Targeted Approaches for Advanced BRAF V600, N-RAS, c-KIT, and GNAQ Melanomas
Abstract
The introduction of a newly developed target therapy for metastatic melanomas poses the challenge to have a good molecular stratification of those patients who may benefit from this therapeutic option. Practically, BRAF mutation status (V600E) is commonly screened although other non-V600E mutations (i.e., K-R-M-D) could be found in some patients who respond to therapy equally to the patients harboring V600E mutations. Furthermore, other mutations, namely, N-RAS, KIT, and GNAQ, should be sequenced according to distinct melanoma specific subtypes and clinical aspects. In our report, a practical flow chart is described along with our experience in this field.
After decades of unsatisfactory treatments for advanced melanoma, in the last five years, new treatment modalities have been explored that dramatically change the current clinic scenario. The introduction of targeted therapies for melanoma is based on the discovery of genes that are linked to the initiation, progression, and invasion of the tumor [1]. More specifically, somatic mutations in the BRAF, NRAS, KIT, and GNAQ genes are critical to correctly stage and manage patients with metastatic disease who can nowadays benefit from these modern molecular targeted therapies. The mutations affect receptor tyrosine kinases and the MAPK and MTOR pathways display different frequencies in distinct histopathological subtypes of melanoma [2].
Somatic mutations in BRAF have been found in almost 50% of all melanomas [3, 4] and most commonly in melanomas derived from skin without chronic sun-induced damage [5]. The result of these mutations (mainly V600E) is enhanced BRAF kinase activity and increased phosphorylation of downstream targets, particularly MEK.
In particular, BRAF inhibitors, targeting the common V600E mutations, have become increasingly popular since they have a high objective response rate and few side effects.
In a previous study we demonstrated that patients harboring uncommon BRAF V600R-M-D mutations, not included in the original experimental protocols of BRAF selective inhibitors, were the responders to the therapy. Surprisingly, patients harboring non-V600E BRAF mutations revealed an objective clinical response similar to V600E melanoma patients [6, 7].
In the clinical setting, BRAF mutations are routinely screened but when BRAF mutation is not detected, melanomas should be screened for N-RAS, KIT, and GNAQ mutations.
RAS genes are mutated in up to 20% of melanomas which are typically thicker and have a higher mitotic rate [8]. Higher frequency of KIT mutation in melanoma is associated with older patients and the acral and mucosal melanoma subtypes [8]. Somatic mutations in the GNAQ and GNA11 genes are found in 80% of uveal melanomas [9]. Nowadays, patients with N-RAS, KIT, and GNAQ mutated tumors can be enrolled in clinical trials of specific inhibitors [2, 8–11].
In the experience of our institution, thirty-two BRAF mutated melanomas (32%) were detected among 99 melanomas screened for genetic mutations. Among BRAF mutation-negative melanomas, 6 N-RAS mutations (four Q61R, one Q61K, and one Q61L) and 3 KIT mutations (N822K) were found. The lower BRAF mutation rate found in our study compared to the literature might be due to a selection bias since we screened only patients with metastatic disease.
Hot spot V600E mutations were found in 27 patients. V600R mutation and double (V600E-V600M) mutation were identified in two melanomas. In five cases, V600K mutations were found. Two screening failures were noted.
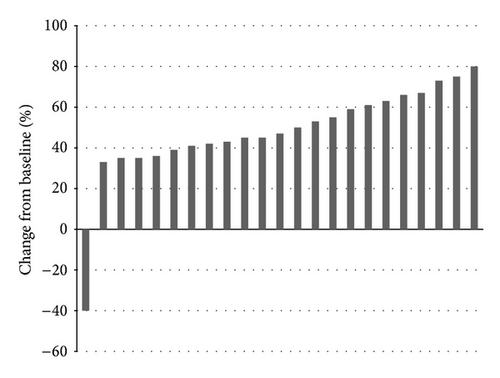
Twenty-three patients with BRAF mutated metastatic melanoma were enrolled in the protocol with BRAF inhibitors for compassionate use at the University of Modena. Two N-RAS mutated patients were enrolled in an alternative anti-NRAS protocol in another University. Mean progression-free survival for BRAF positive patients at followup of 8 weeks was 7.6 months (Table 1) (Figure 1). There was no statistically significant difference in the duration of the objective tumor response among different BRAF status groupings. An objective response with few side effects was observed in all except one patient (Table 2).
| Patient ID | Gender | Age | Treatment duration (months) | Objective response | Time to progression (months) | Followup (months) | Status |
|---|---|---|---|---|---|---|---|
| 1 | F | 60 | 6 | Partial | 6 | 18 | Dead |
| 2 | F | 61 | 26 | In response | 26 | 29 | Alive |
| 3 | F | 66 | 4 | Partial | 4 | 13 | Dead |
| 4 | F | 51 | 14 | Partial | 14 | 19 | Alive |
| 5 | M | 59 | 4 | Partial | 4 | 5 | Dead |
| 6 | F | 67 | 6 | Partial | 6 | 7 | Dead |
| 7 | M | 51 | 6 | Partial | 6 | 18 | Alive |
| 8 | M | 70 | 6 | Partial | 6 | 11 | Dead |
| 9 | F | 68 | 23 | Partial | In response | 23 | Alive |
| 10 | F | 62 | 6 | Partial | 6 | 11 | Alive |
| 11 | F | 81 | 8 | Partial | 7 | 8 | Dead |
| 12 | M | 56 | 2 | None | 3 | 3 | Dead |
| 13 | F | 51 | 5 | Partial | 5 | 5 | Dead |
| 14 | F | 58 | 18 | Partial | In response | 18 | Alive |
| 15 | M | 68 | 6 | Partial | 14 | 6 | Dead |
| 16 | M | 43 | 9 | Partial | 3 | 16 | Dead |
| 17 | M | 62 | 6 | Partial | In response | 6 | Alive |
| 18 | M | 58 | 8 | Partial | In response | 18 | Dead |
| 19 | M | 38 | 2 | Partial | 2 | 3 | Dead |
| 20 | M | 66 | 12 | Partial | 10 | 12 | Alive |
| 21 | M | 65 | 6 | Partial | In response | 6 | Alive |
| 22 | M | 79 | 7 | In response | Stable disease | 7 | Alive |
| 23 | M | 75 | 8 | Partial | 6 | 8 | Dead |
| 1 N-ras | M | 69 | 10 | Partial | 10 | 12 | Alive |
| 2 N-ras | M | 56 | 6 | Stable | 6 | 7 | Alive |
| Side effects | Frequency (%) |
|---|---|
| Arthralgia | 54% |
| Nausea | 34% |
| Skin erythema | 28% |
| Vomiting | 14% |
| Headache | 13% |
| Fatigue | 11% |
| Keratoacanthomas | 2% |
| Hypertransaminasemia | 2% |
| Alopecia | 1% |
| QTc prolongation | 1% |
- (1)
Screen for V600E BRAF mutation in melanoma patients with advanced disease (i.e., unresectable stages III and IV) as well as those at high risk of disease progression (stages IIIB and IIIC).
- (2)
In case of negative-V600E BRAF mutation, look for other non-V600E BRAF mutations (i.e., K, M, R, D).
- (3)
Melanomas not showing BRAF mutations should be investigated for N-RAS mutations.
- (4)
Double-negative BRAF and N-RAS melanomas should be further explored for KIT mutations or amplifications. This is even more relevant for acral and mucosal melanomas that should be investigated for both BRAF and KIT mutations at the first step.
- (5)
Triple-negative melanomas may benefit from GNAQ mutation evaluation, especially for uveal melanoma.
For melanoma, like other cancers, tailored therapies are dramatically changing the current approaches for treating patients with metastatic disease. However, the heterogeneous molecular defects in melanoma account for the development of drug resistance and thus the different clinical objective responses of targeted therapies. It is known that resistance to BRAF inhibitors is due to either the acquisition of secondary mutations in the BRAF gene or upregulation of other molecular pathways such as platelet-derived growth factor receptor β or N-RAS, the consequences of which lead to resistance to MEK and ERK inhibitors [12, 13]. Independent research teams have identified three mechanisms by which melanoma can develop resistance to BRAF inhibitors [13, 14]. The findings suggest that BRAF inhibitors will need to be combined with other types of drugs, although future studies will have to determine the relative frequency of each mechanism. To conclude, future efforts will be directed not only to develop multitargeted therapies (i.e., BRAF and MEK inhibitors) but also to further investigate the combination of target treatments and promising immune-therapy approach.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.