Predicting and Preventing Flow Accelerated Corrosion in Nuclear Power Plant
Abstract
Flow accelerated corrosion (FAC) of carbon steels in water has been a concern in nuclear power production for over 40 years. Many theoretical models or empirical approaches have been developed to predict the possible occurrence, position, and rate of FAC. There are a number of parameters, which need to be incorporated into any model. Firstly there is a measure defining the hydrodynamic severity of the flow; this is usually the mass transfer rate. The development of roughness due to FAC and its effect on mass transfer need to be considered. Then most critically there is the derived or assumed functional relationship between the chosen hydrodynamic parameter and the rate of FAC. Environmental parameters that are required, at the relevant temperature and pH, are the solubility of magnetite and the diffusion coefficient of the relevant iron species. The chromium content of the steel is the most important material factor.
1. Introduction
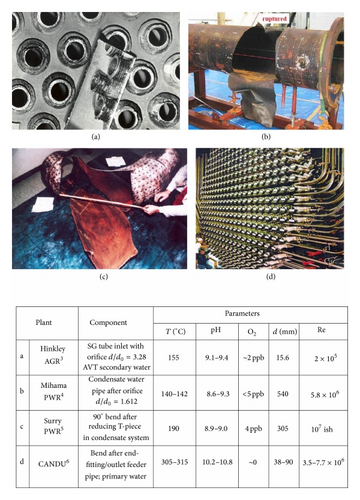
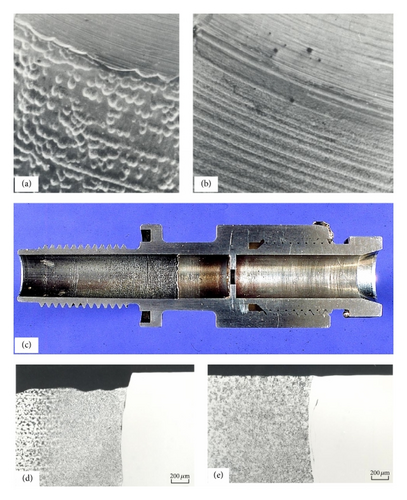
Flow accelerated corrosion (FAC) of carbon steels in water has been a major concern in civil nuclear power production for over 40 years [1, 2]. The important features of FAC are the linear or increasing rate with time and the generation of a scalloped surface. Its effects have been unique in two important ways. Firstly it has affected nearly every reactor type worldwide and sometimes in more than one location; Figure 1 shows some examples [3–6]. Secondly it is probably the only corrosion mechanism that has led to accidents that have caused fatalities. There have been pipe ruptures leading to a release of steam and deaths of workers, but it must be emphasized that such fatalities are not unique to nuclear plants.
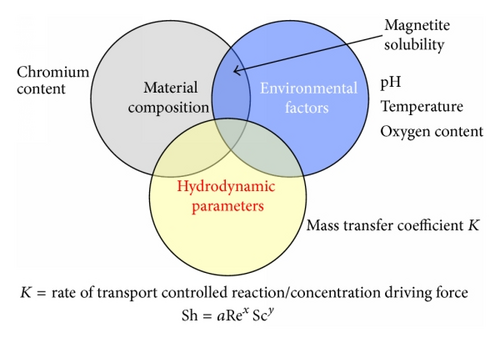
The occurrence of FAC, or erosion corrosion as it is sometimes known, is critically dependent on the following: the temperature and chemistry of the environment (pH and oxygen content), the hydrodynamics of the system, and the composition of the steel particularly the chromium content; this is shown schematically in Figure 2.
There are various approaches to predicting the possibility and the rate of flow accelerated corrosion. Testing has involved either actual components or a chosen specimen. In addition there are theoretical or empirical models, some computer based, available to allow the prediction of attack.
It is readily apparent that any review must be very selective; other reviews are available [7–10]. This review has tried to cover areas that the author has been involved with over the last 40 years that have been neglected or are contentious. It follows earlier reviews [11–14] but focuses on FAC of steels in the nuclear power plant industry. For both practical and mechanistic reasons there is a need to identify the hydrodynamic parameter which controls the occurrence and rate of erosion corrosion. It was previously suggested [12] that there was a spectrum of mechanisms which could be involved in erosion corrosion. This review concentrates on the corrosion end of the spectrum. It is argued that for dissolution based mechanisms the important hydrodynamic parameter is the mass transfer coefficient (K). The relationship between the rate of FAC and K is discussed in some detail and the development and effect of surface roughness on both K and FAC are considered. Finally some aspects of the solubility of magnetite and the prediction and prevention of FAC are discussed. This review is not a best buy guide to predictive programs.
2. Hydrodynamics
- (i)
Velocity (V).
- (ii)
Reynolds number (Re).
- (iii)
Mass transfer coefficient (K).
- (iv)
Surface shear stress (τ).
- (v)
Intensity of turbulence (TI).
- (vi)
Freak energy density (FED).
The mass transfer coefficient is intimately linked to wall shear stress, and the two cannot be practically separated either experimentally or mathematically [16].
| Parameter | Some measuring techniques | References |
|---|---|---|
| The velocity (V). |
|
[11, 17, 18]. |
| Reynolds number (Re). | Calculated from velocity, diameter of tube, and kinematic viscosity | [11, 17, 18]. |
|
|
[12, 19–23]. |
|
|
[12, 18–20, 24, 25]. |
|
|
[11, 18–20]. |
|
Complex analysis of fluctuations in limiting current density on isolated small electrode, single author use. | [26–28]. |
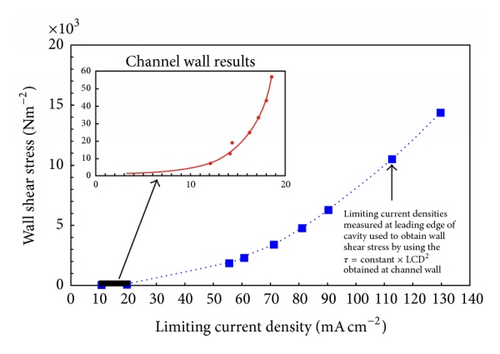
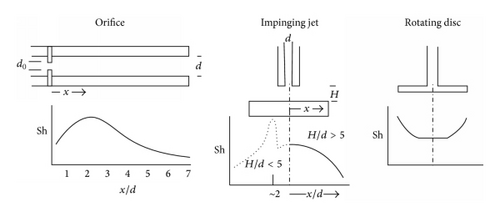
This view has been challenged by a number of workers [25, 29] based on the breakdown of the analogy between mass, heat, and momentum in detached flow. The consequences of this are that in detached flow the surface shear stress cannot be calculated from the measured limiting current density (LCD) at an isolated microelectrode, where in normal flow τ is proportional to the cube of the limiting current density (Leveque equation); this is demonstrated in Figure 3. This problem seems to have been ignored by Schmitt and Gudde [30] whose approach was to obtain a functional relationship between shear stress and limiting current density for a normal flow situation (Figure 4). Then to use the same relationship to convert LCDs measured in disturbed flow to shear stresses (Figure 4). Apart from being invalid this approach involved the extrapolation of a maximum shear stress in the channel wall of under 60 N/m2 to over 14000 N/m2 in the disturbed flow.
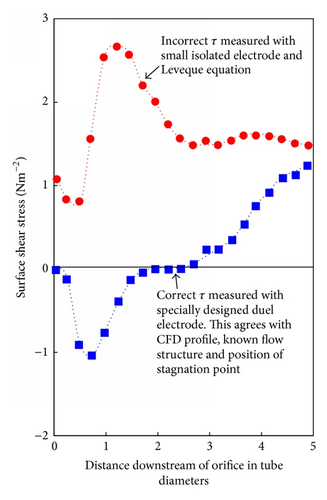
One way of refuting the importance of τ is its variation, for example, downstream of an orifice where the FAC and shear stress profiles are fundamentally different (see Figures 3 and 5). This refutation of the importance of τ has apparently been misunderstood [32]. A similar rejection of the dominant role of τ has been made by Matsumura et al. [33, 34] in work with an impinging jet. Another reason to believe that τ is unimportant is because it seems that the surface shear stress is not sufficient to produce the effects ascribed to it, namely, mechanically disrupt a surface film.
For copper alloys in seawater Matsumura showed that the FAC profile did not correspond to the measured shear stress distribution on an impinging jet specimen but could be explained by the forces, measured with a pressure transducer, due to the peak in turbulence intensity at about 2 jet diameters from the centre of the specimen. Of course mass transfer is caused by bulk convection and turbulent convection, so while ruling out the primacy of the importance of shear stress this does not prove the importance of mechanical factors. It must be remembered that increased mass transfer can dissolve away a protective surface layer; this was elegantly analysed by Coney [35].
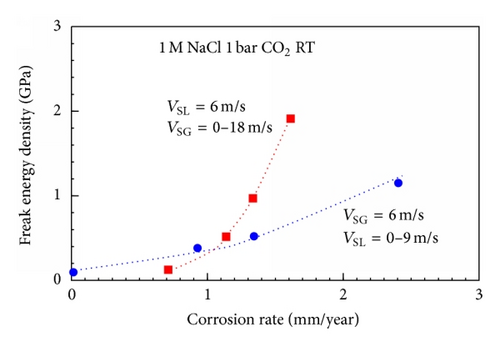
It was found that forces in high energy microturbulence elements oriented perpendicularly to the wall are finally responsible for the scale destruction (freak energy density (FED) Model). The maximum FED in a flow system can be measured with appropriate tools; however, in practical cases this is not necessary, because in a given flow system the FED is proportional to the wall shear stress by a factor in the order of 105 to 106. Thus, for practical application and flow system evaluations wall shear stresses can be used to quantify flow intensities in given flow systems [27].
- (1)
Although there are time response limitations involved in LCD measurements it might be expected that some freak events could at least be partially detected, after all real freak waves can be observed.
- (2)
No attempt to measure such high stresses was made, for example, by fast response pressure transducers, as Matsumura did.
- (3)
The use of the Leveque equation to obtain freak energy densities from freak current densities is highly questionable.
- (4)
There is not a single relationship between τ and FED [27, 28] and from a practical point of view this would have to be obtained for each geometry of interest. And measuring surface shear stresses in detached flow is, as outlined earlier, not possible using Schmitt’s technique.
- (5)
How rough surfaces that develop naturally during corrosion are dealt with is unclear.
Once removed the high local flow intensities prevent the re-formation of protective layers and, hence, start fast mass transport controlled local metal dissolution (FILC, also called erosion corrosion).
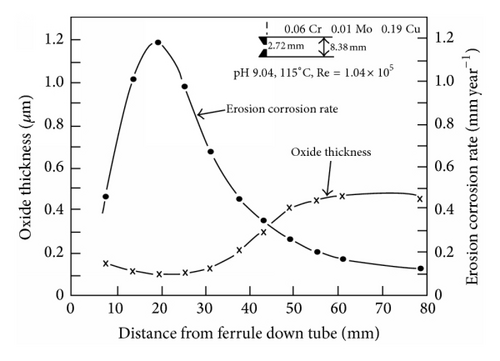
For steels in pure boiler water there is good evidence that the protective magnetite layer is thinned (not cracked) and the FAC rate correlates with this thinning [31] and the mass transfer coefficient (Figure 5). There is also the possibility that the mass transfer could be large enough to lead to the film being completely removed by dissolution. In this case the subsequent rate of attack would probably be limited by activation kinetics. This film dissolution appears to have occurred on carbon steel exposed to sodium nitrate solutions under an impinging jet [11].
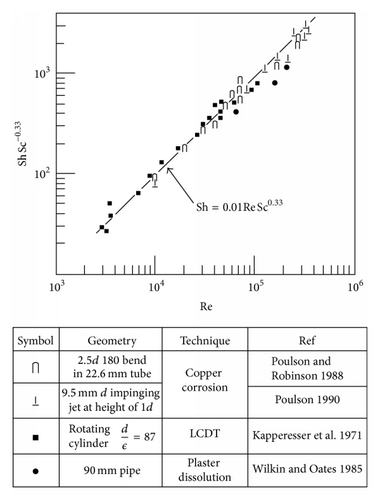
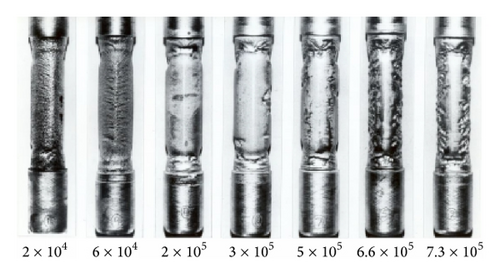
Of course in most situations in which corrosion is occurring the surface undergoes some roughening; this then raises the question of how to measure the changes in the hydrodynamic parameter as the roughness develops. Roughness development and other considerations (Table 1) led the current author to develop, justify, and apply the dissolution of copper in ferric chloride solutions [21, 37–41] (Figure 7), to the measurement of mass transfer coefficients; typical specimens from single-phase and two-phase studies are shown in Figures 8 and 9.
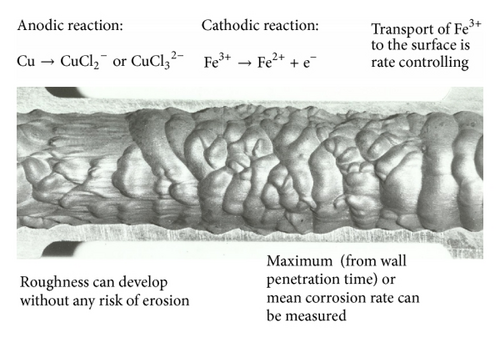



Because some predictive models use an enhancement factor to describe a geometries effect relative to a straight tube such a factor is often quoted. It must be emphasised that this factor is usually a function of the Reynolds number and its use has nothing to do with—the mass transfer coefficient at the point of interest becomes difficult to measure or even define, so “enhancement factors” over the straight-pipe values are employed [32].
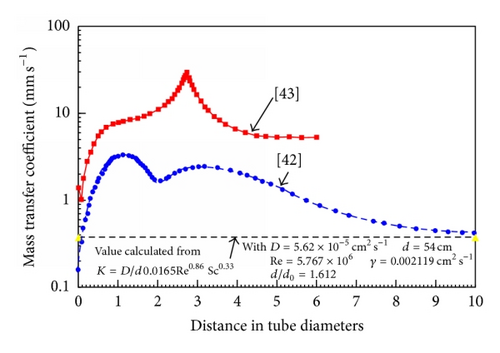
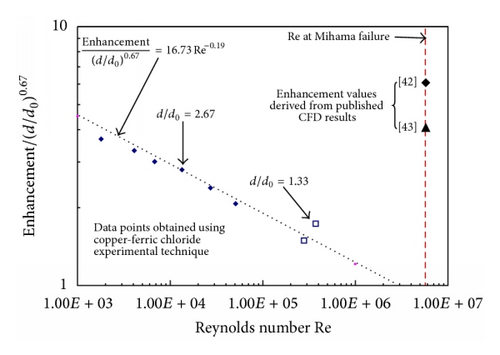
Recently there has been an increasing tendency to use computation fluid dynamics (CFD) techniques to obtain hydrodynamic parameters for subsequent use in FAC assessments. CFD has the ability to investigate very high Re’s, which often cannot be reached in experimental studies. However CFD results must be shown to agree with well established data before it can be applied to the higher flows. A number of applications of CFD are listed in Table 2; unfortunately some of these computations have been carried out without any comparison to well established experimental data; in some cases there are significant differences. This is particularly evident after the Mihama-3 FAC failure downstream of an orifice; the mass transfer characteristics of which have been studied by a number of workers and reviewed by others. Figures 10 and 11 are a summary of and comparison with excepted correlations. The difference between the two CFD predictions is of some interest; the higher results may be a misprint in units, as the other data agrees very well with a simple calculation for fully developed flow. It is of great interest that the CFD predicted enhancements are much higher than those from expected correlations downstream of an orifice, yet no comparison with the well established lower Reynolds number data were made. Also in this authors’ view CFD has not been shown to be capable of modelling the roughness and its effects that develops naturally by metal dissolution or oxide deposition.
| Authors | Purpose | Comments | References |
|---|---|---|---|
| Uchida et al. | Prediction of FAC with Mihama as test case | No comparison with existing data and its extrapolation to high Re’s or effects of roughness | [42] |
| Hoashi et al. | Prediction of FAC with Mihama as test case | No comparison with existing data and its extrapolation to high Re’s or effects of roughness. | [43] |
| Pietralik and Smith, Pietralik and Schefski |
|
Compares with some but not all bend data. Attempts to deal with roughness development and component interactions | [44, 45] |
| Nesic and Postlethwaite | Predict e-c following sudden expansion | Makes key point that profiles of K and shear stress do not correlate | [29] |
| Zinemams and Herszaz | Predict FAC in bifurcation and nozzle | Limited detail to check results but claims there is agreement, effects of roughness not considered. | [46] |
| Yoneda | Predict FAC in PWR and BWR, of 45° elbow | Interesting but again no comparison with others or the effects of roughness | [47] |
Roughness development in situations where a solid is dissolving under mass transfer control has been investigated by a number of workers. One theory attributes roughness to the imprinting of the fluid on the surface [48] and the other to the role of surface defects [49]. Criteria for the development of roughness have not been fully developed and it is perhaps overlooked that there is a tendency for leveling of any surface which is dissolving under diffusional control [39]. For example, consider an isolated roughness element exposed to single phase flow. It would be expected that the rates of dissolution of both the peak of the roughness element and the region after the roughness element were both higher than the region upstream. For roughness to develop the region after the roughness peak must dissolve faster than the roughness peak itself. In two phase flow it is probable that the annular film would leave the wall after the peak rather than inducing separated flow as in single phase conditions. Thus it would seem reasonable that in annular two-phase flow roughness development would be more difficult and would tend to occur only when the thickness of the wall film was such that a recirculation zone could form.
- (1)
While defects can produce surface roughness, they are not a requirement. The roughness that develops usually reflects the flow structure. For example, Figure 7 illustrates the symmetrical peaks at the inlet to the 180° bend and evidence of counter rotating vortices in the bend region.
- (2)
There appears to be a critical Re required for roughness to develop. The critical Re value ~5 × 104 in 8 mm diameter tubing [40] is significantly higher than that found using dissolving plaster of Paris [22], where roughness developed at the lowest Re tested of 1.9 × 104 in 25 mm diameter tubing.
- (3)
There are other indications [39] that using the dissolving plaster of Paris technique can give misleadingly high values of K due to erosion of the plaster occurring.
- (4)
If the surface roughens the mass transfer can increase and there are indications [40] that the roughness becomes more important then than the geometry in influencing mass transfer.
- (5)
An upper bound mass transfer correlation for all rough surfaces has been proposed [38] as shown in Figure 12:
() - (6)
There is some evidence, Table 3 [41], that the development of roughness has a smaller effect on geometries where flow is separated, as compared to normal flow. In the former turbulence is generated away from the wall, while in normal flow it is generated close to the wall. This was first realized with the region downstream of an orifice in that some enhancement in mass transfer occurred in some tests but again nowhere near a Reo dependency (Figure 10). Also for a multi-impinging jet and a cylinder in restricted cross flow the rough surface mass transfer correlations all have a Reynolds number dependency with an exponent less than one, Figure 13, with
() - (7)
If roughness develops, the height (ε) and wavelength (λ) of the roughness elements decrease with increasing Re [40]. This means that the enhancement in mass transfer caused by roughness does not increase with increases in ε/d. There is a suggestion [50] that the roughness that develops at any Re produces the maximum resistance to flow.
- (8)
If roughness develops the enhancement factor of any geometry over a straight tube is not a constant [37] and will usually increase with the Reynolds number. For the region downstream of an orifice the enhancement decreases with increases in Re for both smooth and rough surfaces.
| Normal flow | Separated flow | |
|---|---|---|
| Shear stress and K | Related | Not related |
| Turbulence created | Near wall | Away from wall |
| Roughness effects on K and ΔP | Both increase | Evidence for increase lacking |
| Roughness effects on FAC | Limited clear evidence of increase | Evidence for increase lacking |
- But in all probability there will be a number of situations that are between these two extremes.

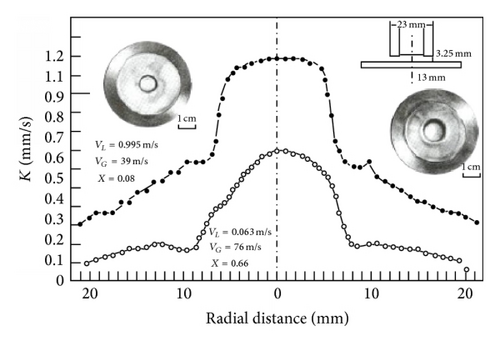
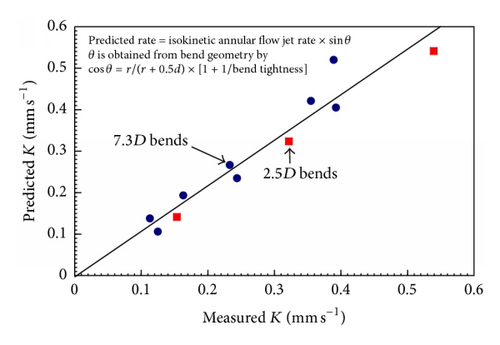
In two-phase flow it is not immediately obvious how the mass transfer data can be simply incorporated since there is some evidence [39] that Chen’s correlation is not valid. Figure 14 shows a simple model can apparently be used to relate the tightness of bends to the resulting influence of the annular flow on the mass transfer at bends. There is evidence that mass transfer effects dominate at least up to 50 m s−1; but clearly mechanical damage will occur above some critical velocity which needs to be defined; this leads to the concept of a spectrum of mechanisms as suggested earlier [12]. Models ascribing droplet impingement causing mechanical damage are referenced later in predictive models but are outside the scope of this paper.
The relationship between K and FAC must not be assumed to be linear; this is discussed later in this review.
3. Environmental Variables
- (i)
Temperature.
- (ii)
pH.
- (iii)
Oxygen content.
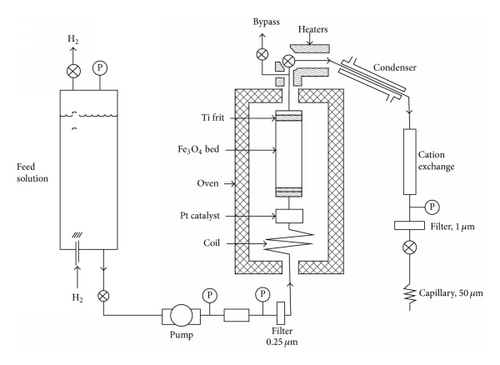
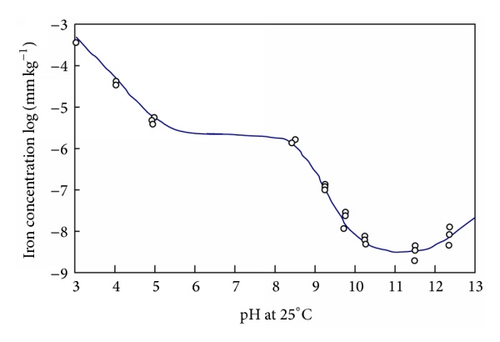
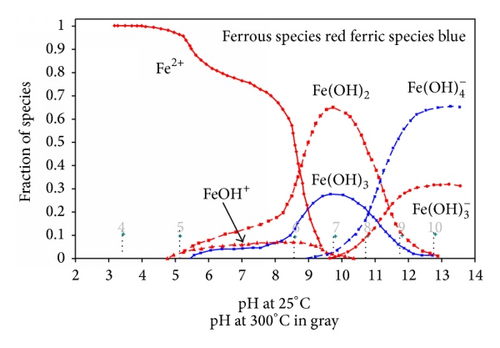
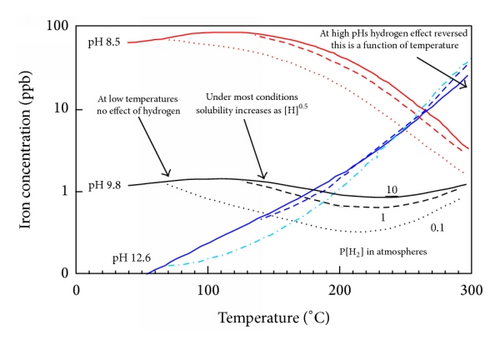
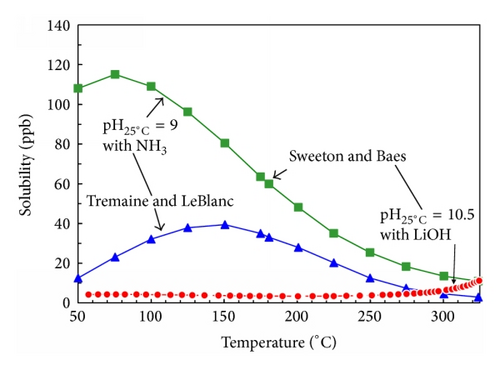
In general solubility’s can be calculated from available thermodynamic data or measured. For a very sparingly soluble oxide like magnetite the preferred option has been to experimentally determine it using equipment such as that shown in Figure 15. There are a number of experimental difficulties such as the need to establish steady state conditions under controlled redox conditions, the problems with colloidal material, and the influence of trace impurities. Such solubility data, for example, Figure 16, is then used to obtain thermodynamic data for the various possible dissolving species, for example, Figure 17. The total solubility of iron species can then be modeled and predictions can be made (Figure 18).
- (1)
The gradient of solubility with respect to pH changes from negative to positive at a critical pH, producing a typical U-shaped curve, Figure 16. The pH value this occurs at is of intense interest; an estimate (using T&LeB data [53]) can be obtained from
() - (2)
The gradient of solubility with respect to increasing temperature changes from negative at lower pH to positive at higher pH, Figure 18; this occurs at ~9.4 and 9.9 at temperatures of 300°C and 150°C, respectively [53].
- (3)
There are various functional relationships between iron solubility and hydrogen content which arise because of the details of the stable species and the nature of the oxide dissolution reaction, Table 4 and Figure 18.
- (i)
Usually [53, 56] the solubility is proportional to :
() - (ii)
As pHs increases the solubility is predicted [53] to become proportional to , and that will occur above a certain temperature [55]. Consider
()However the data appears to be insufficient to make accurate predictions of pH’s and temperature’s and the expectation is that the transition will be gradual. - (iii)
Solubility is independent of [H2] at low temperatures below ~83°C [55]. But this temperature increases with increases in [H2]. This is due to the activity of ferrous ions in solution being controlled by a hydrous ferrous oxide phase rather than magnetite [55].
- (i)
- (4)
There appear to be significant differences between the two most quoted data sets as shown in Figure 19. In particular the values of Tremaine and LeBlanc (T&LeB) are lower than those of Sweeton and Baes (S&B) and mirror the temperature dependency of FAC more closely.
- (5)
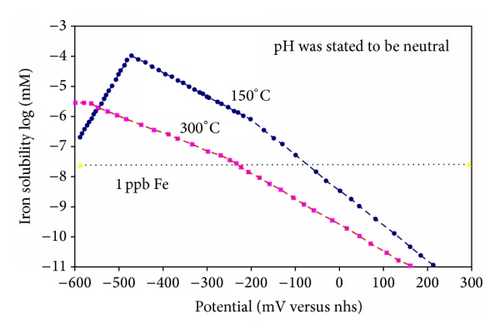
Under conditions where ferrous ions are dominant the effect of potential [59], Figure 20, or oxygen content of the water is profound and of great practical importance; the solubility of haematite is exceedingly small.
- (6)
There is no solubility data available for magnetite containing chromium; such data might allow the role of chromium to be clarified.
| Regiem | Hydrogen dependency | Reference |
|---|---|---|
| Normally | [H]1/3 | [53, 55, 56] |
| At high pHs above ~220°C | [H]−1/6 | [53, 55] |
| At temperatures below ~80–110°C | Independent | [55] |
| At high temperatures above pH~9.8 | Less than [H]1/3 | [55] |
- Such effects could influence both dissolution and deposition.
In Figure 21 the results of Coney are shown and compared to the recalculated values for FeOH+, which Coney suggests is the most relevant species. It appears that the recalculated values are approximately half of the earlier values and a correlating equation obtained using Table Curve* is given.
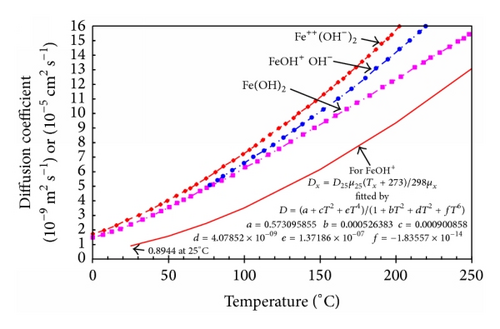
Temperature also influences the density and viscosity of water; such values are readily available in the literature.
4. Material Influences
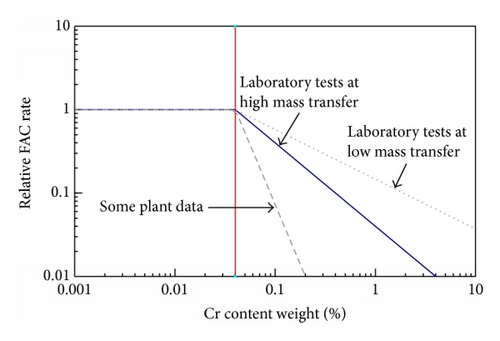
In general water chemistry changes are so much easier to implement than material changes; it is only in new or replacement components that material changes can be made.
It is believed that the first instance when the possibility of FAC influenced, indeed determined, the choice of material (1%Cr1/2%Mo) was for the economizers for the British advanced gas cooled reactors (AGRs) once through boilers at Torness and Heysham 2. This was substantiated by long term tests (22800 hour) in laboratory rigs, on-site rigs, and in situ SGs exposure. Both tight 180° bends and orifice geometries were utilized, Figure 24. Interestingly the feedwater system for the British PWR at Sizewell, ordered later, was specified as carbon steel. While there is no dispute as too the benefits of chromium additions there have been at least three suggestions as to its mechanism.

5. Predictive Approaches
5.1. Testing
There are two possible approaches. The first is to test all geometric features of interest under realistic and accelerated conditions to obtain margins of safety. Figure 24 shows such a rig used to test AGR components at design and twice design velocities for times up to 22800 hours.
The alternative approach is to use a specimen with a range of mass transfer rates as illustrated in Figure 25, or a single specimen over a range of flow rates. In all cases it is good practice to check any relationship, between the suspected hydrodynamic parameter and the rate of FAC, with tests using a different specimen geometry.
- (1)
Tests are carried out long enough to obtain realistic steady state rates.
- (2)
The method of measuring the corrosion rate has the required precision; various methods have been suggested [12].
- (3)
The environmental conditions in the test rig really do correspond to the practical situation. The use of a marker geometry, that is, one which corrodes at a known rate, has much to commend itself.
5.2. Theoretical Based Models
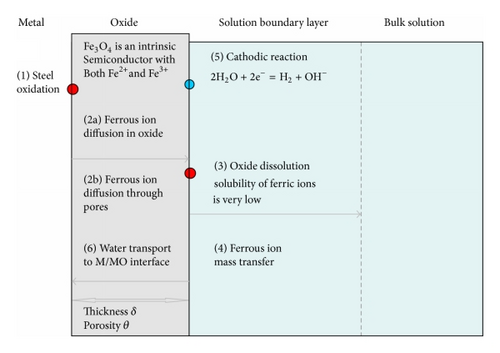
There appears to be reasonably widespread agreement that the basic mechanism of FAC is the enhanced transport of dissolved ferrous ions away from the metal surface. This causes the protective magnetite film to be thinned down and results in essentially linear kinetics. This is to be compared to parabolic kinetics during normal corrosion.
Re (the dimensionless Reynolds number) is given by V(d/γ) and Sc (the dimensionless Schmitt number) is given by γ/D.
V is the relevant velocity (m s−1), d is the characteristic specimen length (m), γ is the kinematic viscosity (m2 s−1), and D is the diffusivity of the relevant species (m2 s−1).
However, most if not all the predictive models involve additional processes shown in Figure 25. The differences in the various mechanistic models arise in the way the processes are quantified and combined, in particular in the explanation of the key environmental parameters especially the temperature and how the flow dependency of the measured FAC rates can be explained.
From a predictive aspect a very important difference is that some theories predict a linear relationship between mass transfer and flow accelerated corrosion rate: a nonlinear rate is predicted by other models particularly the CEGB theory of Bignold, Garbett, and Woolsey. Other workers have argued that a greater-than-linear dependency of rate on mass transfer equates to a mechanical contribution probably in removing oxide.
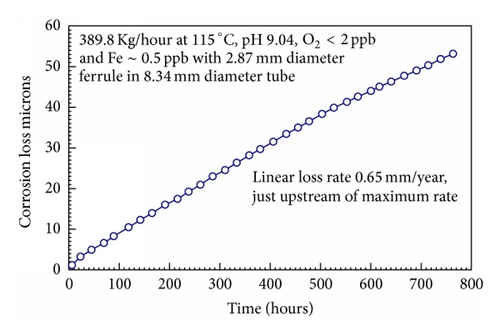
High resolution measurements of the FAC rate downstream of a ferrule or orifice suggested that the rate did not increase with time as the surface roughened, Figure 27, and roughness effects do not appear to have been incorporated into the CEGB model.
The porosity behavior with temperature is difficult to model. It appears to be temperature dependent and not linear. Based on many experiments using the CIROCO test loop, taking into account different temperatures, mass transfers and chemicals, (16) has been simplified as
where H(T) is a temperature-dependent function normalized to K and which leads to a hydrogen content (in mg kg−1) equivalent for increasing the iron solubility. Only experiments can lead to this function and data are not shown.
The Sanchez Calder model [77] was developed to predict the rate of FAC in steam extraction lines. It incorporated most of the processes shown in Figure 26, with an equation similar to the EDF model, and highlighted their view of the importance of the oxide porosity, which was subsequently incorporated into some other models.
- (i)
improved magnetite solubility data,
- (ii)
assumption that porosity was a function of oxygen content of water,
- (iii)
introduction of several piping configurations and surface finishes.
Burrill and Cheluget [80] followed a similar approach to CEGB workers in having the magnetite solubility (calculated using S&B) as a function of potential and thus the corrosion rate. However under CANDU condition of high pH and high temperature the dependency of magnetite solubility could be less than the normally accepted [H]1/3 relationship.
- (1)
Some magnetite dissolution occurs.
- (2)
Dissolution loosens magnetite crystals.
- (3)
Surface shear stress spalls or erodes crystals.
- (4)
Thinned magnetite is less protective.
Subsequent work in collaboration with Japanese workers, including Uchida, has subsequently developed their own evaluation program because Unfortunately, details of their (other models) theoretical basis and of the data bases of the program packages are classified due to intellectual property rights. Both traceability of the computer program package and its validation are required for making policy on plant reliability when applying the package calculations.
A paper covering the evaluation of FAC simulation code based on verification and validation has been published [82], which describes the steps involved as follows.
Step 1. Define flow velocities and temperatures in system: 1D CFD code RELAPS used.
Step 2. Calculate O2 levels ECP, and so forth: 1D O2-hydrazine reaction code CORRENV used.
Step 3. Distribution of mass transfer coefficients: 3D CFD code PLASY MTCEXTRA used.
Step 4. Danger zone evaluation: chart analysis DRAWTHREE-FAC.
Step 5. Distribution of wall thinning: wall thinning code WATH.
Step 6. Evaluation of residual life and effect of counter measures: final evaluation DRAWTHREE-FAC.
Interestingly this paper appears to indicate the importance of obtaining the mass transfer rate but does not mention the importance of the surface shear stress.
5.3. Empirical Models
- (a)
Environmental factors.
- (b)
A modified Kc, which also included downstream influences:
()
5.4. Other Models
The combination of a deterministic model which reflects a mechanistic picture of FAC and a purely nondeterministic ANN model which reflects best experimental representation of the phenomenon are the best tools for extracting information from complex phenomenon such as FAC [79].
A Russian program ECW-02 based on CHECWORKS has been developed, and a Ukrainian program KASESC based on WATHEC has also been produced. RAMEK is a more original Russian approach that has been described and reviewed [8] that appears to combine a loss rate due to mass transfer with a loss rate due to the surface shear stress peeling of the oxide layer.
Various models [89–91] have been developed to predict droplet attack at bends in two-phase flow, but they appear to ascribe damage to be mechanical in nature. As outlined earlier the conditions causing such attack, as opposed to corrosion, need defining.
The relative usage of the various programs is that CHECWORKS is used in USA, Canada, Taiwan, Japan, South Korea, Czechia, Slovakia, and Slovenia ~153 units, EDF is only used in France but in all 58 units, and COMSY is used in Germany, Spain, Finland, Hungary, and Bulgaria ~17 units.
6. Discussion
A simplified summary of how the prediction schemes deal with some of the variables that have been dealt with in this review is given in Table 5, and suggested reasons for the bell shaped dependency of FAC rate with temperature are summarized in Figure 29.
| Model | FAC function of Vm or Kn | Roughness effects | Solubility relationship | Cause of FAC reduction at Low T and High T |
|---|---|---|---|---|
|
Acknowledged but not specifically integrated. | S and B | MDR * Solubility | |
|
Yes and specified relationship | S and B |
|
|
| Sanchez Calder [77] | Linear n = 1 or less | Apparently not | S and B |
|
|
|
Yes but not specified | Their own which is bell shaped at pH 7 and increases with temperature at pH 9 | Unclear |
| Not stated but m apparently between 0.6 and 0.7 | Yes but suggested and did not occur | Unclear which or if incorporated into model, though clearly identified differences between S and B and T and LeB data | MDR Solubility | |
| Siemens KWU [84–86] |
|
Apparently not | Unclear which or if incorporated into model | Unclear |
| Lister and Lang [15] and Uchida et al [81] | Nonlinear n > 1 due to shear stress on oxide | Yes | S and B or T and LeB? | Unclear |
- *MDR is magnetite dissolution reaction.
6.1. Importance of Mass Transfer
It is widely agreed that the mass transfer coefficient is the hydrodynamic parameter that controls the occurrence and rate of FAC. A relationship between rate of FAC and the mass transfer coefficient, with a power dependency of greater than one, is an erroneous reason to invoke the importance of τ the surface shear stress. It is believed that there are more cogent reasons to reject the involvement τ as an important parameter. These have been dealt with in detail earlier and elsewhere. Briefly the variations in τ, K, turbulence level, and flow assisted corrosion rate with distance downstream of an orifice are sufficient to confirm that τ is not important. It is probable that a similar statement will be found for other regions where detached flow occurs and the relationship between mass transfer heat-transfer and pressure drop breaks down.
6.2. Relationship between FAC and Mass Transfer and Magnetite Solubility
It is clear that there is not always a simple linear relationship between the FAC rate and the mass transfer rate, for example, the variation in FAC downstream of an orifice. Of the various predictive schemes only the EdF model appears to suggest a linear model, and the position of the EPRI code is unclear. Figure 28 compares some CEGB and EDF data.

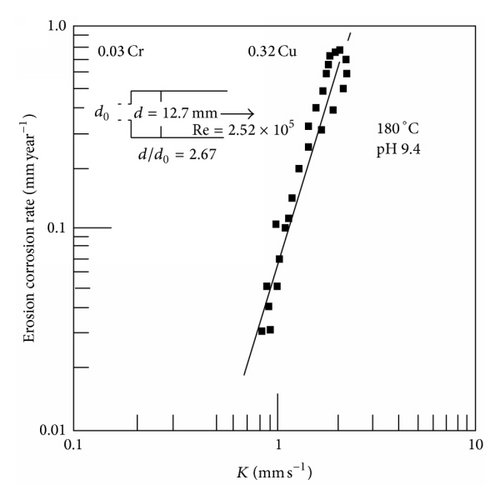
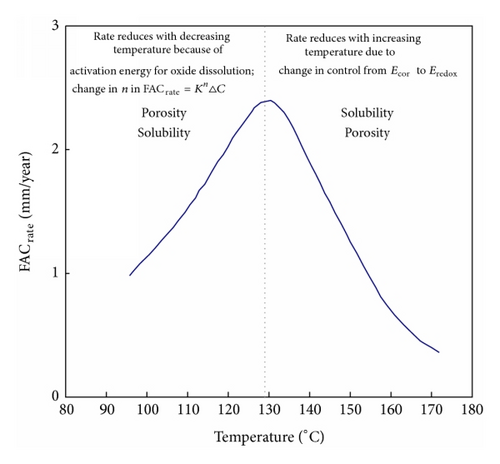
It has been suggested that at low temperatures the FAC rate is limited by the magnetite dissolution rate. However an empirical fit of the data produced an activation energy that was a function of the mass transfer rate [70]. At temperatures above the FAC peak it has been suggested that redox reactions could become more important than the corrosion potential in determining the rate of FAC. Both of these situations would produce a lower dependency on mass transfer than at the peak temperature where the highest value of n is associated with the maximum feedback between the FAC rate, the corrosion potential, the production of hydrogen, and the solubility of magnetite.
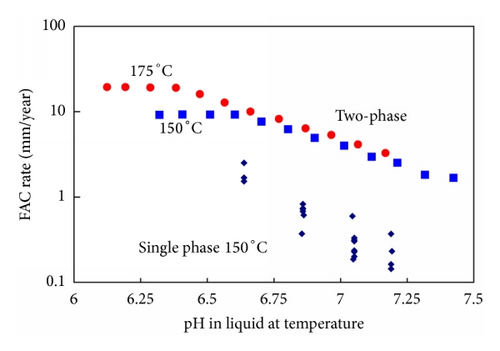
Except for very high plateau FAC rates at low pH’s (Figure 30) there is no convincing evidence for the importance of any processes other than mass transfer and magnetite solubility. The importance of magnetite dissolution kinetics being limiting at low temperatures appears to result from the widespread use of the solubility data of Sweaton and Baes. Most importantly this data suggests that magnetite solubility increases with decreasing temperatures down to about 60°C. However the data of Tremaine and LeBlanc show a bell shaped magnetite solubility curve (Figure 19), which more closely resembles the temperature dependence of FAC. In addition there are theoretical reasons why solubility should not be related to hydrogen partial pressure at low temperatures. Other reasons proposed to explain the temperature dependence of the FAC rate are summarized in Figure 28.
6.3. Influence of Roughness Development
The way the different models deal with roughness development, mass transfer, and the influences on the rate of FAC is summarised in Table 5. It appears that only the WATHEC model does not incorporate roughness effects. However it is not clear how the smooth to rough surface transition is handled by any of the models. The EdF model incorporates a formulation very similar to Figure 12 with the factor 0.01 being replaced with 0.193(ε/d) 0.2. This would appear a reasonable formulation in line with pressure drop formulations. However it is clear that that as Re increases the size of scallops produced by FAC decreases, although the mass transfer enhancement over a smooth surface is increased; this appears inconsistent, except possibly during the initial stages of attack.
6.4. Mechanistic Comments on Prediction Models
- (1)
The relevance of various processes particularly oxide porosity.
- (2)
The relationship between FAC and K.
- (3)
Which solubility data to use.
- (4)
The mechanism of improvement due to Cr content of the steel.
- (5)
The importance and treatment of roughness development.
6.5. Relationship of FAC to Deposition and SCC?
The difficulties predicting the occurrence of deposition have been indicated [64], although a velocity below 1.6–2.9 m/s was stated [93] as necessary to prevent the occurrence in straight tubes; otherwise pressure drop increases of 20 times at a Re of 106 could occur. Deposition appears preferentially at regions of high mass transfer and it has been suggested [94] that this is due to electrokinetically generated currents at regions of separated flow, but deposition does occur on straight pipes and bends, Figure 31. If electrokinetically currents are important deposition might be expected to occur at any dissolved iron levels (like electrodeposition). However there is still some debate about the roles of soluble or particulate iron. Like FAC deposition can be controlled by the addition of oxygen to the water or pH changes reducing the iron solubility.
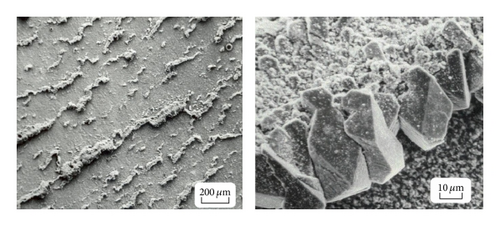
The SCC of highly stressed experimental orifice holders undergoing FAC was reported [12]. It was suggested that the electrochemical conditions for FAC and SCC are often similar. For example it was shown that the potential range promoting SCC and FAC, of carbon steels in carbonate solution, is identical. Also both SCC and FAC of carbon steels occur in carbonate, nitrate, and caustic solutions. The relationship between oxide solubility and crack propagation has also been suggested [95]. However from a practical viewpoint for carbon steels in water it is clear that cracking is favored by oxidizing conditions, which inhibit the occurrence of FAC. The occurrence of both FAC and SCC assumed importance by the occurrence of both FAC and SCC on the outlet headers of a CANDU plant; as yet there does not seem to be a full understanding of such cracking.
7. Preventing the Occurrence of FAC
If the three major influences on FAC are considered, that is, material, environmental, and hydrodrodynamic, as shown in Figure 2, then how to prevent its occurrence is straightforward and depends if at the design stage or postconstruction and into operation.
The history of the British AGRs once through steam generators illustrates this rather well. Damage was first observed at and downstream of the flow control orifice assemblies situated in the feedwater inlet headers. These are accessible and the problem was corrected by a redesign which included a stainless steel section of tubing downstream of the orifice. However this problem highlighted the risk to the carbon steel bends in the economizer section of the boiler, which was controlled initially by raising the pH. Although this appeared successful it limited the life of ion exchange beds. It was known that oxygen additions to the feedwater could be beneficial in terms of general corrosion [96, 97]. A large program demonstrated that adding a small amount of oxygen prevented FAC [78, 98]. High resolution surface activation measurements of FAC rate confirm this as shown in Figure 32. In addition it was shown that additions of oxygen and hydrazine could be made together and that the oxygen would inhibit FAC in the economizer prior to being reduced by the hydrazine and thus minimise the possibility of SCC at oxidising potentials. Thus very cleverly two additions can be made to the secondary water, having opposite effects and preventing two forms of corrosion occurring in different regions of the boiler.
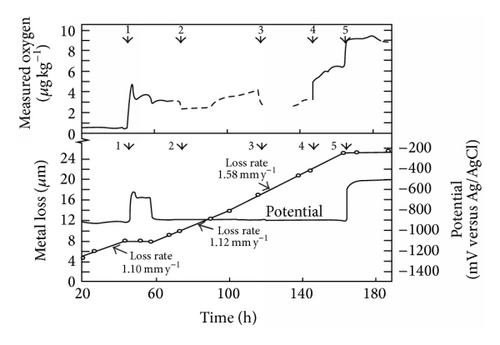
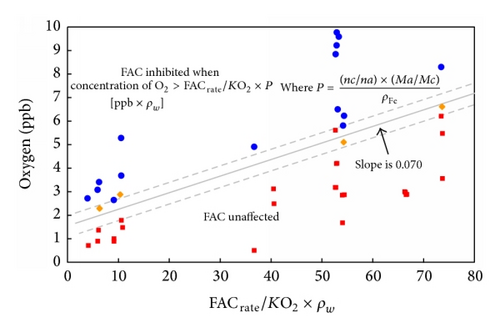
The success of such oxygen additions has been described [99] and the approach has been adopted in other situations [100]. Later AGR boilers were constructed using a 1%Cr 0.5%Mo steel after extensive testing had confirmed its suitability. The use of 1%Cr 0.5%Mo steel has the advantage that no postweld heat-treatment is usually required unlike 2.25%Cr 1%Mo and higher alloyed steels.
8. Conclusions
- (1)
The available evidence continues to support the use of the mass transfer coefficient (K) as the best hydrodynamic parameter to characterize FAC in power plant.
- (2)
The fundamental relationship between K and the rate of flow accelerated corrosion continues to be a significant difference between the different predictive schemes. However, there is strong evidence that the rate of FAC is not always a simple linear function of the mass transfer rate.
- (3)
With normal flow as roughness develops mass transfer increases and the rate of FAC increases. There is now more evidence that this is much less important with detached flow.
- (4)
It is clear that the influence of the environmental parameters such as temperature, pH, and oxygen content is through their effect on the solubility of magnetite. However, the details of this and the variation of magnetite solubility deserve closer attention. In particular it might prove possible to explain the temperature dependency of FAC in terms of magnetite solubility and its relationship to hydrogen partial pressure, without having to invoke a change in the rate controlling step.
- (5)
There is the possibility that at higher pH’s and temperatures, for instance those in the CANDU primary system the effect of hydrogen partial pressure, on magnetite solubility might not be proportional to . As pH increases, at high temperatures, there will be a gradual reversal in the effect of hydrogen content on solubility, from being proportional to to .
- (6)
The beneficial effect of chromium continues to be demonstrated under all conditions. However, it is not clear if this effect is due to a reduction in the solubility of the magnetite, a change in the kinetics of oxide dissolution or its influence on the oxide porosity.
- (7)
It is clear that oxygen additions can prevent FAC but such additions to the environment might lead to other problems.
- (8)
There is still no agreement on a number of important mechanistic aspects. The mechanistic models appear to have adjustable parameters particularly the f(T) in the EDF code and the n in the Kn dependency of the FAC rate, in the CEGB model.
- (9)
The pragmatic models do not give enough information to completely understand their operation.
Conflict of Interests
The author declares that there is no conflict of interests regarding the publication of this paper.