Availability of Wild Edible Fungi in La Malinche National Park, Mexico
Abstract
The aim of this paper is to compare edible mushroom availability between the two slopes of La Malinche National Park in central México, and to discuss the possible relation between their availability and traditional use. Eight transects were set up. Samples were collected weekly during the rainy seasons of years 1998–2000. Sixty-one edible mushroom species were collected from a total area of 3200 m2 (0.32 ha). Over the three-year period, the diversity of mushrooms ranged from 21 to 28 taxa per transect line. Sporocarps were produced at a rate from 2.06 to 6.05 kg/401.51 m2. The highest species richness and production values for spatio-temporal frequency were obtained in Southeast slope. Edible mushrooms availability in the Southeast slope showed a strong dominance, driven mainly by Laccaria trichodermophora and Hebeloma mesophaeum. The Southwest slope had more diversified availability in time and space, with the most representative species, being L. trichodermophora. The characteristics of traditional management on each slope determined the differences found.
1. Introduction
“La Malinche” volcano (altitude 4460 m) is one of the most important mountains in central México. Located in the Trans-Mexican Volcanic Belt, in the southern part of the state of Tlaxcala, it has been considered one of its eldest mountains (INEGI 1986). Most of its forests are protected as a National Park. However, timber and nontimber forest products are extracted as part of the subsistence strategy of local communities. People gather firewood, edible and medicinal plants, seeds, and moss and mushrooms and hunt small preys [1]. 226 species of macromycetes have been listed [2], 93 of which are used by local people as food, fuel, cosmetics, medicines, and insecticides [2, 3]. In the surroundings of La Malinche, there are 236 villages [4], some inhabited by Nahua and Otomí indigenous descendants and others settled by mestizo people. In consequence, East and West forests are under different management practices [5]. In many of these localities, Amanita basii, Lyophyllum decastes, and Boletus pinophilus are the species with the highest cultural significance (cultural significance refers to the importance of the role that the organism plays within a particular culture [6]) [5]. As a preliminary suggestion, it has been proposed that both fruit body abundance and price are related to the cultural significance of species. Montoya et al. [7] found a negative correlation between the fruit body abundance and the mention frequency, suggesting that the most valued resources are not always the most abundant.
It has been proposed that the volcano is regionalized into two cultural areas, based on the different valuations of mushroom species. There are several differences in the uses of the forest. In Javier Mina, a community located on Southeast slope of the volcano, 73.5% of the total population collects and sells mushrooms every year [4, 5]. In the Southwest slope, in San Isidro Buensuceso, 21% from a total of 220 persons sell wild mushrooms [4, 5]. Available information shows that mushrooms are used and granted value by people from both slopes; however, the use and importance of particular species are different in both sides. Nevertheless, there is scarce information about ecology parameters such as the fruit body production [8] and their relation with mushroom traditional use. The aim of this paper is to compare wild edible mushroom availability in the two slopes of “La Malinche” volcano and to assess the possible relation between availability and traditional use.
2. Materials and Methods
2.1. Study Area
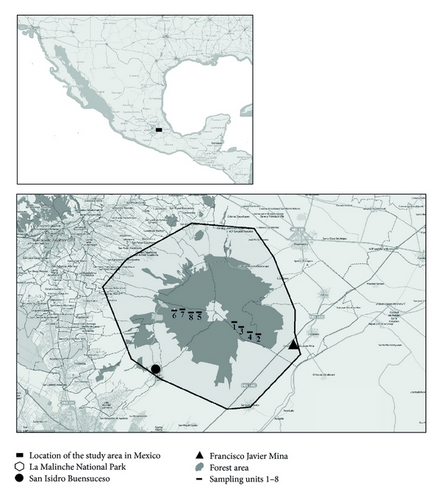
La Malinche National Park is located between northern latitudes 97° 55′ and 98° 08′ and between western longitudes 19° 20′ and 19° 08′. The local climate is temperate subhumid with a rainy season in the summer [C(w2)(w)]; the pressure/temperature ratio is 41.9 and there is little annual variation in average monthly temperatures, with fluctuations between 5° and 7°. The annual mean temperature is 15.3°C. May is the hottest month (mean temperature = 17.7°C) and January is the coldest (mean temperature = 11°C). Over 4000 m, weather tends to be very cold, type E (T) H, with temperatures under 0°C in the coldest month [9].
There are three main vegetation kinds: a forest dominated by P. hartwegii in higher altitudes; a forest dominated by Pinus montezumae and P. teocote mixed with Alnus jorullensis, Quercus laurina, and Q. crassifolia in lower altitudes; and an Abies religiosa forest sometimes mixed with individuals of P. montezumae, P. hartwegii, Salix cana, S. paradoxa, and Juniperus monticola in some gullies.
2.2. Sampling
Eight sample units (SUs) were established for this study (Table 1). Four SUs (1–4) were placed in Southeast slope (4–7 km west of Francisco Javier Mina) and the other four (5–8) in the Southwest slope (6-7 km north of San Isidro Buensuceso) (Figure 1). SUs were placed in locations usually visited by mushroom collectors. This had the purpose to reproduce not the natural production of mushrooms but their real availability, since there is a strong competition among mushroom collectors. To reduce the impact of mushroom collection in our data, transects were always visited as early as possible.
| Sampling unit (SU) | Location | Vegetation | Altitude (p = plot) |
Geographical coordinates | |
|---|---|---|---|---|---|
| North | West | ||||
| SU1 | 7.5 km east from Francisco Javier Mina | Pinus montezumae—Abies religiosa forest. 50 plots are located in Pinus and the other 50 in Abies. Abies is located in a ravine. Pinus area is subject to frequent harvesting of wild mushrooms during the rainy season. |
|
|
|
| SU2 | 4.5 km east from Francisco Javier Mina | P. montezumae—A. religiosa forest. 50 plots are located in Pinus and the other 50 in Abies. Abies is located in a ravine. Pinus area is subject to frequent harvesting of wild mushrooms and firewood during the rainy season. |
|
|
|
| SU3 | 7 km east from Francisco Javier Mina | P. montezumae forest. The forest is subject to frequent harvesting of wild mushrooms and firewood during the rainy season. |
|
|
|
| SU4 | 5.5 km east from Francisco Javier Mina | P. montezumae forest. The forest is subject to frequent harvesting of wild mushrooms during the rainy season. |
|
|
|
| SU5 | 14.5 km west from San Luis Teolocholco | A. religiosa forest. The forest is subject to frequent tree cutting. |
|
|
|
| SU6 | 11.5 km west from San Luis Teolocholco | A. religiosa forest with some individuals of P. montezumae and Salix sp. |
|
|
|
| SU7 | 12 km west from San Luis Teolocholco | Open forest dominated by P. montezumae. The forest is subject to frequent tree cutting. |
|
|
|
| SU8 | 13 km west from San Luis Teolocholco | Mixed forest dominated by P. montezumae mixed with Alnus jorullensis, A. religiosa, and Salix sp. The forest is subject to frequent tree cutting. |
|
|
|
- (For an integer (n), SUn = sampling unit n and pn = plot n.)
The SUs were sampled at one week intervals during the rainy seasons (July to October). Both areas were visited during three years, from 1998 to 2000; SUs 1–4 (Southeast slope) were visited 40 times, and SUs 5–8 (Southwest slope) were visited 37 times. At each visit, all fruit bodies were counted, picked up, and weighed, to avoid double counting at the next visit. At least one sample of each species was taken to the laboratory, processed as a voucher specimen for identification, and deposited at TLXM herbarium.
Each SU was composed of two parallel transects of 250 m each. Both transects were separated by a 50 m distance. Transects were permanently marked every 5 m, using sticks surrounded by black pieces of plastic on one side. We had a total of 100 sampling plots on each SU. Each plot had a radio of 1.13 m and a total area of 4.011 m2 [10]. The total area sampled each year was of 3,200 m2.
Edibility of each species was determined through local information, literature from the area [3], literature from México [11], and literature from other parts of the world [12]. The complete list of the material reviewed was published previously by Montoya et al. [3].
2.3. Data Analysis
Species richness was determined by the number of species registered in each SU. Abundance of fruit bodies was defined as the number of fruit bodies of each species in each SU during the three-year period. Production was calculated as the total fresh weight of each species. Biomass was calculated by measuring the dry weight of each species (fruit bodies were dehydrated at least 24 h at 105°C). Spatiotemporal frequency was calculated as the sum of the number of sampling plots where a species was found in each sampling date. Spatial frequency is the number of different plots in which a species was found during the three-year period in each SU. Spatial frequency was categorized in exponential classes: very infrequent (1–3), infrequent (4–9), frequent (10–21), very frequent (22–45), and extremely frequent (46–100). We looked for statistical differences in fruit body abundance and fruit body production between the two slopes. For this purpose, either the total number of fruit bodies or the total fresh weight in each SU (8) was considered as independent observations, while the data in each SU along the years (3) were considered as repeated measures, having twelve observations per slope. Means were compared by a bifactorial ANOVA for mixed designs in STATISTICA10 [13]. Availability of each species was determined by means of its ecological importance value, which equals the sum of its relative abundance, relative spatiotemporal frequency, and relative production [14].
Similarity between the SUs, according to their species composition, was computed using the species spatiotemporal frequency. A distance matrix was built, where rows corresponded to the species and columns to the eight SUs. The correlation index (Pearson product moment) was computed and SUs were clustered with the UPGMA method; then, the cophenetic value was computed. An ordination of the eight OTUs (=SUs) in a multidimensional space of characters was made by means of a Principal Component Analysis (PCA). Analyses were done in NTSYS-pc [15]. The diversity was calculated by using the Shannon-Wiener index. Since it is not possible to know the number of individuals, fruit bodies were counted and, instead of using abundance rates, spatiotemporal frequency was used. These analyses were done in the past software, version 2.16 [16].
3. Results
3.1. Species Richness
During the three sampling years, 61 edible mushroom species were found (Table 2): 48 species in the Southeast slope and 49 in Southwest slope. The species belonged to 37 genera. Fifty-one species were Basidiomycetes and the best represented families were Russulaceae with 9 species and Amanitaceae with 5 species. We identified 9 Ascomycetes, the family Helvellaceae being the best represented, with 4 species. 44 species were mycorrhizal, 15 were saprotrophs, and 2 were parasitic.
| Species | Total abundance | Relative abundance | Total fresh weight | Relative fresh weight | Total dry weight | Relative dry weight | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SESa | SWSb | SES | SWS | SES | SWS | SES | SWS | SES | SWE | SES | SWS | |
| Agaricaceae | ||||||||||||
| Agaricus augustus Fr.S | 0 | 1 | 0 | 0.00073 | 0 | 18 | 0 | 0.00134 | 0 | 1 | 0 | 0.00080 |
| Cystoderma amianthinum (Scop.) FayodS | 15 | 14 | 0.00549 | 0.01020 | 51.7 | 4.3 | 0.00321 | 0.00032 | 3.58 | 0.38 | 0.00225 | 0.00225 |
| Lycoperdon perlatum Pers.S | 5 | 18 | 0.00183 | 0.01311 | 3.98 | 141.45 | 0.00025 | 0.01052 | 0.64 | 14.25 | 0.00040 | 0.01137 |
| Amanitaceae | ||||||||||||
| Amanita aff. vaginata (Bull.) Lam.M | 2 | 0 | 0.00073 | 0 | 9.7 | 0 | 0.00060 | 0 | 1.33 | 0 | 0.00084 | 0 |
| Amanita basii Guzmán and Ram.-Guill.M | 4 | 0 | 0.00146 | 0 | 289 | 0 | 0.01795 | 0 | 22.59 | 0 | 0.01422 | 0 |
| Amanita franchetii (Boud.) FayodM | 50 | 2 | 0.01831 | 0.00146 | 829.9 | 105.2 | 0.05155 | 0.00783 | 65.99 | 8.47 | 0.04153 | 0.00676 |
| Amanita fulva Fr.M | 0 | 2 | 0 | 0.00146 | 0 | 19.4 | 0 | 0.00144 | 0 | 1.74 | 0 | 0.00139 |
| Amanita rubescens Pers.M | 43 | 4 | 0.01574 | 0.00291 | 818.2 | 112 | 0.05083 | 0.00833 | 58.11 | 10.62 | 0.03657 | 0.00847 |
| Auriculariaceae | ||||||||||||
| Auricularia auricula-judae (Bull.) Quél.P | 2 | 5 | 0.00073 | 0.00364 | 0.6 | 5.6 | 3.7E − 05 | 0.00042 | 0.039 | 0.39 | 2.4E − 05 | 0.00031 |
| Boletaceae | ||||||||||||
| Boletus luridus SchaeffM. | 0 | 2 | 0 | 0.00146 | 0 | 76.4 | 0 | 0.00568 | 0 | 1.03 | 0 | 0.00082 |
| Boletus pinophilus Pilát and DermekM | 5 | 2 | 0.00183 | 0.00146 | 920.5 | 243.7 | 0.05718 | 0.01813 | 84.95 | 24.53 | 0.05346 | 0.01957 |
| Cantharellaceae | ||||||||||||
| Cantharellus cibarius Fr.M | 0 | 2 | 0 | 0.00146 | 0 | 25.4 | 0 | 0.00189 | 0 | 2.34 | 0 | 0.00187 |
| Clavariadelphaceae | ||||||||||||
| Clavariadelphus truncatus DonkM | 10 | 0 | 0.00366 | 0 | 33.3 | 0 | 0.00207 | 0 | 4.12 | 0 | 0.00259 | 0 |
| Clavulinaceae | ||||||||||||
| Clavulina cinerea (Bull.) J. Schröt.M | 0 | 1 | 0 | 0.00073 | 0 | 5.4 | 0 | 0.00040 | 0 | 0.93 | 0 | 0.00074 |
| Clavulina coralloides (L.) J. Schröt.M | 0 | 1 | 0 | 0.00073 | 0 | 8.7 | 0 | 0.00065 | 0 | 1.37 | 0 | 0.00110 |
| Cortinariaceae | ||||||||||||
| Cortinarius glaucopus (Schaeff.) Fr.M | 1 | 15 | 0.00037 | 0.01092 | 60.5 | 412.83 | 0.00376 | 0.03071 | 3.71 | 32.02 | 0.00233 | 0.00233 |
| Hebeloma mesophaeum (Pers.) Quél.M | 316 | 36 | 0.11571 | 0.02622 | 758.05 | 86.95 | 0.04709 | 0.00647 | 93.57 | 9.43 | 0.05888 | 0.05888 |
| Discinaceae | ||||||||||||
| Gyromitraínfula (Schaeff.) Quél.S | 1 | 37 | 0.00037 | 0.02695 | 11.2 | 160.4 | 0.00070 | 0.01193 | 1.2 | 16.48 | 0.00075 | 0.00076 |
| Entolomataceae | ||||||||||||
| Entoloma clypeatum (L.) P. Kumm.M | 72 | 12 | 0.02636 | 0.00873 | 733.2 | 130.8 | 0.04554 | 0.00973 | 48.72 | 11.69 | 0.03066 | 0.03066 |
| Gomphaceae | ||||||||||||
| Ramaria sp. 1M | 1 | 0 | 0.00037 | 0 | 52.99 | 0 | 0.00329 | 0 | 3.93 | 0 | 0.00247 | 0 |
| Ramaria sp. 2 M | 1 | 0 | 0.00037 | 0 | 336.8 | 0 | 0.02092 | 0 | 25.61 | 0 | 0.01612 | 0 |
| Ramaria sp. 3 M | 2 | 0 | 0.00073 | 0 | 107.9 | 0 | 0.00670 | 0 | 12.28 | 0 | 0.00773 | 0 |
| Turbinellus floccosus (Schwein.) Earle ex Giachini and Castellano M | 0 | 9 | 9 | 0.00655 | 0 | 395 | 0 | 0.02938 | 0 | 32.41 | 0 | 0 |
| Gomphidiaceae | ||||||||||||
| Chroogomphus jamaicensis (Murrill) O. K. Mill.M | 6 | 2 | 0.00220 | 0.00146 | 26.3 | 9.4 | 0.00163 | 0.00070 | 3.39 | 0.72 | 0.00213 | 0.00057 |
| Helvellaceae | ||||||||||||
| Helvella acetabulum (L.) Quél. M | 0 | 20 | 0 | 0.01457 | 0 | 121.44 | 0 | 0.00903 | 0 | 15.47 | 0 | 0 |
| Helvella crispa (Scop.) Fr.M | 39 | 80 | 0.01428 | 0.05827 | 213 | 472.4 | 0.01323 | 0.03514 | 33.43 | 68.54 | 0.02104 | 0.02104 |
| Helvella elastica Bull.M | 3 | 12 | 0.00110 | 0.00874 | 5.5 | 30.6 | 0.00034 | 0.00228 | 1.32 | 1.98 | 0.00083 | 0.00083 |
| Helvella lacunosa Afzel.M | 49 | 122 | 0.01794 | 0.08886 | 211.8 | 730.4 | 0.01316 | 0.05433 | 38.98 | 100.3 | 0.02453 | 0.02453 |
| Hydnangiaceae | ||||||||||||
| Laccaria amethystina CookeM | 15 | 0 | 0.00549 | 0 | 7.6 | 0 | 0.00047 | 0 | 1.02 | 0 | 0.00064 | 0 |
| Laccaria trichodermophora G.M. Muell.M | 1678 | 225 | 0.61443 | 0.16387 | 3215.12 | 773.05 | 0.19972 | 0.05750 | 367.73 | 65.57 | 0.23142 | 0.05230 |
| Hygrophoraceae | ||||||||||||
| Hygrophorus hypothejus (Fr.) Fr.M | 0 | 4 | 0 | 0.00291 | 0 | 15.9 | 0 | 0.00118 | 0 | 1.72 | 0 | 0 |
| Hygrophorus chrysodon (Batsch) Fr.M | 13 | 50 | 0.00476 | 0.03642 | 44.9 | 86.31 | 0.00279 | 0.00642 | 3.14 | 7.76 | 0.00198 | 0.00198 |
| Hygrophorus purpurascens (Alb. and Schwein.) Fr.M | 7 | 1 | 0.00256 | 0.00073 | 216.7 | 18.6 | 0.01346 | 0.00138 | 15.37 | 0.51 | 0.00967 | 0.00967 |
| Hygrophoropsidaceae | ||||||||||||
| Hygrophoropsis aurantiaca (Wulfen) MaireS | 0 | 2 | 0 | 0.00146 | 0 | 5.1 | 0 | 0.00038 | 0 | 0.46 | 0 | 0 |
| Lyophyllaceae | ||||||||||||
| Lyophyllum decastes (Fr.) SingerS | 46 | 12 | 0.01684 | 0.00874 | 355.3 | 71.2 | 0.02207 | 0.00530 | 26.2 | 6.59 | 0.01649 | 0.00526 |
| Lyophyllum sp. 1S | 1 | 0 | 0.00037 | 0 | 138.5 | 0 | 0.00860 | 0 | 6.32 | 0 | 0.00398 | 0 |
| Morchellaceae | ||||||||||||
| Morchella elata Fr. S,M* | 6 | 151 | 0.00220 | 0.10998 | 33 | 468.6 | 0.00205 | 0.03486 | 2.95 | 68.59 | 0.00186 | 0.05471 |
| Morchella esculenta (L.) Pers* | 1 | 10 | 0.00037 | 0.00728 | 16.5 | 55.7 | 0.00102 | 0.00414 | 2.3 | 3 | 0.00145 | 0.00239 |
| Omphalotaceae | ||||||||||||
| Gymnopus dryophillus (Bull.) MurrillS | 16 | 42 | 0.00586 | 0.03059 | 39.15 | 152.2 | 0.00243 | 0.01132 | 3.75 | 8.78 | 0.00236 | 0.00236 |
| Pezizaceae | ||||||||||||
| Sarcosphaera coronaria (Jacq.) J. Schröt.M | 0 | 104 | 0 | 0.07575 | 0 | 2834.2 | 0 | 0.21081 | 0 | 219.8 | 0 | 0.17532 |
| Pluteaceae | ||||||||||||
| Pluteus cervinus (Schaeff.) P. Kumm.S | 2 | 8 | 0.00073 | 0.00583 | 32.9 | 93.1 | 0.00204 | 0.00693 | 1.52 | 10.3 | 0.00096 | 0.00822 |
| Pyronemataceae | ||||||||||||
| Geopora sp.M | 0 | 1 | 0 | 0.00073 | 0 | 18.3 | 0 | 0.00136 | 0 | 3.24 | 0 | 0 |
| Physalacriaceae | ||||||||||||
| Armillaria aff. mellea (Vahl) P. Kumm.P | 44 | 0 | 0.01611 | 0 | 342.03 | 0 | 0.02124 | 0 | 28.1 | 0 | 0.01768 | 0 |
| Rhizopogonaceae | ||||||||||||
| Rhizopogon sp.M | 31 | 2 | 0.01135 | 0.00146 | 48.1 | 17.3 | 0.00299 | 0.00129 | 9.14 | 3.09 | 0.00575 | 0.00246 |
| Russulaceae | ||||||||||||
| Lactarius deliciosus (L.) Gray M | 15 | 8 | 0.00549 | 0.00583 | 224.6 | 229.3 | 0.01395 | 0.01706 | 18.66 | 16.18 | 0.01174 | 0.01290 |
| Lactarius salmonicolor R. Heim and Leclair M | 21 | 13 | 0.00769 | 0.00947 | 354.53 | 183.32 | 0.02202 | 0.01364 | 29.7 | 21.19 | 0.01869 | 0.01690 |
| Russula acrifolia Romagn.M | 21 | 18 | 0.00769 | 0.01312 | 613.95 | 1677 | 0.03814 | 0.12474 | 58.65 | 147.49 | 0.03691 | 0.11764 |
| Russula albonigra (Krombh.) Fr.M | 2 | 0 | 0.00073 | 0 | 144.7 | 0 | 0.00899 | 0 | 8.52 | 0 | 0.00536 | 0 |
| Russula americana SingerM | 19 | 7 | 0.00696 | 0.00510 | 214.9 | 136.8 | 0.01335 | 0.01018 | 16.05 | 10.86 | 0.01010 | 0.00866 |
| Russula brevipes PeckM | 19 | 19 | 0.00696 | 0.01384 | 1249.5 | 1222 | 0.07762 | 0.09090 | 128.38 | 131.86 | 0.08079 | 0.10518 |
| Russula integra (L.) Fr.M | 16 | 0 | 0.00586 | 0 | 452.9 | 0 | 0.02813 | 0 | 44.74 | 0 | 0.02816 | 0 |
| Russula olivacea (Schaeff.) Fr.M | 1 | 0 | 0.00037 | 0 | 57.2 | 0 | 0.00355 | 0 | 5.28 | 0 | 0.00332 | 0 |
| Russula xerampelina (Schaeff.) Fr.M | 5 | 5 | 0.00183 | 0.00364 | 162.1 | 105.8 | 0.01007 | 0.00787 | 20.13 | 11.34 | 0.01267 | 0.00905 |
| Strophariaceae | ||||||||||||
| Pholiota lentaS | 5 | 16 | 0.00183 | 0.01165 | 27.5 | 108.9 | 0.00171 | 0.00810 | 2.22 | 8.49 | 0.00140 | 0.00677 |
| Stropharia coronilla (Bull. ex DC.) Quél.S | 0 | 14 | 0 | 0.01020 | 0 | 62.5 | 0 | 0.00465 | 0 | 4.01 | 0 | 0.00320 |
| Suillaceae | ||||||||||||
| Suillus pseudobrevipes A. H. Sm. and ThiersM | 70 | 52 | 0.02563 | 0.03787 | 702.8 | 690.55 | 0.04366 | 0.05136 | 55.33 | 49.06 | 0.03482 | 0.03913 |
| Tricholomataceae | ||||||||||||
| Clitocybe gibba (Pers.) P. Kumm.S | 21 | 152 | 0.00769 | 0.11071 | 96.4 | 565.1 | 0.00598 | 0.04203 | 9.63 | 49.62 | 0.00606 | 0.03958 |
| Clitocybe odora (Bull.) P. Kumm.S | 1 | 4 | 0.00037 | 0.00291 | 10.2 | 21.2 | 0.00063 | 0.00158 | 0.09 | 0.68 | 5.6E − 05 | 0.00054 |
| Lepista ovispora (J. E. Lange) GuldenS | 16 | 1 | 0.00586 | 0.00073 | 1784.3 | 17.4 | 0.11084 | 0.00129 | 210.4 | 3.11 | 0.13241 | 0.00248 |
| Melanoleuca melaleuca (Pers.) Murrill Kühner and MaireS | 11 | 45 | 0.00403 | 0.03277 | 33.7 | 422.5 | 0.00209 | 0.03143 | 3.14 | 37.69 | 0.00198 | 0.03006 |
| Tricholoma equestre (L.) P. Kumm.M | 1 | 8 | 0.00037 | 0.00583 | 4.9 | 76.2 | 0.00030 | 0.00567 | 3.09 | 6.63 | 0.00194 | 0.00529 |
- aSoutheast slope; bSouthwest slope; S: saprobic; M: mycorrhizal; P: parasitic.
During the three years of sampling, the highest species richness was found in the Pinus-Abies forest (SU2 and SU6) and the lowest value was observed in the Pinus forest (SU7) of the Southwest slope. Despite the sampling year, the highest species richness was always observed in the Pinus-Abies forests (in 1998 at SU6; in 1999 at SU1 and SU5; in 2000 at SU5, SU6, and SU8). In 1999, a Pinus forest (SU3) located in the Southeast slope also showed a high species richness. The presence of different tree host species offers more possibilities to find a higher diversity of ectomycorrhizal mushrooms and more substrates for saprotrophic mushrooms. Likewise, microhabitats, produced by the soil humidity and mosses associated to Abies, produce several differences for the mushroom community.
Species exclusive to the Southeast slope were Amanita basii, Amanita vaginata, Armillaria aff. mellea, Cantharellus cibarius, Laccaria amethystina, Lyophyllum sp. 1, Ramaria sp. 1, Ramaria sp. 2, Ramaria sp. 3, Russula integra, and Russula olivacea. Species exclusive to the Southwest slope were Agaricus augustus, Amanita fulva, Boletus luridus, Clavulina cinerea, Clavulina coralloides, Geopora sp., Turbinellus floccosus, Helvella acetabula, Russula albonigra, Hygrophoropsis aurantiaca, Hygrophorus hypothejus, and Sarcosphaera coronaria.
3.2. Abundance of Fruit Bodies
During the three sampling years, the highest number of fruit bodies (1,319) was found in the SU4, in a Pinus forest on Southeast slope. This means that they were 5.6 times more than those recorded at SU1, where the less number of fruit bodies was found (230). The lowest abundance was observed in the Pinus-Abies forest (SU1) of the same area. More fruit bodies were found in the year 2000 than in the two previous years. Southeast slope produced almost twice as many fruit bodies as the Southwest slope (Table 2). The most abundant species in the three years were L. trichodermophora, Hebeloma mesophaeum, Clitocybe gibba, Helvella lacunosa, Morchella elata, Suillus pseudobrevipes, Helvella crispa, and S. coronaria.
Although the mean of fruit bodies produced in the Southeast slope (256.83 fruit bodies/SU year) doubled those produced in the Southwest slope (114.42 fruit bodies/SU year), no statistical differences were found between slopes (F(1,18) = 3.77, P = 0.06), nor between years (F(2,18) = 0.291, P = 0.750), because of the high standard deviation in the data of Southeast slope (233.882). The interaction between slopes and years also showed any difference (F(2,18) = 1.034, P = 0.375).
3.3. Production
Comparing the values obtained for the two areas, higher values (16.10 Kg/3200 m2) were found on the Southeast slope, L. trichodermophora being the most productive species, whereas, on the Southwest slope (13.44 Kg/3200 m2), S. coronaria showed the highest values. The total fresh weight recorded at the SUs during the three-year period was 29.54 Kg/3200 m2 (Table 2). This amount means 92.10 Kg/ha/3 years of edible wild mushrooms. SU3, located in a Pinus forest, had the highest values of fresh weight. Year 2000 had the greatest production of edible mushroom fresh weight.
The species with the highest values of fresh weight were in 1998 L. ovispora, R. acrifolia, R. brevipes, H. mesophaeum, and L. trichodermophora; in 1999 L. trichodermophora, R. brevipes, R. acrifolia, and A. rubescens; and in year 2000 S. coronaria, L. trichodermophora, S. pseudobrevipes, R. acrifolia, C. glaucopus, and B. pinophilus. No statistical differences were found between the means of fresh weight of edible mushrooms produced in each slope (F(1,18) = 0.417, P = 0.526) nor between the years (F(2,18) = 2.24, P = 0.135). The interaction between mushrooms abundance by slopes and years did not show any difference (F(2,18) = 1.19, P = 0.325).
3.4. Biomass
The highest biomass production (1.59 Kg/3200 m2/3 years) was recorded in the SUs located in Southeast slope, while 1.25 Kg/3200 m2/3 years was produced in the Southwest slope. L. trichodermophora and L. ovispora were the species with the highest biomass production values in the Southeast slope and S. coronaria and R. brevipes in the Southwest slope. The total biomass was 2.84 Kg/3200 m2/3 years, which would mean 8.87 kg/ha. SU3 had the highest values. The highest values were recorded in year 2000 (Table 2).
3.5. Spatiotemporal Frequency
Southeast slope had a higher spatiotemporal frequency (STF), presenting 905 plots with mushrooms, while Southwest slope had 590 plots with mushrooms. SU4, located in a Pinus forest, had the highest overall STF with 371, while SU7, also in a Pinus forest, had the lowest with 96. Year 2000 had the highest overall STF with 642 plots. The species observed in the biggest number of sampling plots were L. trichodermophora, H. mesophaeum, H. lacunosa, H. crispa, S. pseudobrevipes, and C. gibba; then, they were the species most widely distributed in the study area (Table 3).
| Species | Total spatial frequency | Relative spatial frequency | Total spatiotemporal frequency | Relative spatiotemporal frequency | Availability index | Availability index | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SESa | SWSb | SES | SWS | SES | SWS | SES | SWS | SES | SWS | |
| Agaricus augustus | 0 | 1 | 0.00425 | 0.00257 | 0 | 1 | 0 | 0.00169 | 0 | 0.00633 |
| Amanita aff. vaginata | 2 | 0 | 0.00849 | 0 | 4 | 0 | 0.00442 | 0 | 0.01000 | 0 |
| Amanita basii | 4 | 0 | 0.04246 | 0 | 31 | 2 | 0.03425 | 0.00339 | 0.06216 | 0.00339 |
| Amanita franchetii | 20 | 2 | 0 | 0.00514 | 0 | 2 | 0 | 0.00339 | 0.11232 | 0.01781 |
| Amanita fulva | 0 | 1 | 0.03609 | 0.00257 | 20 | 2 | 0.02219 | 0.00339 | 0.02211 | 0.00886 |
| Amanita rubescens | 17 | 1 | 0.01274 | 0.00257 | 2 | 0 | 0.00221 | 0 | 0.10487 | 0.01381 |
| Armillaria sp. 1 | 6 | 0 | 0.00212 | 0 | 10 | 0 | 0.01105 | 0 | 0.06115 | 0 |
| Auricularia auricula | 1 | 2 | 0 | 0.00514 | 1 | 3 | 0.00110 | 0.00508 | 0.00400 | 0.01428 |
| Boletus luridus | 0 | 2 | 0.00849 | 0.00514 | 0 | 2 | 0 | 0.00339 | 0 | 0.01567 |
| Boletus pinophilus | 4 | 2 | 0.00849 | 0.00514 | 5 | 2 | 0.00552 | 0.00339 | 0.07303 | 0.02811 |
| Cantharellus cibarius | 4 | 2 | 0.00425 | 0.00514 | 0 | 2 | 0 | 0.00339 | 0.00849 | 0.01188 |
| Chroogomphus jamaicensis | 2 | 2 | 0.00425 | 0.00514 | 4 | 0 | 0.004412 | 0 | 0.01250 | 0.00730 |
| Clavariadelphus truncatus | 2 | 0 | 0 | 0 | 1 | 1 | 0.00110 | 0.00169 | 0.01108 | 0.00169 |
| Clavulina cinerea | 0 | 1 | 0 | 0.00257 | 1 | 1 | 0.00110 | 0.00169 | 0.00110 | 0.00540 |
| Clavulina coralloides | 0 | 1 | 0.02123 | 0.00257 | 13 | 42 | 0.01436 | 0.07119 | 0.01436 | 0.07513 |
| Clitocybe gibba | 10 | 23 | 0.00212 | 0.05913 | 1 | 2 | 0.00110 | 0.00339 | 0.03601 | 0.21526 |
| Clitocybe odora | 1 | 1 | 0.00424 | 0.00257 | 1 | 7 | 0.00110 | 0.01186 | 0.00423 | 0.01893 |
| Cortinarius glaucopus | 1 | 5 | 0.00212 | 0.01285 | 11 | 3 | 0.01215 | 0.00508 | 0.01840 | 0.05957 |
| Cystoderma amianthinum | 6 | 2 | 0.01274 | 0.00514 | 3 | 2 | 0.00331 | 0.00339 | 0.02476 | 0.01905 |
| Entoloma clypeatum | 25 | 2 | 0.05308 | 0.00514 | 32 | 5 | 0.03536 | 0.00847 | 0.16035 | 0.03209 |
| Geopora sp. | 0 | 1 | 0 | 0.00257 | 0 | 1 | 0 | 0.00169 | 0 | 0.00636 |
| Gymnopus dryophillus | 3 | 9 | 0.00637 | 0.02314 | 5 | 12 | 0.00552 | 0.02033 | 0.02018 | 0.08539 |
| Gyromitra infula | 1 | 9 | 0.00212 | 0.02314 | 1 | 17 | 0.00110 | 0.02881 | 0.00429 | 0.09083 |
| Hebeloma mesophaeum | 57 | 15 | 0.12102 | 0.03856 | 99 | 21 | 0.10939 | 0.03559 | 0.39321 | 0.10684 |
| Helvella acetabulum | 0 | 9 | 0 | 0.02314 | 0 | 12 | 0 | 0.02034 | 0 | 0.06707 |
| Helvella crispa | 18 | 33 | 0.03822 | 0.08483 | 25 | 50 | 0.02762 | 0.08475 | 0.09335 | 0.26298 |
| Helvella elastica | 1 | 7 | 0.00212 | 0.01799 | 3 | 8 | 0.00331 | 0.01356 | 0.00688 | 0.04257 |
| Helvella lacunosa | 17 | 47 | 0.03609 | 0.12082 | 25 | 62 | 0.02762 | 0.10508 | 0.09482 | 0.36909 |
| Hygrophoropsis aurantiaca | 0 | 1 | 0 | 0.00257 | 0 | 1 | 0 | 0.00169 | 0 | 0.00610 |
| Hygrophorus hypothejus | 0 | 1 | 0 | 0.00257 | 8 | 21 | 0.00884 | 0.03559 | 0.00884 | 0.04226 |
| Hygrophorus chrysodon | 8 | 10 | 0.01699 | 0.02571 | 0 | 2 | 0 | 0.00339 | 0.02453 | 0.07193 |
| Hygrophorus purpurascens | 2 | 1 | 0.00212 | 0.01285 | 5 | 2 | 0.00552 | 0.00339 | 0.02580 | 0.00807 |
| Laccaria amethystina | 2 | 0 | 0.00425 | 0.00257 | 3 | 0 | 0.00331 | 0 | 0.01353 | 0 |
| Laccaria trichodermophora | 109 | 33 | 0.00425 | 0 | 380 | 70 | 0.41989 | 0.11864 | 1.46546 | 0.42485 |
| Lactarius deliciosus | 7 | 3 | 0.23142 | 0.08483 | 11 | 5 | 0.01215 | 0.00847 | 0.04646 | 0.03907 |
| Lactarius salmonicolor | 10 | 8 | 0.01486 | 0.00771 | 12 | 12 | 0.01326 | 0.02034 | 0.06420 | 0.06401 |
| Lepista ovispora | 9 | 1 | 0.04671 | 0.01542 | 15 | 1 | 0.01657 | 0.00169 | 0.15238 | 0.00629 |
| Lycoperdon perlatum | 4 | 7 | 0.02123 | 0.02057 | 4 | 11 | 0.00442 | 0.01864 | 0.01499 | 0.06027 |
| Lyophyllum decastes | 22 | 6 | 0.00849 | 0.01799 | 29 | 7 | 0.03204 | 0.01186 | 0.11767 | 0.04132 |
| Lyophyllum sp. 1 | 1 | 0 | 0.01911 | 0.00257 | 1 | 0 | 0.00197 | 0 | 0.01220 | 0 |
| Melanoleuca melaleuca | 7 | 28 | 0.00212 | 0 | 8 | 34 | 0.00884 | 0.05763 | 0.02982 | 0.19381 |
| Morchella elata | 3 | 17 | 0.01492 | 0.07198 | 3 | 23 | 0.00331 | 0.03898 | 0.01393 | 0.22752 |
| Morchella esculenta | 1 | 5 | 0.00637 | 0.04370 | 1 | 6 | 0.00110 | 0.01017 | 0.00462 | 0.03444 |
| Pholiota lenta | 5 | 9 | 0.00212 | 0.01285 | 5 | 11 | 0.00552 | 0.01864 | 0.01968 | 0.06153 |
| Pluteus cervinus | 2 | 6 | 0.01062 | 0.02314 | 2 | 7 | 0.00221 | 0.01186 | 0.00923 | 0.04004 |
| Ramaria sp. 1 | 1 | 0 | 0.00425 | 0.01542 | 1 | 0 | 0.00110 | 0 | 0.00689 | 0 |
| Ramaria sp. 2 | 1 | 0 | 0.00212 | 0 | 1 | 0 | 0.00110 | 0 | 0.02452 | 0 |
| Ramaria sp. 3 | 1 | 0 | 0.00212 | 0 | 1 | 0 | 0.00110 | 0 | 0.01067 | 0 |
| Rhizopogon sp. | 7 | 2 | 0.01486 | 0.00514 | 6 | 2 | 0.00663 | 0.00339 | 0.03583 | 0.01127 |
| Russula acrifolia | 13 | 8 | 0.02760085 | 0.02057 | 19 | 11 | 0.02099 | 0.01864 | 0.09442 | 0.17706 |
| Russula albonigra | 1 | 0 | 0.00212 | 0 | 1 | 0 | 0.00110 | 0 | 0.01295 | 0 |
| Russula americana | 8 | 5 | 0.01699 | 0.01285 | 10 | 6 | 0.01105 | 0.01017 | 0.04834 | 0.03830 |
| Russula brevipes | 6 | 13 | 0.01274 | 0.03342 | 23 | 17 | 0.02541 | 0.02881 | 0.12273 | 0.16697 |
| Russula integra | 12 | 0 | 0.02548 | 0 | 15 | 0 | 0.01657 | 0 | 0.07604 | 0 |
| Russula olivacea | 1 | 0 | 0.00212 | 0 | 1 | 0 | 0.00110 | 0 | 0.00715 | 0 |
| Russula xerampelina | 3 | 2 | 0.00637 | 0.00514 | 5 | 2 | 0.00552 | 0.00339 | 0.02379 | 0.02004 |
| Sarcosphaera coronaria | 0 | 18 | 0 | 0.04627 | 0 | 31 | 0 | 0.05254 | 0 | 0.38538 |
| Stropharia coronilla | 0 | 3 | 0 | 0.00771 | 0 | 7 | 0 | 0.01186 | 0 | 0.03442 |
| Suillus pseudobrevipes | 22 | 12 | 0.04671 | 0.03085 | 36 | 26 | 0.03978 | 0.04407 | 0.15578 | 0.16415 |
| Turbinellus floccosus | 0 | 4 | 0 | 0.01028 | 0 | 5 | 0 | 0.00847 | 0 | 0.05469 |
| Tricholoma equestre | 1 | 6 | 0.00212 | 0.01542 | 1 | 8 | 0.00110 | 0.01356 | 0.00390 | 0.04048 |
- aSoutheast slope; bSouthwest slope.
3.6. Spatial Frequency
Southeast slope had the highest spatial frequency (SF) (471 plots) and Southwest slope showed a relative SF of 389 plots. The SUs with the highest values of frequency were SU4, SU3, SU6, and SU5. SU7 presented the lowest SF. Species with the highest percentage of SF throughout all the sampled area were L. trichodermophora (17.75%), H. mesophaeum (9.00%), H. lacunosa (8.00%), H. crispa (6.38%), M. melaleuca (4.38%), S. pseudobrevipes (4.25%), and C. gibba (4.13%) (Table 3).
The SF values for A. basii, A. rubescens, B. pinophilus, H. mesophaeum, L. trichodermophora, and L. decastes were higher in the Southeast slope, while, for T. floccosus, H. crispa, H. lacunosa, M. elata, and M. esculenta, higher SFs were registered in the Southwest slope. In both cases, those species have been determined to be the most important from a cultural perspective [5].
3.7. Availability
Values obtained as the availability index for each species are showed in Table 3. Species with the highest values in this study were L. trichodermophora, S. coronaria, H. lacunosa, H. crispa, M. elata, C. gibba, M. melaleuca, R. acrifolia, R. brevipes, and S. pseudobrevipes. The information obtained from the availability index shows the presence of several different environments adequate for the fruiting of mushrooms. In the Southwest slope, Abies forests are located in a lower altitude than those in the Southeast slope, where Pinus forests are predominant, so there are differences in species between the two sites.
L. trichodermophora, H. mesophaeum, E. clypeatum, and S. pseudobrevipes had the highest values in the Southeast slope, while L. trichodermophora, S. coronaria, H. lacunosa, C. gibba, and H. crispa had the highest values on the Southwest slope. As for the Southwest slope, Figure 2 shows a greater diversity of species with considerable availability. These were present in space and time in a differential way. As well as in the Southeast slope (Figure 3), the significance of L. trichodermophora stands out. In this case, S. coronaria, because of its consistency, showed high production values considering its low abundance.
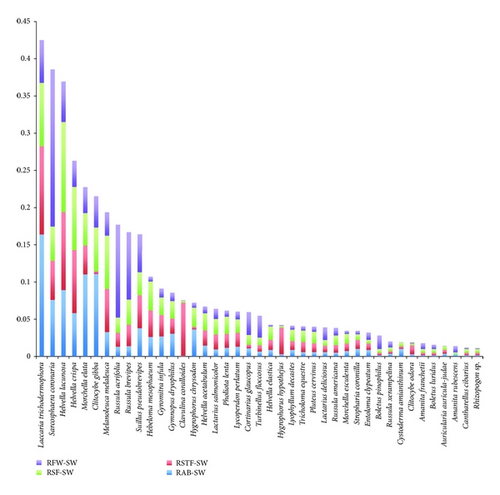
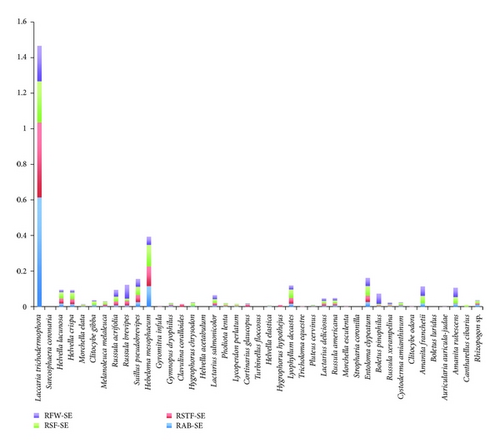
The availability of species measured by the ecological importance value did show remarkable differences between the two slopes. The Southeast slope has two dominant species: L. trichodermophora and H. mesophaeum. The other species registered on this area showed low values, suggesting their scarce availability in the three sampling years. L. trichodermophora was very abundant; it was widely distributed in the said space and time. In contrast, its production was not very high because of the size of its fruit bodies. It is interesting to notice that mushrooms as B. pinophilus have relatively high values of production due to the consistence and size of their fruit bodies, despite their low abundance and distribution in time and space. These characteristics contribute to the increase of the high production values in the Southeast slope.
3.8. Similarity
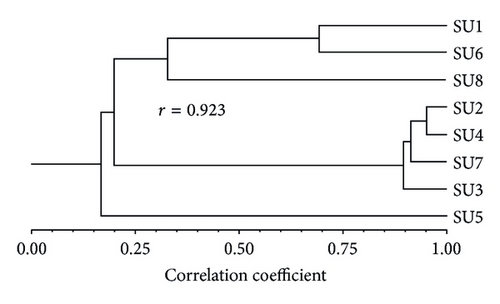
The cluster analysis (Figure 4) shows the similarity between SUs based on the values of the spatiotemporal frequency of species. Two main clusters can be observed. The first is composed of three SUs, two from the Southwest slope (SU6, SU8) and one from the Southeast slope (SU1). The two most similar SUs of this group are SU1 and SU6 and are related to SU8; half of SU1 and all SU6 are located in an Abies forest and SU8 which is the most different SU is in a mixed forest. The second cluster includes SU2, SU4, SU7, and SU3, three of them from the Southeast slope, and SU7 is from the Southwest slope, all of which are set up on Pinus forests. SU2 and SU4 are the two most similar. SU3 is the most different within this group. SU5 is the most different of all SUs.
As shown in Figure 5, the results of PCA provide a sharper definition of the different clusters described above. The results of PCA indicate that the species that contributed to cluster formation (which have a loading >0.7 on the first two PCs) were M. aff. melaleuca, L. trichodermophora, A. basii, H. mesophaeum, C. cibarius, and C. amianthinum in PC1. A. vaginata, Geopora sp., G. dryophilus, G. infula, M. elata, and S. coronaria in PC2 are all absent from SU3. The first two Principal Components explain cumulatively 44.9% of data variation.
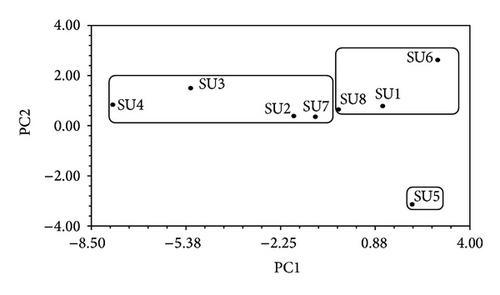
The representation of the OTUs in a three-dimensional space of characters (Figure 5) shows that SUs studied are closer to one another by vegetation type. In the clusters formed by these SUs, it is possible to identify subgroups, according to the species of edible mushrooms present or absent. Sampling units 1 and 6 showed 17 species in common, some are characteristic of Abies forests, for example, C. gibba, C. odora, H. crispa, H. elastica, H. lacunosa, L. salmonicolor, and M. esculenta. And some others also grow in Pinus forests, for example, A. rubescens and E. clypeatum. SU8 presented six exclusive species, which had the highest values in the analysis of PCA. Conforming a subgroup distinct from the previous, SUs 2 and 4 presented 19 species in common, most of them are mushrooms associated with Pinus forests (e.g., A. basii, A. franchetii, H. mesophaeum, L. trichodermophora, and S. pseudobrevipes, among others), and SUs 3 and 7 share 16 species which are mushrooms associated with Pinus forests (e.g., A. franchetii and B. pinophilus). SU 5 was the most different; it had two exclusive species (Geopora sp. and S. coronaria) and is located higher in altitude than other SUs.
Comparing information obtained for both slopes of La Malinche National Park, the highest values, in all parameters considered, were observed in the Southeast slope. However, we did not find statistical differences.
3.9. Diversity
Based on the abundance of fruit bodies, the Shannon-Wiener diversity index (H′) in the Southeast slope was 1.78, with a max H′ of 3.87. H′ in Southwest slope was 3.00, with a max 3.89 H′. Based on the abundance of plots, H′ was 2.53 for Southeast slope and 3.26 for Southwest slope. In summary, considering the abundance of fruit bodies or plots, the greatest diversity values were found in the Southwest. The calculation of the weighted diversity index (Hp) showed that both slopes are statistically different with respect to one another (Table 4).
| Abundance of fruit bodies | Abundance of plots | |||
|---|---|---|---|---|
| Southwest slope | Southeast slope | Southwest slope | Southeast slope | |
| S = species richness | 49 | 48 | 49 | 48 |
| N = number of fruit bodies/plots | 1373 | 2731 | 590 | 903 |
| H′ = Shannon-Wiener diversity | 3.00 | 1.78 | 3.26 | 2.55 |
| = maximum diversity | 3.89 | 3.87 | 3.89 | 3.87 |
| Hp = weighted diversity | 2.98 | 1.77 | 3.22 | 2.50 |
| Var = variance | 0.000930 | 0.001224 | 0.00173 | 0.00296 |
| t = Student’s t-test | −26.055 | −10.573 | ||
| df = degree of freedom | 3937.4 | 1488.8 | ||
| P (same) = probability | 3.3256e188 | 3.0487e25 | ||
The highest value for the Shannon-Wiener diversity index was obtained in SU7 (H′ = 3.43) located in the Southwest slope, with 21 species. The lowest value of diversity was obtained in SU4 (H′ = 1.81) located in the Southeast slope, with 25 species. The evenness ranged from 0.88 in SU1 (Pinus-Abies forest) to 0.78 in SU8 (mixed forest dominated by Pinus) (Table 4).
4. Discussion
The area located in Southeast slope of La Malinche National Park presented the highest values of abundance, production, biomass, STF, and SF of fruiting bodies of edible wild mushrooms, while the values obtained in the SUs located in the Southwest slopes were lower. Southeast slope is an area influenced by mestizo communities, contrary to the indigenous condition in the Southwest region; this is a relevant fact in terms of forest management. The other difference between both slopes, related to the management of mushrooms, is the level of commercialization, which is made in great scale in some communities of the Southeast slope, for example, in Javier Mina, opposite to the Southwest region, where there exists a low-level trade of mushrooms, and in San Isidro Buensuceso, where their use is mainly for self-consumption. By this way, different extractive techniques and uses have different impacts on the availability of mushrooms in the forest areas surrounding the communities [5].
Both locations had almost the same number of species. In year 2000, higher values were found in all variables measured. With regard to the SUs, the highest values were recorded for SU4 and the lowest for SU1. Highest values in production (fresh weight) and biomass (dry weight) were recorded in SU3. Highest species richness was detected in SUs 2 and 6. Largest number of exclusive species was found in SU8 and SU2. Mycorrhizal fungi were more abundant than saprobes, since families with more species observed were Russulaceae, Tricholomataceae, Amanitaceae, Gomphaceae, and Helvellaceae.
It should be noticed that H. mesophaeum and M. elata had their highest abundance in 1998; this was probably a result of the fires before the rainy season. Fires had a favorable effect in stimulating fruiting and in increasing the number of sporocarps. Moser [17] mentions the carbonicolous habit of H. mesophaeum, and Lincoff et al. [18] describe the preference of M. elata to fruit in areas that have been burned prior to the rainy season. That is the reason why such species presented high values of abundance during the three years of sampling. M. elata was collected from the Pinus-Abies forest (SUs 1, 5, and 8) and H. mesophaeum from both Pinus and Pinus-Abies forests (SUs 1–8).
Most significant species in the Southeast slope have the highest values in production, abundance, and spatial frequency in this area, compared to the same species in the other slope. The same behavior was observed in the Southwest slope. Then, the possibility to make a more comprehensive research is suggested, that takes into consideration the monitoring of ecology of mushrooms for a long period, including the measurement of structural characteristics of vegetation and weather variables. It would also be very important to include the measurement of the impact of harvesting and other traditional management practices as ecological variables. Intentional fires increase the production of some species as H. mesophaeum and Morchella spp., but there is no information of their effect on other species in the area.
Investigations made about the ecology of wild edible fungi in Mexico have used different methods, cannot make any kind of comparisons. However in some forests of Central and Southern Mexico, production values obtained are very variable compared to the present study. We recorded 29.53 kg/3200 m2, or 92.101 kg/ha/3 years, and Zamora-Martínez and Nieto de Pascual-Pola [19] reported a production of 76.3 kg/ha/year and, for the other year, 52.4 kg/ha of edible wild mushrooms in a Christmas trees plantation (Abies religiosa) in Topilejo, Mexico. The authors suggest that the annual variations in the production of mushrooms were due to temperature and precipitation, as well as the age of the trees. Also, in the Malinche Volcano, Hernández-Díaz [8] assessed the production of wild edible mushrooms in a pine and fir forest, sampling two permanent plots of 900 m2 each. There were 35 species of fungi: 28 in fir and 22 in pine. The total production was 55.50 kg/ha/year of weight fresh. Anahid [20] reported 49 species of wild edible mushrooms in the fir forest of La Malinche volcano, with a production of 27.34 kg/ha/year.
Garibay-Orijel et al. [14] recorded 81 species of wild edible mushrooms in the pine-oak forest of Ixtlan de Juárez, Oaxaca. The production was of 59.01 kg/105,600 m2 or 5.58 kg/ha/2 years. Availability is very heterogeneous in dense areas within the same forest. Species composition is very different, abundance and production are contrasting. This was not the case with La Malinche where the species composition in both slopes compared was very similar, and the availability of species shows two patterns, few available species in Southeast slope and greater availability of many species in Southwest slope. Both in Ixtlan and in La Malinche, L. trichodermofora is one of the most abundant species. Garibay-Orijel et al. [14] suggest that the utilization of the species must be done using different strategies taking into account their availability.
It is necessary to remark the importance of designing an ecological method more adequate to sample mushroom species. Because of the way that data were obtained with in this study, the real values in all parameters are underestimated. It is possible to say the above, if comparisons are made between the amounts of mushrooms which collectors obtain during their travels. Montoya et al. [21] reported 219.6 Kg of A. basii, in one rainy season, and Pacheco-Cobos [22] showed a value of 2,494 fruit bodies of T. floccosus and 2,066 of C. gibba on 55 fungi search paths with persons from San Isidro Buensuceso. This means that there are considerable differences between those species, for the values found in this study.
On the other hand, climatic conditions are one of the key factors for fructification [23], and the climatic information of La Malinche volcano suggests several differences between the two slopes. This is important because rain is one of the most important factors that could affect the soil humidity, nutriment availability, and temperature. However, rains have an irregular distribution in the studied area. Comparing the rain regime with the annual average precipitation, it is observed that San Pablo del Monte (in Southwest slope) is the area with more rains with annual values of 942.7 mm. Values in the municipality of Zitlaltepec, located in the East part, are of 800 mm of annual rains. These differences affect the availability of plants and other organisms as mushrooms. Another important weather element is temperature because, depending on its values, it could affect the assimilation of several nutrients, minerals, and water. The lowest temperature in Zitlaltepec is 0°C during the coldest months, and the maximum temperature is from 20 to 28°C. San Pablo del Monte is the warmest area, with maximum temperatures from 22 to 28°C throughout the year, and its coldest temperature is never under 5°C. Frosts affect negatively the fruiting of mushrooms; this was observed in this study, during three years of collection. In La Malinche, the highest incidence of frosts is registered from November to February, with an incidence of 60 to 80 days per year [24]. In addition, the characteristics of climatic variables in the study area explain the differences found in the two sampled areas. Information about temperature is important because it affects the level of humidity retention in the soil throughout time, with a beneficial effect in the fruiting of some mushroom species. Apparently, mushroom collection did not affect abundance, production, and frequency of mushrooms, even though there were more frequent visits from mushroom collectors in the Southeast slope than in the Southwest region; nevertheless, it would be convenient to test their actual effect, in experimental plots in the park.
5. Conclusions
The results show differences between the two La Malinche slopes regarding production, abundance, richness, and diversity of edible species of mushrooms. Southeast slope presented, in all variables measured, higher values than Southwest slope. However, the availability of mushroom species in space and time is more homogeneous in the Southwest slope, where it is possible to find more species and better distribution during the rainy season. There are few species that dominate the fruit body production in the Southeast slope. We believe that the management of forests by people of different origins (indigenous in the West and mestizo in the East) and the level of commercialization of mushroom species that are important in each slope, as well as the type of forests with their microenvironments, are determinants of those differences.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
Thanks are due to Trinidad Romero, from Javier Mina, who kindly collected mushrooms in the forest with the authors. The authors are also grateful to José Jiménez-López for assisting them with weather information and to Andrea Vera Reyes for the support at soil’s Laboratory in Centro de Investigaciones en Ciencias Biológicas, Universidad Autónoma de Tlaxcala (CICB, UAT). The authors are grateful to Héctor Luna for his assistance during field trips. Special thanks are due to the staff of Mycorrhiza Laboratory in the CICB, UAT. This research was supported by CONACyT (Reference no. 980022) and PROMEP (code P/PROMEP UATLAX-2000-07). Thanks are due to Coordinación General de Ecología, Tlaxcala, for the permissions to enter the Park.




