Crystal Structures and Magnetic Properties of Two New Cobalt(II) Complexes with Triazole-Substituted Nitronyl and Imino Nitroxide Radicals
Abstract
Two new cobalt(II) complexes of formula [Co(hfac)2(NITphtrz) 1 and Co(hfac)2(IMphtrz) 2] have been prepared and characterized structurally [where NITphtrz = 4,4,5,5-tetramethyl-2-(2-phenyl-1,2,3-triazole-4-yl)imidazoline-1-oxyl-3-oxide and IMphtrz = 4,4,5,5-tetramethyl-2-(2-phenyl-1,2,3-triazole-4-yl)imidazoline-1-oxyl]. All of the complexes crystallize in an isomorphous triclinic space group with the Co(II) ion octahedrally coordinated via the bidentate chelating mode. The magnetic measurements show that two complexes exhibit antiferromagnetic interactions between the metal ions and the nitroxide radicals.
1. Introduction
Metal-radical complexes have been well investigated toward molecule-based magnets, where a radical center is directly bonded to the metal ion and affording appreciable magnetic exchange coupling [1]. The predominant magnetic features of metal-nitroxide complexes are determined by the donor properties of the nitronyl group and the coordination geometry of the resulting complex. In the past decades, growing interest has been paid to metal complexes with nitronyl nitroxide (NITR: 4,4,5,5-tetramethyl-2-R-imidazolin-1-oxyl-3-oxide) and imino nitroxide (IMR: 4,4,5,5-tetramethyl-2-R-imidazolin-1-oxyl) radicals [2–5]. The feature of R-group linked to the nitronyl nitroxide not only influences the intermolecular spin-spin interactions but also affects the coordination mode of the nitronyl nitroxides with metal ions [6, 7]. Because the auxiliary heteroaromatic N-donor forces the nitroxide O-atom to bind to a poor electrophilic metal center by the chelating effect, the complexes of nitronyl or imino nitroxides substituted by N-heteroaromatic groups have drawn great interests. Cobalt(II) complexes have been rarely investigated due to large spin-orbital coupling, although cobalt(II) is a very effective spin carrier. In order to extend our knowledge of the extremely rich chemistry of such systems, it was thus of interest to further explore the reactions between Co(hfac)2 and nitroxide radicals.
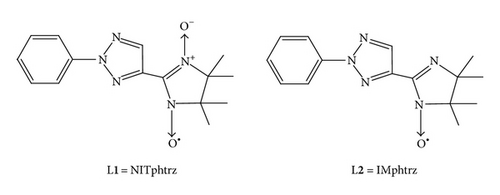
In this paper, we will report two new nitroxide ligands L1 and L2 (Scheme 1). Due to the presence of the N-triazole moieties, L1 and L2 can coordinate to metal ions as chelate ligands. Reactions of L1 and L2 with Co(hfac)2 (hfac = hexafluoroacetylacetonate) afforded two heterospin complexes. Crystal structures and magnetic properties of the related compounds (Co(hfac)2(NITphtrz) 1 and Co(hfac)2(IMphtrz) 2) will be described and discussed.
2. Experimental
2.1. Materials and Equipment
2-phenyl-1,2,3-triazole-4-carboxaldehyde was prepared according to the literature method [8]. Co(hfac)2·2H2O (hfac = hexafluoroacetylacetone) was obtained as of previously reported [9]. All the other chemicals purchased were of reagent grade and used without purification. Elemental analyses (C, H, and N) were carried out with a Perkin Elmer 240C elemental analyzer. IR spectra were recorded from 400 to 4000 cm−1 on an Avatar-360 spectrophotometer using KBr pellets. Variable-temperature magnetic susceptibilities were measured with an MPMS-7 SQUID magnetometer. Diamagnetic corrections were made with Pascal’s constants for all constituent atoms.
2.2. Synthesis of NITphtrz (L1) and IMphtrz (L2)
NITphtrz (L1) was prepared as previously described [10]. Yield: 76%. Anal. Calcd. for C15H18N5O2: C, 60.00; H, 6.00; N, 23.33%. Found: C, 60.31; H, 6.12; N, 23.18%. Important IR absorptions (KBr, cm−1): 1368 (s, νN–O).
IMphtrz (L2) was prepared according to [11]. Yield: 52%. Anal. Calcd. for C15H18N5O: C, 63.38; H, 6.34; N, 24.65%. Found: C, 63.51; H, 6.21; N, 24.50%. Important IR absorptions (KBr, cm−1): 1360 (s, νN–O).
Reactions of Co(hfac)2·2H2O with L1 or L2 yielded complexes (Co(hfac)2(NITphtrz) 1 and Co(hfac)2(IMphtrz) 2), respectively.
2.3. Synthesis of Co(hfac)2(NITphtrz) 1 and Co(hfac)2(IMphtrz) 2
Co(hfac)2(NITphtrz) 1. Co(hfac)2·2H2O (0.1 mmol) was dissolved in boiling n-heptane (30 mL). The solution was left to boil for 30 min and then cooled down to 60°C, whereupon 0.1 mmol NITphtrz (L1) was added under stirring followed by the addition of 5 mL of CH2Cl2. The mixture was stirred for 40 min and then filtered. The deep brown filtrate was set aside at a refrigerator for two weeks. Crystals of complex 1 suitable for X-ray crystallographic analysis were obtained and filtered. Yield: 58%. Anal. Calcd. for C25H22F12CoN5O6 1: C, 38.73; H, 2.86; N, 9.03%. Found: C, 38.68; H, 2.83; N, 8.95%. Important IR absorptions (KBr, cm−1): 1370 (s, νN–O).
Co(hfac)2(IMphtrz) 2 was synthesized using the same procedure as that of complex 1, which started from IMphtrz and Co(hfac)2·2H2O. Yield: 36%. Anal. Calcd. for C25H22F12CoN5O5 2: C, 39.55; H, 2.92; N, 9.22%. Found: C, 39.42; H, 2.83; N, 9.13%. Important IR absorptions (KBr, cm−1): 1345 (s, νN–O).
3. Results and Discussion
3.1. Descriptions of the Structure
For two title complexes Co(hfac)2(NITphtrz) 1 and Co(hfac)2(IMphtrz) 2, the corresponding crystallographic and refinement data are listed in Table 1. Selected bond distances and angles are given in Table 2.
| Complex 1 | Complex 2 | |
|---|---|---|
| Formula | C25 H22 F12 Co N5 O6 | C25 H22 F12 Co N5 O5 |
| Formula weight | 775.11 | 759.106 |
| Crystal system | Triclinic | Triclinic |
| Space group | ||
| Unit cell dimensions (Å, °) | ||
| a | 10.208(2) | 10.107(2) |
| b | 11.678(9) | 10.983(2) |
| c | 14.680(3) | 15.168(3) |
| α | 79.02(3) | 86.19(3) |
| β | 76.53(3) | 76.17(3) |
| γ | 67.98(3) | 77.53(3) |
| Volume (Å3), Z | 1567.4(5), 2 | 1596.2(5), 1 |
| F(000) | 1160 | 748 |
| θ range for data collection (°) | 1.89 to 28.37 | 1.90 to 28.37 |
| Limiting indices |
|
|
| Reflections collected | 37545 | 28519 |
| Independent reflections | 7808 | 7899 (Rint = 0.0223) |
| Completeness (%) | 99.3 | 98.7 |
| Goodness-of-fit on F2 | 1.097 | 1.515 |
| Final R indices (I > 2 σ (I)) | R1 = 0.0622; wR2 = 0.1864 | R1 = 0.0621; wR2 = 0.1973 |
| R indices (all data) | R1 = 0.0782; wR2 = 0.2035 | R1 = 0.0781; wR2 = 0.2100 |
| Complex 1 | |||
| Co(1)–O(1) | 2.025(3) | Co(1)–O(6) | 2.031(2) |
| Co(1)–O(4) | 2.030(3) | Co(1)–O(5) | 2.054(2) |
| Co(1)–O(3) | 2.057(2) | Co(1)–N(3) | 2.251(2) |
| N(1)–O(1) | 1.289(3) | N(2)–O(2) | 1.265(4) |
| O(1)–Co(1)–O(6) | 89.30(10) | O(1)–Co(1)–O(4) | 177.82(10) |
| O(6)–Co(1)–O(4) | 91.26(10) | O(1)–Co(1)–O(5) | 86.09(11) |
| O(6)–Co(1)–O(3) | 175.71(8) | O(5)–Co(1)–O(3) | 167.92(10) |
| Complex 2 | |||
| Co(2)–O(5) | 2.028(2) | Co(2)–O(3) | 2.045(2) |
| Co(2)–O(2) | 2.044(2) | Co(2)–O(4) | 2.069(2) |
| Co(2)–N(1) | 2.088(2) | Co(2)–N(5) | 2.287(2) |
| N(2)–O(1) | 1.275(3) | ||
| O(5)–Co(2)–O(3) | 89.69(9) | O(5)–Co(2)–O(2) | 174.56(7) |
| O(4)–Co(2)–N(5) | 173.43(9) | O(3)–Co(2)–N(1) | 170.37(9) |
| O(3)–Co(2)–O(4) | 87.28(11) | N(1)–Co(2)–N(5) | 77.07(9) |
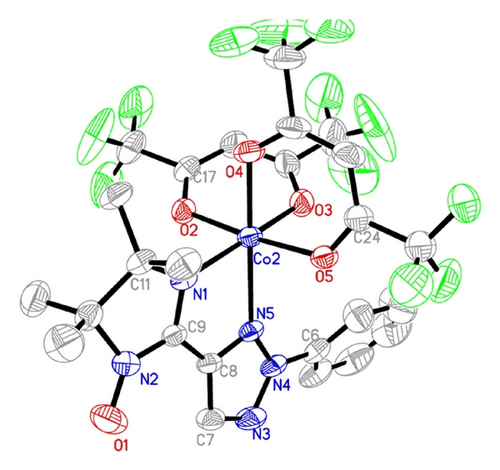
ORTEP drawings of complex 1 and complex 2 are depicted in Figures 1 and 2, respectively.
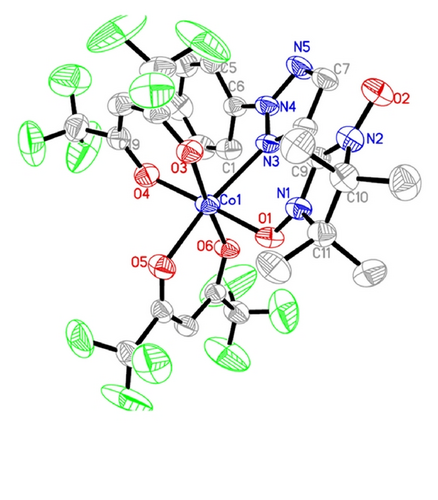
In complex 1, the Co(II) atom is six-coordinated in a distorted octahedron N1O5 environment. The equatorial plane is formed by N(3) from NITphtrz and by O(3), O(5), and O(6) from two hfac ligands. The Co–O bond lengths in the basal plane are 2.031(2), 2.057(2), and 2.054(2) Å, respectively. The Co–N3 distance is 2.251(2) Å. The axial positions are occupied by two oxygen atoms from hfac and NITphtrz, respectively. The Co–O bond lengths are 2.025(3) and 2.030(3) Å for Co–O(NITphtrz) and Co–O(hfac), respectively. The O4–Co–O1 angle is 177.82°, and the N3–Co–O1 angle is 91.03°. The dihedral angle between the triazole rings from the phtrz part and the O–N–C–N moieties (O1, N1, C9, and N2) is 11.9°. The fragment O(1)–N(1)–C(9)–N(2)–O(2) forms a dihedral angle of 54.5° with the equatorial plane.
In complex 2, the Co(II) ion is also in a distorted octahedron N2O4 environment. The equatorial plane is formed by N(1) and N(5) from IMphtrz and by O(3) and O(4) from two hfac molecules. The Co–O bond lengths in the basal plane are 2.045(2) and 2.069(2) Å, respectively. The Co–N distances are 2.287(2) and 2.088(2) Å, respectively. The axial positions are occupied by two oxygen atoms from two hfac ligands. The Co–O bond lengths are 2.044(2) and 2.028(2) Å, respectively. The O2–Co–O5 angle is 174.56°, and the N1–Co–N5 angle is 77.07°. The dihedral angle between the triazole rings for the phtrz part and the O–N–C–N moieties (O1, N2, C9, and N1) is 1.4°. The fragment O(1)–N(2)–C(9)–N(1) forms a dihedral angle of 7.5° with the equatorial plane.
3.2. Descriptions of the Magnetic Properties
The temperature dependence of the magnetic susceptibility for complexes 1 and 2 was investigated in the temperature range 1.8–300 K under a magnetic field of 2000 G. For both complexes, the variation curves of χM and χMT versus T are represented in Figures 3 and 4, respectively.
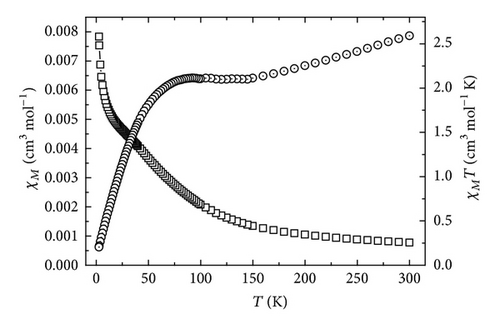
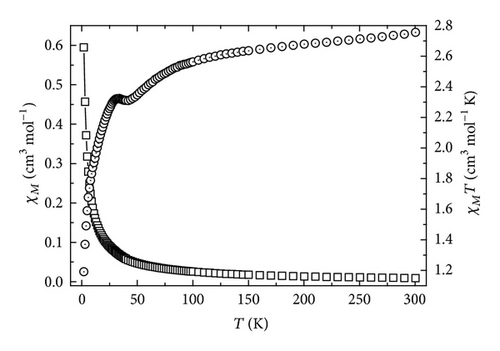
For complex 1, the value of χMT at 300 K is 2.59 cm3 ·K·mol−1, lower than the spin-only value expected for the uncorrelated spin system with Co(II) (S = 3/2) ion and the nitronyl nitroxide unit (S = 1/2). The χMT values decreased steadily with decreasing temperature to give a plateau around 150 K, where the χMT value is about 2.11 cm3·K·mol−1. This behavior can be accounted for by a strong intramolecular antiferromagnetic coupling between high-spin Co(II) and nitronyl nitroxide. Below 75 K, the χMT value decreases markedly, coming close to zero at the lowest temperature. These results show that there exists strong antiferromagnetic coupling between the cobalt(II) ion and the directly coordinated nitroxide group.
For complex 2, the value of χMT at 300 K is 2.80 cm3·K·mol−1, slightly lower than the spin-only value expected for the uncorrelated spin system with Co(II) (S = 3/2) ion and the nitronyl nitroxide unit (S = 1/2). The χMT values decreased steadily with decreasing temperature to give a plateau around 50 K, where the χMT value is about 2.32 cm3·K·mol−1. This behavior can be accounted for by a strong intramolecular antiferromagnetic coupling between high-spin Co(II) and imino nitroxide. Below 30 K, the χMT value decreases markedly, coming close to zero at the lowest temperature. These results show that there exists strong antiferromagnetic coupling between the cobalt(II) ion and the directly coordinated nitroxide group.
The best-fit parameters for complex 1 are J = −158.18 cm−1, D = −3.56 cm−1, and g = 2.84 with R = 1.74 × 10−4, where R is defined as . The result (J = −158.18 cm−1) indicates that a strong antiferromagnetic interaction exists between Co(II) and NITphtrz via Co(II)⋯O coordination bonding. For complex 2, the best-fit parameters are J = −142.03 cm−1, D = −3.44 cm−1, and g = 2.92 with R = 1.49 × 10−4. The magnitudes of the exchange integrals of CoII-radical complexes are basically dependent on the extent of overlap of the SOMO π*orbital of the radical with the magnetic orbital of metal ions [13].
4. Conclusion
Two chelate nitroxides ligands (L1 and L2) have been synthesized and characterized structurally. Reactions of L1 and L2 with Co(hfac)2 yielded two new cobalt(II) complexes. The temperature dependence of magnetic susceptibilities was studied and fitted according to suitable models. Strong antiferromagnetic couplings have been observed.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 21071006), the Natural Science Foundation of Henan Province (Grant no. 102102210457), and the Natural Science Foundation of the Henan Higher Education Institutions of China (Grant no. 2010B150001). Detailed crystallographic data in CIF format for the title complexes are available from Cambridge Crystallographic Data Center (CCDC IDs: 895284 (C25 H22 Co1 F12 N5 O5) and 895285 (C25 H22 Co1 F12 N5 O6)).




