Cloning, Purification, and Partial Characterization of Bacillus subtilis Urate Oxidase Expressed in Escherichia coli
Abstract
Urate oxidase (EC 1.7.3.3) is an enzyme involved in purine metabolism which is used in the treatment of gout and as diagnostic reagent for detection of uric acid. In order to produce this enzyme in large quantities for biotechnological purposes, the gene coding for the Bacillus subtilis urate oxidase was cloned and heterologously expressed in Escherichia coli. Time course induction in E. coli showed an induced protein with an apparent molecular mass of ∼60 kDa. Soluble recombinant enzyme was purified in a single-step procedure using Ni-NTA column. The enzyme was purified 2.1-fold with a yield of 56% compared to the crude extract. MALDI-TOF analysis revealed an ion with a mass of 58675 Da which is in agreement with the expected mass of the recombinant protein. The purified enzyme showed an optimal pH and temperature of 8.0 and 37∘C, respectively, and retained 90% of its activity after 72 hours of incubation at −20∘C and 4∘C.
1. Introduction
Gout is a disease characterized by joint and kidney inflammation caused by high levels of uric acid in the bloodstream and tissues. Uric acid is water soluble and is normally eliminated by the kidneys in quantities between 600 and 700 mg/day when the organism receives a normal diet. Plasma concentrations of uric acid higher than 6 mg/dL and 7 mg/dL in women and men, respectively, cause hyperuricemia. This medical condition may be caused by a diet rich in proteins, fat, and alcohol as well as hereditary factors (inborn errors of purine-pyrimidine metabolism) [1]. There are other diseases associated to hyperuricemia such as type 2 diabetes which, in the presence of higher levels of uric acid, causes insulin absorption resistance in tissues, increase of nephropathy progression, and tumor lysis syndrome (TLS) [2–4].
Urate oxidase (uricase, EC 1.7.3.4) is an enzyme with copper bonds that catalyze the oxidative opening of the purine ring of uric acid to form allantoin which is 5–10 times more soluble than uric acid [5, 6]. This enzyme can be used therapeutically to reduce toxic urate accumulation. Rasburicase (Fasturtec/Elitek) is a market name given to the Aspergillus flavus urate oxidase expressed in Saccharomyces cerevisiae which has been approved for clinical use. Studies showed that Rasburicase is more effective than other drugs in the treatment of hyperuricemia due to low incidence of hypersensibility reaction [7, 8]. Urate oxidase is also used as a reagent in clinical diagnostic kits for enzymatic determination of uric acid. Native enzyme may be obtained from several microorganisms of the genus Micrococcus, Brevibacterium, Streptomyces, Candida, Bacillus, Pseudomonas, Arthrobacter, and Aspergillus [9–15]. Urate oxidase from thermophilic Bacillus sp. TB-90 has been extensively studied for diagnostic purposes since it exhibits high activity and thermostability in a wide range of pHs [16]. Purified recombinant Bacillus subtilis urate oxidase has been tested with success in a biochip system for diagnostic purposes [17]. In this case, the pucLM gene was cloned in-frame with the maltose-binding protein (MPB) coding sequence resulting in a large protein fusion (~98 kDa) which was not significantly overexpressed in Escherichia coli. This result prompted us to employ a different approach to improve the expression of B. subtilis urate oxidase in E. coli by using a smaller 6x His tag which would also enable simple and fast purification of the recombinant enzyme for further biochemical characterization and large-scale production.
2. Materials and Methods
2.1. Strains and Reagents
Bacillus subtilis subtilis LMD 69.3 was used as a source of the urate oxidase gene. For heterologous expression, E. coli BL21 (DE3) pLysS and the expression vector pET21a (Novagen) were used. Ni Sepharose 6 Fast Flow (GE Healthcare) resin was used to purify recombinant urate oxidase. Restriction enzymes and the pGEM-T cloning vector were from Promega. Plasmid extraction was performed by the use of QIAPrep Spin Mini Kit (Qiagen). T4 DNA ligase was obtained from New England Biolabs. Taq Platinum was purchased from Invitrogen. Monoclonal antipoly histidine AP antibody was purchased from Sigma. Other reagents were obtained from standard commercial sources.
2.2. DNA Manipulations
B. subtilis chromosomal DNA was extracted as described elsewhere [18]. The urate oxidase coding sequence (1485 pb) was obtained by PCR using primers PUCL5 (5′-CGGATCCATGTTCACAATGGATGACCTG -3′) and PUCL3 (5′-GCTCGAGGGCTTTCAGGCTCCGACAT-3′) which were designed based on the B. subtilis pucLM gene sequence (GeneBank gi: 32468813). Primer PUCL5 contained a BamHI site (underlined) and the translation initiation codon (bold), whereas PUCL3 incorporated an engineered XhoI site (underlined). Amplification was performed using the following conditions: 30 cycles of 1 minute at 94°C, 45 seconds at 50°C, and 1.5 minutes at 72°C. The purified PCR product was ligated into pGEM-T resulting in plasmid pGEMURI. The sequence of the cloned gene was verified by automated DNA sequencing on the Genetic Analyzer Mega Bace 1000 (GE Healthcare) using the Mega Bace Dye Terminator kit. Plasmid pGEMURI was digested with BamHI and XhoI and the pucLM gene was ligated into pET21a digested with the same enzymes. Because primer PUCL3 lacks a stop codon, the urate oxidase gene was cloned in-frame with the 6x His tag coding sequence present in pET21a. The resulting plasmid, pETURI, was used to transform E. coli BL21 (DE3) pLysS.
2.3. Heterologous Expression
A single E. coli colony transformed with pETURI was inoculated in 6 mL Luria-Bertolin (LB) medium containing 100 μg/mL ampicillin and incubated at 37°C with shaking (200–300 rpm) overnight. Five milliliters of this preculture were transferred to 50 mL of LB medium in a 250 mL shake flask. The culture was grown under the same condition until OD600 reached 0.6 when IPTG was added to a final concentration of 1 mM. One milliliter samples were collected at different intervals and cell pellet was resuspended in 50 μL of SDS-PAGE sample buffer and boiled for 5 minutes prior to gel electrophoresis analysis. Typically, 1–5 μg total protein were loaded in each lane.
2.4. Western Blotting
Protein samples were separated on 12% SDS-PAGE gels and electroblotted onto PVDF membrane. The membrane was blocked with 5% milk in 10 mM Tris-HCl with 150 mM NaCl (pH 8.0) and 0.1% Tween 20 (TBST) for 2 hours at room temperature. The membrane was then incubated for 2 hours at room temperature with monoclonal mouse antipoly histidine AP antibody diluted 1 : 1000 in TBST. After three washes with TBST, bands were visualized by using the NBT/BCIP detection method.
2.5. Recombinant Enzyme Purification
In order to purify and analyze the recombinant protein, one bacterial clone was induced with 1 mM IPTG in 100 mL LB during 3 hours. The cells were harvested by centrifugation at 5000 rpm for 20 minutes at 4°C. Cell pellet (1.5 g) was resuspended in 2 mL lysis buffer (10 mM imidazole, 50 mM NaH2PO4 [pH 8.0], 300 mM NaCl) and lysed by sonication (6 cycles of 10 seconds of pulses at 45% amplitude—59W). The suspension was centrifuged at 13000 rpm for 10 minutes and the supernatant loaded into an Ni-NTA column with a volume of 1 mL resin preequilibrated in lysis buffer. The column was washed 8 times with 1 mL wash buffer (20 mM imidazole, 50 mM NaH2PO4 [pH 8.0], 300 mM NaCl) and 4 times with 500 μL elution buffer (200 mM imidazole, 50 mM NaH2PO4 [pH 8.0], 300 mM NaCl). All fractions were stored at 4°C for further analysis.
2.6. MALDI TOF Mass Determinations
Molecular mass determinations were performed on an Ultraflex II spectrometer (Bruker Daltonics) using lyophilized sample dissolved in Milli-Q water and mixed separately with two different saturated matrices solutions, α-cyano-4-hydroxycinnanic acid, and 3,5-dimethoxy-4-hydroxycinnamic acid (sinapinic acid). MALDI spectra were acquired in linear mode with external calibration.
2.7. Urate Oxidase Assay
Urate oxidase degrades uric acid forming hydrogen peroxide and allantoin. Hydrogen peroxide reacts with 4-aminoantipyrine and 3,5-dichloro-2-hydroxybenzenesulfonic acid and forms quinoneimine, a red compound that is detected by an increase in absorbance at 555 nm. The enzymatic reaction was carried out at 37°C and contained 200 μL uric acid solution (10 mg in 100 mL of 10 mM Tris-HCl, pH 8.0), 50 μL distilled water, and 50 μL enzyme samples. After 15-minute reaction, the reagent solution (4 mM 4-aminoantipyrine, 2 mM 3,5-dichloro-2-hydroxybenzenesulfonic acid, 2 mM horseradish peroxidase, and 10 mM Tris-HCl buffer) was added, incubated at room temperature for 5 minutes, and measured at 555 nm. One unit of urate oxidase activity was defined as the amount of enzyme that catalyzes the transformation of 1 μmol of urate per minute at 37°C (pH 8.0). Urate oxidase activity was calculated using the math formula found at the Kikkoman Biochemicals website (http://www.kikkoman.co.jp/bio/e/common/rinsyou.html). Protein concentration was determinate using the Bradford method [19].
2.8. Enzyme Characterization
Enzyme activity as a function of pH was tested with 50 mM sodium citrate for the followings values of pH: 2.5, 3.0, 4.5, 5.0; 50 mM sodium phosphate for pH values 6.0 and 7.0; 50 mM Tris-HCl for pH values 8.0, 9.0 and 10.0. The optimum temperature was evaluated by measuring urate oxidase activity for 30 minutes at different temperatures: 25, 37, 40, 60, and 80°C. The effect of temperature on enzyme stability was determined by measuring the residual activity after 0, 12, 24, 48 and 72 hours of preincubation in 10 mM Tris-HCl (pH 8.0) at −20, 4 and 37°C.
3. Results
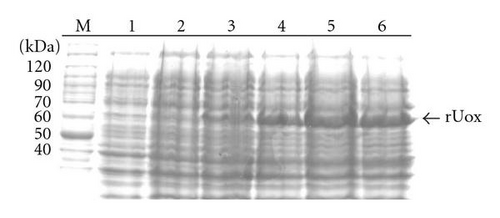
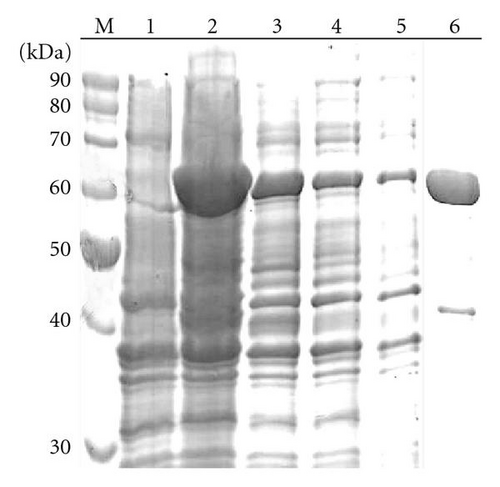
A ~1.5 kb amplicon corresponding to the B. subtilis pucLM gene was successfully cloned, sequenced and transferred to the bacterial expression vector pET21a (data not shown). In order to analyze the kinetics of urate oxidase expression, a colony of E. coli cells harbouring pETURI was grown to mid-log and induced with IPTG for 0, 15, 30, 60, 90, and 120 minutes. Samples analyzed by SDS-PAGE revealed a ~60 kDa induction band (Figure 1(a)). The size of this band was consistent with the predicted mass of the recombinant enzyme. Western blot analysis showed that the induction band represented a protein which contained a 6x His tag (Figure 1(b)). Two smaller (<50 kDa) and less abundant bands were also observed in the Western blot (Figure 1(b)). Induction profile showed a progressive accumulation of the enzyme starting 15 minutes after addition of IPTG and reaching the highest peak 90 minutes later. After induction in 100 mL, the cleared cell lysate was chromatographed in a Ni-NTA resin column in order to purify recombinant urate oxidase in a single-step. Fractions collected in different steps of purification were analyzed by SDS-PAGE (Figure 2). In order to optimize the purification procedure of the enzyme different elution buffers containing increasing concentrations of imidazole (50, 100, 150, 200 mM) were tested (data not shown). After elution with 200 mM imidazole, a predominant band with an apparent molecular mass of ~60 kDa was observed (Figure 2, lane 6). The data presented in Table 1 shows that the enzyme was purified with a 2.1 fold increase in specific activity with a yield of ~58% as compared to the crude extract.
| Step | Total activity | Total protein | Specific activity | Purification | Yield |
|---|---|---|---|---|---|
| (IU) | (mg) | (IU/mg) | (fold) | (%) | |
| Crude Extract | 54.0 | 3.0 | 18.0 | 1 | 100 |
| Ni-NTA | 31.2 | 0.8 | 39.0 | 2.1 | 56 |

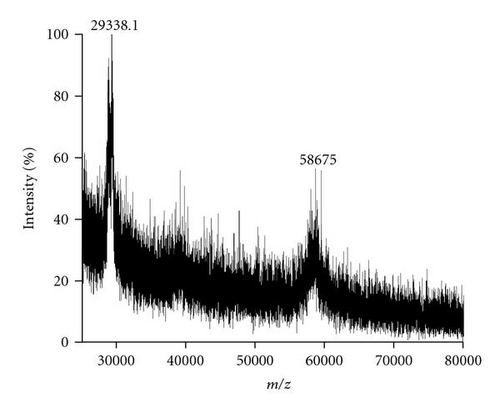
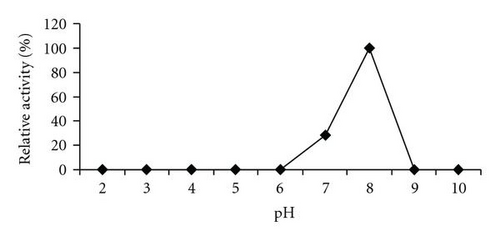
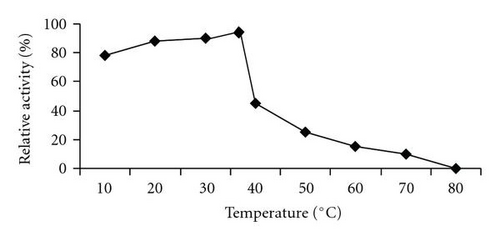
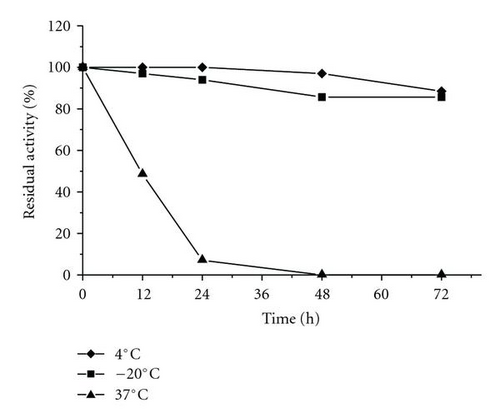
MALDI-TOF/MS analysis of the purified enzyme fraction revealed two major ions M+H+ = 58675 Da and its double charged M+2H+ = 29338.1 Da (Figure 3). The effects of pH and temperature on enzyme activity are shown in Figures 4(a) and 4(b), respectively. Purified urate oxidase showed maximum activity at pH 8.0 and 37°C (Figure 4(b)). As shown in Figure 5, the residual activity was practically constant at −20°C and 4°C after a 72 hours incubation period but was significantly affected by incubation at 37°C.
4. Discussion
Urate oxidase is a enzyme conserved in many species ranging from microorganisms to mammals; however, primates have lost this activity as consequence of evolutionary gene mutations [20, 21]. This enzyme catalyzes the oxidative reaction that converts urate to allantoin, a more soluble and easily excreted compound. Due to this property urate oxidase may be used for both therapeutical and diagnostic purposes [22]. Many sources of urate oxidase are available, but commercial production may be hampered by low productivity and difficulties in protein purification for clinical applications [22, 23]. DNA technology has been employed to overcome these difficulties by expressing urate oxidase genes in heterologous systems [24].
In B. subtilis the genes for purine degradation are clustered at positions 284 to 285° in the genome map [25, 26]. Mutational analysis of this region showed that inactivation of pucLM resulted in a defect in the utilization of guanosine, hypoxanthine, and uric acid and was thus proposed to code for urate oxidase [26]. Although the protein coded from the puLM gene shows amino acid identity to other urate oxidases, this similarity is restricted to the C-terminal domain (amino acids 171–494) [26] with the highest identities being with Bacillus sp. TB-90 (66%), Bacillus clausii (63%), and Bacillus fastidiosus (55%). It has been proposed that the first 170 amino acids correspond to a peroxide reductase domain that could be involved in the removal of hydrogen peroxide, a product of the oxidation of uric acid to allantoin catalyzed by urate oxidase [26]. Unlike eukaryotic urate oxidases and similar to Bacillus sp. TB-90, the B. subtilis enzyme does not have the typical type 2 copper binding motif H-X-H-X-F [27].
In this work, the pucLM was successfully expressed in E. coli in a soluble and active form. This result is particular important for the commercial production of this enzyme since it eliminates the laborious denaturing/refolding steps often necessary to solubilize proteins present as inclusion bodies. Furthermore, the strategy used in this work involved the fusion of B. subtilis urate oxidase to a 6x His tag in order to allow simple one-step purification using Ni-NTA column. A derivation of this method using Ni ion-chelating magnetic beads to increase yield was reported for the purification of recombinant Bacillus sp. TB-90 urate oxidase [28]. The results presented in Table 1 show that the recombinant enzyme was purified almost to homogeneity and the yield was about 58% as compared to the crude extract. This yield was close to that reported for commercial Rasburicase (61%) and native B. subtilis urate oxidase (67%) [28, 29]. The smaller protein contaminants that copurified with the recombinant enzyme represent minor breakdown products because they were recognized by the antipoly histidine antibody (Figure 1(b)). The recombinant enzyme was submitted to biochemical characterization (molecular mass determination, thermostability, optimum temperature and pH) and in vitro enzymatic activity. The experimental molecular mass value of 58675 Da obtained by MALDI-TOF/MS for the recombinant enzyme is close to the theoretical values calculated for the predicted recombinant protein (58.9 kDa). The optimum temperature (37°C) and pH (8.0) were similar to that observed with A. flavus urate oxidase produced in E. coli [30] and the B. subtilis MPB-uricase fusion protein [17]. In enzymatic assays the B. subtilis urate oxidase exhibited a specific activity of 39 IU/mg protein, a value higher than those observed for other urate oxidase described in the literature under optimal conditions. For example, native B. subtilis urate oxidase specific activity was 5.29 IU/mg protein, the specific activity for the A. flavus urate oxidase expressed in E. coli was 26.96 IU/mg and the commercial Rasburicase was 26.98 IU/mg protein [30]. Thermostability is considered an important and useful criterion for industrial application of enzymes. The stability of the recombinant enzyme at the temperatures used to store commercial enzymatic diagnostic kits (−20°C and 4°C) was evaluated and the enzyme kept 80% of its residual activity after 72 hours of pre-incubation. However, at 37°C, 50% of the residual activity was lost after 12 hours which is in agreement with the Rasburicase half life of 19 hours reported in humans [30, 31]. It has been proposed that the human body temperature is responsible for the shorter circulating half-time exhibited by recombinant urate oxidase [30, 31].
The results presented in this work showed the feasibility of producing soluble recombinant urate oxidase at high levels. The purity of the enzyme together with its high activity and thermostability shows that the production of the B. subtilis urate oxidase in E. coli should be considered for the development of diagnostic kits and for clinical purposes.
Acknowledgment
This work was supported by CNPq and FAP/DF.




