Spatial Transcriptome Analysis Reveals Diverse Human Burn Wound Microenvironment
Funding: This work was supported by NLM training grant to the Computation and Informatics in Biology and Medicine Training Program (NLM5T15LM007359) (to P.K.). Research reported in this publication was supported by National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM145723 (to A.G.) and R35GM150893 (to H.Q.D.).
Mary Junak and Parth Khatri have contributed equally to this work.
ABSTRACT
Histologic analyses of burn tissue are unable to discern reversible injury. Advanced molecular profiling, such as bulk RNA-sequencing, provides more detail; however, these methods lose spatial context. Spatial transcriptomics allows gene transcripts to be mapped to tissue locations, revealing the molecular pathways activated in the burn tissue microenvironment, where the depth of injury guides prognosis. This work demonstrates the capability of spatial transcriptomics to detect spatial gene expression patterns in burn tissue. Specifically, we show that (i) spatially variable expressed genes are distinct across different burn depth regions, which would not be identified with bulk RNA-sequencing, (ii) transcriptionally distinct burn tissue regions are defined by gene signatures associated with diverse cell types and biological pathways, and (iii) these spatial gene signatures are identified in a subset of previously published bulk samples, suggesting their potential application in large-scale and integrated studies. Caveats of this technology in burn tissue are provided to guide future research. This study highlights the promise of spatial transcriptomics to understand the human burn wound microenvironment and identify specific regions with regenerative potential that can be the target of tailored therapeutics, providing an alternative to imprecise excision and skin grafting.
1 Introduction
When a patient sustains a burn injury, management of the wound is primarily determined by visual assessment, relying heavily on a burn surgeon's expertise and subjective interpretation. Visual assessment is used to prognosticate healing potential, determine the need for surgery, and intraoperatively guide tissue excision. Unfortunately, in the early post-burn period, visual assessment of burn depth is accurate in less than 70% of cases [1]. Ultimately, there is a need to determine the regenerative capacity of the remaining tissue in the wound bed to avoid excising recoverable dermis.
Studies of the human burn tissue microenvironment are fairly limited to histologic analysis and primarily use haematoxylin and eosin (H&E) staining to identify damage as a marker of burn depth. Histologic staining has several limitations, including sampling error, operator variability, an unclear correlation between structural and functional damage, and limited clinical practicality [1]. Additional techniques, such as multiplex immunohistochemistry, are technically challenging and are limited to detecting a small subset of proteins in each sample [2]. Other well-established assays, including quantitative reverse transcription polymerase chain reaction (qRT-PCR) or bulk RNA sequencing (RNA Seq), lose spatial information that is particularly important in assessing the heterogeneous burn microenvironment and guiding treatment decisions [3].
Spatial transcriptomics is an emerging technology that profiles transcriptomes at distinct spatial locations in a tissue section, enabling the identification of spatial gene expression patterns and their histopathologic correlates [4]. This technology has been used to study the tumour and immune microenvironment in various skin conditions, including basal cell carcinoma and psoriasis [5, 6]. Spatial transcriptomics offers significant potential for advancing the understanding of the burn wound microenvironment by identifying biologic mechanisms that underpin burn response within intact tissue. Here we present, to our knowledge, the first-to-date report of the spatial transcriptomic analysis in human burn tissue using 10× Genomics Visium technology. Bioinformatics analysis showed spatial gene expression patterns associated with distinct biological pathways, which might be lost in bulk profiling.
2 Methods
Full-thickness tissue biopsies were obtained from a burn wound in the early post-burn period (PBD 3) and on the day of surgery (PBD 8). FFPE tissue slices were mounted on the 10× Visium capture area for sequencing. The resulting spatial transcriptomics FASTQ files were processed, mapped, and aligned to the GRCh38 human reference genome, and linked to H&E images using 10× Genomics SpaceRanger software. Gene count matrices were loaded for downstream analysis using the Seurat single-cell platform in R. The data were normalised using the SCTransform method and underwent linear dimensional reduction using principal components analysis (PCA) based on the 3000 most highly expressed genes. To identify spatially constrained gene expression programs (GEPs), the Python libraries scanpy and sctm were used to run the STAMP algorithm. STAMP leverages the spatial connectivity of spots in the sample with their gene expression profiles to identify gene expression patterns (GEPs) in different spatial domains. We used the ClusterProfiler R library for Gene Ontology Analysis to link topics to biological processes from the org.Hs.eg.db. Data visualisation in R used the ComplexHeatmap, enrichplot, and ggplot2 libraries. Additional materials, references and methods are detailed in the Supporting Information: Materials and Methods section (Appendix).
3 Results
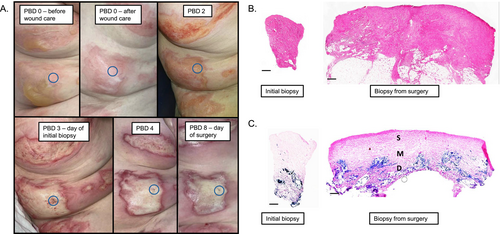
A 73-year-old female presented with a 7% total body surface area (TBSA) flame burn to her right arm and flank. A 4 mm full-thickness tissue biopsy was obtained from the right flank on post-burn day (PBD) 3. The patient underwent surgical excision of the burn on PBD 8, and a full thickness tissue biopsy was obtained adjacent to the initial biopsy site. Despite the full thickness visual appearance of the burn, histologic evaluation of the tissue obtained at the time of surgery revealed necrotic superficial adnexal structures with preserved deeper eccrine glands and vessels; whereas the collagen throughout the dermis appeared altered, thickened, and more homogenised with less distinct collagen fibres (Figure 1). Lactate dehydrogenase (LDH) staining for cell viability at the time of surgery shows that approximately 60% of the total tissue depth lacked viable cells.
To evaluate spatial gene expression, we profiled spot-based transcriptome using Visium technology (Supporting Information: Methods) for the two biopsies (PBD 3 and 8) from the same patient (Figure 2). The PBD 8 sample had more captured spots (2684) with an average of 2316 genes per spot (Figure 2A) compared to the PBD 3 sample (40 spots with 200 or more unique genes profiled). This change in gene expression is likely due to the evolution of the injury over time, including infiltration of inflammatory cells causing a modulation in the regenerative and inflammatory balance. The remainder of the analyses focused on the PBD 8 sample. To assess gene expression variability, we selected 3000 Highly Variable expressed genes (HVGs), typically used as the default for further downstream analysis, and showed they are present similarly across all tissue depths annotated by H&E (superficial, mid, and deep) (Figure 2B, Supporting Information: Methods) [7]. However, only 13.7% of HVGs were identified in all 3 tissue depths, indicating the low number of HVGs that would be correctly captured using bulk RNA-Seq if we collected the tissues across the entire thickness (Figure 2C). Over 23% of genes were only expressed in a single depth level (e.g., deep thickness, 23.7%, Figure 2C), implying the spatial heterogeneity of burn tissue expression that would not be captured by bulk RNA-Seq, even if carefully selected out.
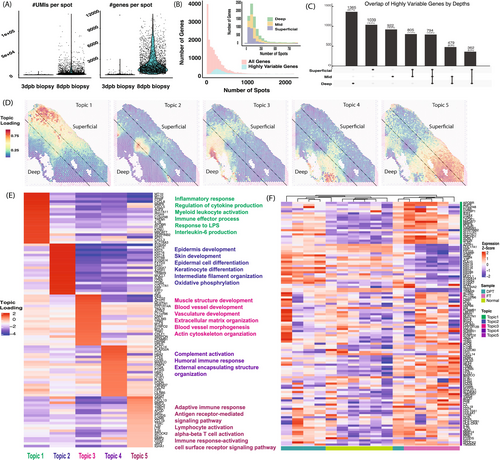
To identify spatially constrained regions with similar gene signatures, we used a machine learning method, STAMP (Simultaneous Training and Model Pruning), to identify 5 transcriptional topics with distinctly located regions in the tissue (Figure 2D) [8]. Physically close spots shared similar gene expression signatures, presented by the topic loadings (Supporting Information: Methods) (Figure 2E), and enriched Biological Process Gene Ontology (GO) terms. Topic 1 expressed inflammatory response and myeloid activation genes, including neutrophil-expressed genes (IL1B, MMP8, and CXCL8), mainly in the superficial region. Topic 2 expressed skin and epidermis development genes, including several keratin genes, in the boundary of mid and deep regions (Figure 2D,E). Topic 3 expressed blood vessel and extracellular matrix organisation genes, while topic 4 expressed complement activation and encapsulating structure organisation genes in the deep region. Topic 5, spread across all 3 depth levels, expressed adaptive immune response and T cell signalling pathway genes. The distinct gene signature and functional GO categories supported spatial gene expression heterogeneity of the burn tissue.
Next, we asked to what extent the spatially defined topics could be found in the previously published bulk RNA-Seq analysis of burn tissue examined in our lab by evaluating the similarity of 16 samples (5 each from deep partial thickness (DPT) and full thickness (FT), and 6 from normal skin) [9]. We found samples were mainly grouped in hierarchical clusters based on the gene signatures of the topics defined from the Visium data. Normal samples expressed the skin and epidermis development topic gene signatures (topic 2), while FT samples expressed all other gene signatures. The DPT samples lacked topic 3 and 4 gene signatures that were present in the deep regions of the spatial sample, which may be due to the absence of damaged tissue in the deeper regions of the DPT samples. Of note, the samples in the prior study were designated FT or DPT by subjective visual evaluation before sequencing was performed.
Together, these data showcase the power of spatial transcriptome data in understanding the diverse burn microenvironment. Bioinformatics analyses identified the extent of spatial gene expression heterogeneity, with distinct gene signatures and biological pathway enrichments. These gene signatures were expressed in bulk RNA-Seq samples, indicating the potential to link spatially defined cluster analysis to biological pathways and functions for different clinically and histopathologically annotated samples in large sample sizes.
4 Discussion
Much of our understanding of the burn wound microenvironment is derived using bulk gene expression or histology-based analyses, which lack the ability to preserve the precise location of biological changes within the tissue. Prior studies have shown that after burn injury there is an immediate and sustained inflammatory response characterised by the release of cytokines, chemokines, and reactive oxygen species often in a hypoxic state [10]. However, the specific locations within the tissue and how they influence the tissue's regenerative capacity of burn injuries remain unknown. This current work, albeit with a single patient, demonstrates the ability to locate distinct transcriptional topic areas within burned tissue, including inflammation, innate and humoral immune responses, and cellular regeneration. Specifically, genes associated with neutrophil-predominant inflammation were localised to the superficial aspect of the tissue biopsy, whereas epidermal and keratinocyte developmental genes were expressed primarily in the mid-depth and deep regions surrounding dermal appendages. We expect that inflammatory cells capable of migration would be found in the region of burn necrosis to facilitate wound debris clearance, whereas the viable region of tissue will contain cells with regenerative potential. The spatial heterogeneity of gene expression in burn tissue would be lost using bulk RNA-sequencing assays as fewer than 15% of genes were highly variable across all three tissue areas, and over 50% of the union of HVGs was unique to individual tissue regions. Histologic staining showed viable cells within the deeper layer of tissue; however, based on clinical judgement, this dermal layer was excised and the patient underwent autografting. The ability to probe the exact spatial gene expression within the tissue would allow us to gain a deeper understanding of the potential therapeutics that could be developed to enhance healing, potentially avoiding or minimising skin grafting and donor sites.
There are important considerations to note when applying this technology to burn tissue. The primary challenge of this analysis is the presence of necrosis in severe burns. Spatial sequencing relies on high-quality RNA, which can be limited in burn tissue as large areas of necrosis lead to RNA degradation. In this study, the PBD 3 sample was unable to be fully analysed due to poor RNA quality in the tissue. Additionally, samples from a patient with a deep partial thickness burn that eventually progressed to full thickness were also unable to be analysed, likely due to the burden of dead and dying cells within the tissue samples or tissue handling post biopsy, which may have led to poor RNA quality (Supplemental Table 1). While some amount of necrosis is likely acceptable, future studies should incorporate tissue samples with viability that could be assayed with a simple DAPI (4′,6-diamidino-2-phenylindole) stain on an adjacent tissue section to confirm viability in a portion of the tissue. Focusing on identifying changes of expression in DPT burns over time may be beneficial in developing topical therapeutics that reduce the need for surgery. Furthermore, the unsupervised analysis of this data identified transcriptional topics with distinctly located regions in the tissue, however, not all topics correlate to a histologic structure identified on H&E and may not necessarily be clinically significant regions of interest.
Spatial transcriptomic analysis of human burn tissue offers the potential to advance our understanding of burn injury, specifically within the zone of stasis, an area of decreased perfusion that is potentially salvageable. Mapping spatial gene expression patterns to identify molecular and cellular changes in this zone may provide insight into the mechanisms driving tissue viability versus necrosis. Future studies will include multiple samples that can be analysed to identify patterns in tissue collected at the same post-injury time point and to assess differences across time points, providing insight into the progression of injury. Ultimately, this technology has the potential to develop a robust description of the microenvironment within human burn wounds and therapeutic biomarkers.
Acknowledgements
The authors would like to acknowledge Bailey Donohue, BS, Zachery Schultz, BS, and Joana Pashaj, BS for their assistance with tissue processing and microscopic imaging. The authors thank the University of Wisconsin Translational Research Initiatives in Pathology Laboratory (TRIP), supported by the UW Department of Pathology and Laboratory Medicine, UWCCC (P30 CA014520) and the Office of The Director—NIH (S10 OD023526) for use of its facilities and services. This work was completed in part with resources provided by the University of Wisconsin Biotechnology Center—Gene Expression Center (RRID: SCR_017757). P.K. was supported by an NLM training grant to the Computation and Informatics in Biology and Medicine Training Program (NLM5T15LM007359). Research reported in this publication was supported by National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM145723 (to AG) and R35GM150893 (to HQD).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




