A pilot feasibility study of non-cultured autologous skin cell suspension for healing diabetic foot ulcers
Funding information: AVITA Medical
Abstract
A prospective, single arm feasibility study was conducted to evaluate healing outcomes of DFUs treated with autologous skin cell suspension (ASCS) in combination with standard therapy. Wounds up to 100 cm2 in size that failed to heal with conventional therapy were included and wound healing, pain, exudate scores, Quality of Life, satisfaction scores, and safety outcomes were evaluated over a period of 26 weeks. Sixteen subjects were enrolled having a mean DFU duration of 60.4 weeks. All ulcers in this study had a positive healing trajectory, with a mean reepithelialization of 84.9% and 12.2 cm2 reduction in ulcer area. For ulcers that did not acquire a soft tissue infection post-treatment, all either healed or achieved ≥95% reepithelialization including some with exposed tendon. Improvements were observed in all aspects of the health-related Quality of Life questionnaire and subjects and clinicians were highly satisfied across all postoperative visits. This preliminary study suggests ASCS is a well-tolerated and promising therapy for the treatment of DFUs as all ulcers evaluated experienced positive healing results regardless of size, depth, and wound duration. Future studies are warranted to investigate ASCS compared to standard of care for all diabetic foot ulcers, inclusive of the evaluation of treatment algorithms and combination products.
1 INTRODUCTION
Diabetes mellitus is a complex metabolic disorder that is on the rise globally and has considerable impact on patients and health services. Diabetic foot ulcers (DFUs) are complicated chronic wounds with a significant long-term impact on morbidity, mortality, and quality of life. Treatment is often difficult with poor healing responses and high rates of complications. Diabetes UK estimates that by the year 2030, 552 million people worldwide will have diabetes. It is estimated that 5% to 7% of people with diabetes currently have or have had a DFU and approximately 25% of people with diabetes will develop a DFU during their lifetime.1-5
Foot ulceration is most commonly associated with neuropathy, deformity, and trauma. Overall, 20% to 40% of persons with diabetes have neuropathy and 20% to 40% have peripheral arterial disease.4, 6 In addition, people with diabetes are twice as likely as the general population to have peripheral arterial disease which is a contributory factor in the persistence of DFUs.7-9 Successful diagnosis and treatment of patients with DFUs requires optimal management of glucose levels, investigation and treatment of vascular insufficiency, effective wound care (dressings and debridement), management of wound infection (antibiotic therapy), and pressure relieving strategies (specialist footwear, orthotics, and casts).4, 6
Despite conventional treatments, there is still a high risk of amputation, which in addition to the impact on the quality of life of the patient, carries with it a mortality rate of 39% to 80% within 5 years.6 The cost to the NHS of managing DFUs was estimated to be between £524 and £728 million.10 More advanced treatment modalities including biological skin substitutes, negative pressure wound therapy, oxygen therapies, and replacement of cell signaling factors in conjunction with standard wound dressing regimens have been evaluated.11-13 It has also been demonstrated that skin grafting and bilayer artificial skin may also improve the rate of healing of DFUs.14, 15 Studies evaluating the use of autologous skin cell suspension (ASCS) as prepared using an Autologous Cell Harvesting Device (ACHD) as part of the treatment algorithm for chronic wounds have demonstrated favorable results.16-20 In a randomized clinical trial in which ASCS was combined with skin grafting for treatment of a mixture of different types of chronic wounds, there was a significant improvement in healing rate (14 days with ASCS vs 20 days without), reduced tendency for wound recurrence, and fewer complications compared to skin grafting alone.18 In a recently published multicenter prospective randomized controlled trial, 52 subjects with venous leg ulcers (VLUs) were randomized into one of two groups: treatment of ASCS with compression therapy, and a control group with compression therapy alone. At 14 weeks, VLUs treated with ASCS plus compression had a statistically greater decrease in ulcer area compared to the control group. This ASCS group also had a significant decrease in pain and consistent improvement in quality of life after treatment with significant improvement in emotional status.20
Limited data exists relating baseline wound characteristics and ASCS treatment strategy with clinical outcomes specifically for treatment of DFUs. Therefore, the purpose of this study was to determine the clinical benefit, feasibility, and safety of ASCS prepared by ACHD for the treatment of DFUs.
2 STUDY METHODS
This was an open label, prospective, single arm study to determine feasibility and obtain clinical experience in the treatment of DFUs in a group of study participants for whom the ACHD (RECELL System, AVITA Medical, Valencia, CA) was used in combination with conventional therapy. The purpose of this study was to obtain an initial clinical evaluation of ASCS treatment specific for DFUs, further develop clinical practice guidelines, and guide future clinical trial design. This study was registered at clinicaltrials.gov under number: NCT02799121.
Subjects with DFUs were recruited from three sites in the United Kingdom between September 2016 and March 2019. All subjects had baseline demographics, medical history, and a physical exam documented at screening. Prior treatments of the ulcer designated to be treated with ASCS were recorded at the screening visit and served as the patient's historical failure-to-heal control. Baseline wound characteristics, and sections of the NeuroQoL quality of life questionnaire were also obtained and documented at the screening visit. Ulcers were to have been present for at least 3 weeks and have an area of between 3 and 100 cm2. Ulcers classified as University of Texas Ulcer grade 1A, 1B, 2A, or 2B classifications were included. Exclusion criteria included any subjects with Peripheral Arterial Disease (PAD) that would compromise healing according to investigator judgment or subjects that were due to have surgical intervention for PAD. Transcutaneous oxygen pressure (TcPO2) and/or toe-brachial pressures were assessed as an indicator of perfusion.
After consenting to study participation, subjects entered a two-week run-in period of standardized dressings. At the end of the two-week run-in period subjects not exhibiting signs of a rapidly healing ulcer (>30% reepithelialization), nor deterioration, infection, or edema underwent a screening visit and received ASCS treatment. After treatment, subjects returned at Week 1, 2, 4, 6, 7 (only if re-treated at Week 6), 10, 14, 20, and 26 after the initial treatment. Study participation lasted for 26 weeks, unless healing occurred at 26 weeks, which necessitated a return healing-confirmation visit at 28 weeks.
Subjects received application of ASCS prepared using the ACHD in accordance with the manufacturer's instructions for use. Briefly, under local anesthetic, a small (1.5-2 cm2) thin (0.15-0.20 mm) skin sample was harvested and incubated in a proprietary enzymatic formulation. After 15 to 20 minutes of enzyme exposure, the skin sample was rinsed in a buffer solution and then manually scraped until both the epidermis and dermis were fully dissociated. The cells were suspended in approximately 2.5 mL of buffer solution, drawn up and filtered, yielding approximately 2 mL of cell suspension. The resulting suspension was applied to the freshly debrided wound bed by dripping from a syringe, followed immediately by placement of nonadherent, nonabsorbent, small pore wound dressing (Telfa Clear, Covidien, Minneapolis, MN). The primary dressing remained in place for 6-8 days and was not manipulated until the first postoperative visit. Secondary dressings were applied and replaced as needed. Conventional dressings and offloading continued until healing and 2 weeks thereafter. Recruited patients were already part of the high-risk diabetic foot team and offloading was optimized and standardized before entry into the study as determined by the anatomical location and individualized to patient needs. The device met the criteria of anatomical location of the ulcer and patient logistics. Subjects received a second ASCS treatment at the 6-week visit, unless the index ulcer had achieved ≥85% reepithelialization or had improved at least 15% from the prior visit.
The effectiveness measures of the study were to evaluate wound healing determined by the surgeon - by visual assessment, with complete wound closure being defined as complete epithelialization without drainage on two consecutive visits. Digital photography via Tissue Analytics Wound Measurement System (Tissue Analytics, Baltimore, MD) was also used to assess wound closure. Donor sites were assessed visually for closure at each follow-up visit until healed. Posttreatment patient and physician ratings of overall satisfaction were captured on a 10-point scale by answering the question: “How satisfied are you today with the ASCS treatment?”. Selected sections of the Neuro-QoL quality of life questionnaire21 were used at each visit to assess health-related quality of life relating mobility, social impact, and patient perceived well-being. Short forms of three components of Neuro-QoL were used: “Lower Extremity Function (Mobility)”, “Ability to Participate in Social Roles and Activities” and “Positive Affect and Well-Being.” In addition, self-reported pain and self-reported exudate levels were assessed using a visual analogue scale (VAS).
Safety and tolerability of the ASCS treatment was confirmed based on the occurrence of local reactions including but not necessarily limited wound infections not present at baseline or previous assessments, worsened ulceration, local allergic response, or other treatment-related adverse events requiring subsequent surgical intervention (Clinical Trial Registry No. NCT02799121).
2.1 Statistical analysis
No formal sample size estimation was performed as this study was designed to be a feasibility study. Sixteen subjects were enrolled and this sample size was considered appropriate for clinical assessments of safety/tolerability and preliminary effectiveness of ASCS when used for healing of DFUs. Descriptive statistics were performed using Microsoft Excel for Windows. The safety analysis population includes all enrolled participants who were treated.
3 RESULTS
3.1 Subject and wound characteristics
Thirty-one subjects were assessed for eligibility at three investigative sites and 15 were excluded (Figure 1). After enrollment of 16 subjects, the study was stopped as the amount of data to make a clinical assessment of safety/tolerability and preliminary effectiveness of ASCS for treating DFUs was obtained. Of the enrolled subjects, 1 was withdrawn from the study by the investigator due to noncompliance and two did not have healing photography assessment and data recorded. Protocol deviations included lost to follow-up, second or third treatment of ASCS not being applied, and missing wound size data (Figure 1).

Table 1 summarizes patient demographics and comorbidities for the 16 subjects enrolled. The mean age for the subject population was 62.5 years and 87.5% were male. The majority of subjects had one ulcer (93.8%), with one subject having three or more ulcers, however, only one ulcer was included as a study ulcer (Table 1). Most subjects (68.8%) had previous amputations (Table 1).
| Age (years) | |
| Mean ± SD | 62.5 ± 12.5 |
| (Range) | (41-87) |
| Sex (% male) | 87.5% |
| Smoker (%) | 18.8% |
| Height (cm) | |
| Mean ± SD | 178.5 ± 8.3 |
| (Range) | (165-193) |
| Weight (kg) | |
| Mean ± SD | 91.0 ± 29.0 |
| (Range) | (55-159) |
| Number of active ulcers (%) | |
| 1 | 93.8% |
| 2+ | 6.3% |
| Number of prior amputations (N) | |
| 1 | 4 |
| 2 | 4 |
| 3 | 1 |
| 4 | 2 |
Subjects enrolled in the study had comorbidities consisting of coronary and peripheral arterial disease, peripheral neuropathy, cancer, Type I or Type II diabetes, as well as other chronic diseases and conditions including those affecting the musculoskeletal, gastrointestinal, and cardiovascular systems.
The ulcer size at baseline ranged from 3.0 to 29.5 cm2 with the mean ulcer area prior to treatment being 15.5 cm2, and median ulcer size of 15.3 cm2 (Table 2). The mean ulcer duration was 60.4 weeks (median: 28.4 weeks; range 4.1-197.1 weeks) for the patient population and wounds included University of Texas Ulcer grade 1A, 1B, or 2A classifications (Table 2). Subjects with infection that were enrolled (University of Texas Ulcer grade 1B) had quiescent osteomyelitis (n = 3) or a mild infection (n = 1), which were all managed with antibiotics.
| Ulcer size (pre-treatment, cm2) | |
| Mean ± SD | 15.5 ± 6.7 |
| Median | 15.3 |
| (Range) | (3.0-29.5) |
| Duration of ulcer (weeks) | |
| Mean ± SD | 60.4 ± 28.4 |
| Median | 28.4 |
| (Range) | (4.1-197.1) |
| Depth (n) | |
| Superficial | 13 |
| To tendon or closed joint capsule | 3 |
| Infection (n) | |
| No | 12 |
| Yes | 4 |
| Ulcer location (n) | |
| Plantar | 6 |
| Dorsal | 10 |
Prior treatments for the ASCS treated ulcer included topical antibiotics (1), systemic antibiotics (8), negative pressure wound therapy (5), vasodilators (1), debridement (11), and absorbent (12), silver nitrate (2), calcium alginate (3), or synthetic occlusive dressings (1).
3.2 Diabetic foot ulcer and donor site healing outcomes
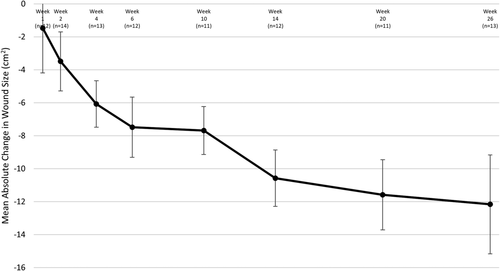
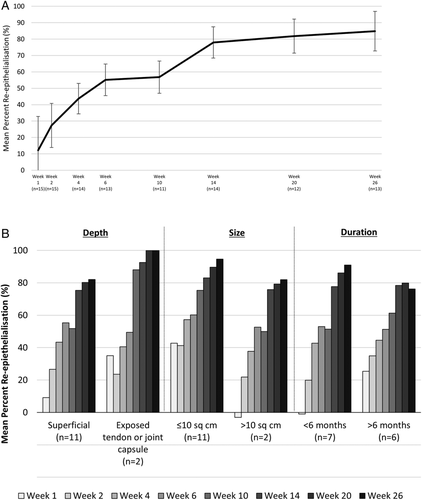
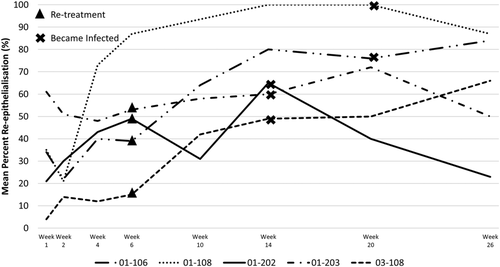
All ulcers were reduced in size at the last visit, with a mean reduction of 12.2 cm2 from post-debridement measurement to Week 26 (Figure 2). The mean percent reepithelialization was evaluated using the 3D wound imaging system. At Week 26, the mean reepithelialization was 84.9% (Figure 3A), with approximately half (7/13) of the assessed ulcers achieving 100% closure. Wounds were stratified based on known predictors of healing, including depth, size, and duration (Figure 3B). Regardless of stratification, all ulcers responded positively throughout the duration of the study. Ulcers were also grouped and assessed by healing status (whether or not they achieved 100% reepithelialization). The subject and wound characteristics were similar between the healed vs unhealed groups (Table 3). When ulcers were grouped by those that avoided an infection prior to healing (n = 9), the majority fully healed (n = 7), with the other two of them achieving greater than 95% reepithelialization (n = 2). Of those that healed, one then suffered an infection and the ulcer recurred, but this went on to fully heal at the 28-week visit, and one recurred, but was still 99% reepithelialized at 28 weeks (Figure 1). None of the four patients who acquired an infection prior to healing went on to completely heal by the end of the 26-week study though the median wound reepithelialization was still 62% (Figures 1 and 4).
| Group | Healed ulcers (n = 7) | Unhealed ulcers (n = 6) |
|---|---|---|
| Hemoglobin A1c (n) | 1 Not done | |
| 6-7.9 | 0 | 3 |
| ≥8 | 7 | 2 |
| Ulcer size (pre-treatment, cm2) | ||
| Mean ± SD | 14.2 ± 8.2 | 16.2 ± 5.5 |
| Median (range) | 14.5 (3.0–29.5) | 15.2 (8.9-24.7) |
| Duration of ulcer (weeks) | ||
| Mean ± SD | 49.3 ± 68.8 | 64.8 ± 68.9 |
| Median (range) | 16.4 (8.1-197.1) | 39 (4.1-174.4) |
| Depth (n) | ||
| Superficial | 5 | 6 |
| To tendon or closed joint capsule | 2 | 0 |
| Infection (managed osteomyelitis) (n) | ||
| No | 6 | 3 |
| Yes | 1 | 3 |
| Re-epithelialization at 26 weeks (%) | ||
| Mean ± SD | 98.1 ± 4.9 | 69.3 ± 29.1 |
| Median (range) | 100 (87.0-100.0) | 75 (23.0-98.0) |
3.3 Pain and exudate
The absolute change in pain ratings from baseline as measured by the subject (VAS) demonstrated no noticeable difference from baseline at any visit posttreatment. The absolute change in exudate ratings from baseline was also measured by the subject (VAS) and there was a −1.6-mean difference from baseline to Week 26. At all other timepoints evaluated, no obvious differences were observed.
3.4 Health related quality of life
The absolute change in all HRQoL ratings as measured by the Neuro-QoL questionnaire (lower extremity function, ability to participate in social roles and activities, and positive affect and well-being) were assessed between baseline and follow-up visits. A consistent trend of improvement in all aspects of the Neuro-QoL was reported by subjects at the Week 26 visit, but when grouped by patients that healed vs those that did not heal, the mean change in score from baseline to Week 26 was always better for those that did heal (Social roles and activities: 6.0 ± 6.5 vs 3.2 ± 5.5; Lower extremity function: 5.8 ± 8.3 vs −3.6 ± 14.6; Positive effect and well-being: 1.3 ± 5.7 vs −0.2 ± 4.6).
3.5 Subject and clinician satisfaction
Subjects and clinicians consistently had high satisfaction scores across all visits. At Week 26, the mean subjects' satisfaction for all wounds was 9.3 ± 1.4 and for clinicians' satisfaction, the mean value at Week 26 was 8.8 ± 1.5.
3.6 Safety
The incidence of subjects with AEs possibly or probably related to ASCS treatment was relatively low (25.0%), with specific AEs listed by number of subjects (Table 4). The most common treatment-related adverse event was infection which is common in this subject population as chronic wounds are susceptible to contamination and colonization by a wide variety of aerobic and anaerobic microorganisms and often the colonization will progress to infection. Overall, infections that occurred during follow-up were low (40.7% of all treatment-related adverse events, and three total infections occurring during the follow-up visits). By the end of the study, new ulcers were reported in two subjects, however, none of the subjects required amputations.
| Total number of subjects with at least one AE (%) | 15 (93.75%) | |
| Specific AE by subject | ASCS site n (%) | Non-study site n (%) |
| Infection | 6 (37.5%) | — |
| Excess exudate | 2 (12.5%) | — |
| Maceration | 4 (25.0%) | — |
| Wound re-opening | 2 (12.5%) | — |
| Cellulitis | 1 (6.3%) | — |
| Soft tissue defect | 1 (6.3%) | — |
| Bleeding | 2 (12.5%) | — |
| Pain | 1 (6.3%) | — |
| Itching at donor site | 1 (6.3%) | — |
| Swelling | 1 (6.3%) | 1 (6.3%) |
| New ulcer | — | 2 (12.5%) |
| Necrotic wound on amputation site | 1 (6.3%) | — |
| Rash/skin reaction | — | 2 (12.5%) |
| Acute kidney injury | — | 1 (6.3%) |
| Exacerbation of COPD | — | 1 (6.3%) |
| Biofilm | 4 (25.0%) | — |
| Most severe AE | ||
| No event reported | 1 (6.25%) | |
| Mild | 10 (62.5%) | |
| Moderate | 3 (18.75%) | |
| Severe | 2 (6.25%) | |
| Subjects with at least one serious AE (details listed below) | 3 (18.75%) | |
| Admitted with breathlessness and non-infective exacerbation of COPD | 1 (6.25%) | |
| Infected ulcer, osteomyelitis and sepsis | 1 (6.25%) | |
| Superficial femoral artery occluded by 50% | 1 (6.25%) | |
| Subjects with at least one AE probably or definitely related to the study device | 4 (25.0%) | |
A total of three SAEs in three subjects occurred during the study. Two SAEs were unrelated to ASCS and one was listed as likely related but was due to an infection that led to osteomyelitis and sepsis; however, this is a common risk in this patient population. The unrelated events included exacerbation of existing unrelated conditions.
4 DISCUSSION
Diabetic foot ulcers are a complex disorder in which damaged areas of tissue can remain unhealed for extended periods of time.6 While there are a multitude of treatments available for DFUs, there is still a high risk of amputation. In the United Kingdom, from 2015 to 2016 17% of patients within the first 12 months of the first presentation of their DFU had at least one amputation.22 Over the 12 months, the mean cost of wound care in the United Kingdom for a DFU was estimated to be £7800 with a healed DFU costing £2140, an unhealed DFU costing £8800, and an amputation costing £16 900.22 As standard therapy does not always result in healing outcomes, with only 35% of DFUs reported healed from 2015 to 2016 in the United Kingdom,22 alternative strategies are often required for the definitive closure of these wounds.
Evidence exists to support the use of split-thickness skin grafting (STSG) as an option to improve healing outcomes for DFUs, however, skin grafting is difficult for ulcers of large area, as a large donor site is required leaving a significant defect subject to potential healing difficulties in this subject population. Furthermore, data suggests that only 66.7% of DFUs treated with a STSG fully heal with 6% requiring amputation.14
The ACHD allows the benefit of treating a skin defect with autologous skin, using minimal donor tissue. At the point-of-care, a 1.5 to 2 cm2 thin skin graft (0.15-0.2 mm) is processed to prepare ASCS which contains a mixed population of keratinocytes, fibroblasts, melanocytes, and Langerhans cells which can treat a wound up to 80 cm2.23 While interactions between keratinocytes and fibroblasts are known to be important for reepithelialization, and melanocytes are necessary for re-pigmentation, a recent study has shown that high numbers of Langerhans cells in the epidermis of DFUs correlates with healing.24 Previous published studies have documented the success of ASCS prepared using the ACHD in treating DFUs18, 25, 26; but this is the first study to date relating patient history (including baseline wound characteristics) and clinical outcomes (incidence of healing, rate of healing, and patient and physician satisfaction).
The study population was comprised of patients with varying wound depths, size, duration, and even some with exposed tendon. Ulcers had a mean area of 15.5 cm2 with a mean ulcer duration of 60.4 weeks. All subjects had a positive healing trajectory with a mean of 12.2 cm2 reduction in wound size and an overall mean of 84.9% reepithelialization at Week 26. In an effort to limit new wounds in this susceptible patient population, the ACHD was employed to spare the amount of donor skin harvested. Additionally, donor sites were treated with ASCS and all were healed by the end of the study. A trend of improved quality of life and high satisfaction was demonstrated. There were no significant differences observed in pain measurements at any visit compared to baseline, likely due to low baseline levels attributed to neuropathy.
Wound duration, size, and depth are well-noted predictors of wound healing.27 When healing was assessed based on stratification of medical history, it was determined that all wounds, regardless of baseline characteristics, had improvement following ASCS treatment. Ulcers that were less than 10 cm2 had improved healing at all visits compared to ulcers >10 cm2, which is not surprising due to the smaller area of reepithelialization required. There was no obvious difference in incidence of healing when ulcers were stratified by duration (>6 months vs >6 months), but more chronic ulcers had a slower healing trajectory compared to less chronic wounds. Ulcers containing exposed tendon/capsule initially had a slower response compared to more superficial wounds and ulcers categorized as initially infected appeared to have less reepithelialization at early time points but recovered at the later visits. In addition, comparison of baseline characteristics of subjects with healed ulcers vs those that did not fully heal demonstrated no difference in ulcer duration or severity of diabetes.
Collectively, the mean time to healing for the patients with definitive closure was 20 weeks with 46% of wounds achieving 100% reepithelialization at Week 26. Although not all ulcers achieved definitive closure, a mean reduction in size of 12.2 cm2 is encouraging considering randomized controlled trials for other DFU treatments reported in the literature assessed mean baseline DFU size under 4 cm2.28, 29 When designing a controlled trial to evaluate effectiveness and understand the clinical impact of ASCS, it is important to assess incidence of healing at 12 weeks with a control arm consisting of standard of care therapy. Additionally, in order to provide meaningful information to assess effectiveness over other therapies, similar inclusion and exclusion criteria based on subject medical history and wound characteristics utilized in other DFU RCTs will be employed. During this study, none of the ASCS-treated ulcers required amputation within the 26 week study period, whereas amputation rate in other published 12-week trials have reported a range of 5.5% to 15.6% amputation of the study limb in either the treatment or control group.30, 31
Acquired infection during the study was found to be a confounding factor for healing. All ulcers that remained infection-free or had managed osteomyelitis progressed to ≥95% reepithelialization, whereas all the ulcers that became infected during the study had a mean reepithelialization of 62% at Week 26. As with all wound care, this suggests a prerequisite for close and careful management of wound bed preparation and subsequent aftercare. Future work is warranted to evaluate the reapplication of ASCS following infection management and the role on reepithelialization and wound closure. In addition, many subjects did not receive multiple applications of ASCS as defined by the protocol. Multiple applications of ASCS may not be necessary, as most of the ulcers achieved complete or near-complete healing, however, the ideal frequency of ASCS application for faster closure of DFUs is not known at this time and may have an impact on clinical results.
Study results support the initial safety of the product for the treatment of DFUs as ASCS was generally well tolerated with no allergic response to the enzyme occurring during the study. The incidence of treatment-related adverse events including infection was low and no treatment-related adverse events were dispositioned as definitely related to the ASCS treatment. The incidence of reported infections was typical for this patient population.
An obvious shortcoming of this study is the lack of a control group available to compare outcomes; however, each subject served as their own historical control, as the study DFU evaluated had not positively responded to standard wound care inclusive of topical antibiotics, systemic antibiotics, negative pressure wound therapy, vasodilators, debridement, and absorbent, silver nitrate, calcium alginate, or synthetic occlusive dressings. We recognize that there is a weakness in use of historical controls and that offloading was not formally assessed in this study which has the potential, along with other multifactorial reasons, to contribute to a positive healing trajectory. Follow-up time of this study was limited to 26 weeks, and evidence of ulcer recurrence and lower limb amputations were not collected past this period. Because this was a feasibility study, the small sample size should be noted and further clinical studies will be necessary to generalize and draw clinically meaningful conclusions.
Overall, the results from this preliminary feasibility study suggest that ASCS, as prepared using the ACHD, appears to be a safe and promising therapy for the treatment of DFUs. All DFUs demonstrated a positive healing trajectory regardless of size, depth, duration, and infection. Future studies are warranted to understand healing outcomes comparing ASCS against standard of care as well as to further define the ASCS treatment algorithm for DFUs and its potential impact on associated health care costs.
ACKNOWLEDGMENT
This study was sponsored by AVITA Medical.
CONFLICT OF INTEREST
Dr. Tawqeer Rashid has received honoraria from Avita Medical.




