DEHT is a suitable plasticizer option for phthalate-free storage of irradiated red blood cells
Abstract
Background and Objectives
Due to increasing concerns about possible endocrine-disrupting properties, the use of the plasticizer di(2-ethylhexyl) phthalate (DEHP) will be banned in future blood storage. Di(2-ethylhexyl) terephthalate (DEHT) provides sufficient red blood cell (RBC) quality during conventional blood bank storage. It is important that a new plasticizer also maintains acceptable quality during exposure to high cell stress, such as irradiation, which is commonly used to prevent graft-versus-host disease.
Materials and Methods
A total of 59 RBC units were collected and processed in polyvinyl chloride (PVC)-DEHT or PVC-DEHP blood bags combined with either saline-adenine-glucose-mannitol (SAGM) or phosphate-adenine-glucose-guanosine-saline-mannitol (PAGGSM) additive solution. All units were X-ray irradiated on day 2 post-collection. Sampling for assessment of parameters of storage lesion was performed on day 2 pre-irradiation and day 14 and 28 post-irradiation.
Results
Though irradiation increased cell stress, DEHT/PAGGSM and current common European preference DEHP/SAGM were equally affected up to 14 days post-irradiation for all measured parameters. At day 28, haemolysis and microvesicle count were slightly increased in DEHT, whereas extracellular potassium ions, glucose, lactate, pH, mean corpuscular volume and microvesicle phosphatidylserine remained unaffected by plasticizer choice throughout storage. No individual unit exceeded 0.8% haemolysis, not even in DEHT/SAGM, the combination overall most affected by irradiation. Of the four combinations, membrane stability was least impacted in DEHP/PAGGSM.
Conclusion
We demonstrate that DEHT is a suitable plasticizer for storage of RBCs after X-ray irradiation cell stress. This strengthens the option of DEHT as a viable non-phthalate substitute for DEHP.
INTRODUCTION
The normal lifespan of a red blood cell (RBC) is around 120 days in circulation, but maximum storage time of a refrigerated RBC concentrate (RCC) is usually limited to five to seven weeks due to a degenerative process called RBC storage lesion. This is a descriptive name for the large number of metabolic, oxidative and morphological changes that RBCs undergo during blood bank storage conditions, which ultimately leads to cell rupture: haemolysis [1]. When RBCs lyse, haemoglobin is released. Haemoglobin is a strong inducer of reactive oxygen species (ROS) that, just like RBC microvesicles (RMV), are linked to pro-inflammatory and pro-thrombotic processes [1, 2]. Therefore, it is crucial to keep the number of haemolysed cells in an RCC as low as possible up until the point of transfusion. The upper limit for haemolysis has been set to 0.8% of red cell mass in the Council of Europe member states [3].
The haemolysis generation is affected by exposure to different manufacturing and storage stressors [4-6]. One of the most extreme stressors is irradiation. Irradiation, usually through X-ray or gamma methods [7], is an important measure to inhibit proliferation of residual white blood cells (WBCs) and thereby prevent transfusion-associated graft-versus-host disease [3, 8, 9]. However, irradiation stimulates the creation of ROS which, in turn, damages the RBC membrane through induction of lipid peroxidation and oxidation of membrane-bound proteins, including inhibition of Na+/K+ ATPase [10-12]. This premature loss of membrane integrity increases the rate of both haemolysis and RMV generation [6, 10-14] as well as the accumulation rate of extracellular potassium ions (K+), associated with hyperkalaemia-linked cardiac arrest [10, 15, 16]. Consequently, the shelf-life of irradiated RCCs is frequently reduced between 24 h and 2 weeks. Among the more stringent practises is the ‘14 + 14 days’ rule (maximum 14 days old RCCs at time of irradiation, maximum 14 days storage post-irradiation), which is advocated in for instance Sweden and the UK. [3, 17, 18].
The use of polyvinyl chloride (PVC) storage containers plasticized with di(2-ethylhexyl) phthalate (DEHP) has, in combination with suitable additive solution (AS), long been a cornerstone to maintain low haemolysis levels throughout storage. DEHP leaches from the PVC matrix and incorporates into the RBC membrane phospholipid bilayer, thereby stabilizing it and prolonging the time to lysis [19, 20]. Due to increasing concerns about possible endocrine-disrupting properties, the regulatory restrictions for medical devices have recently been updated with the aim to abolish the use of DEHP in blood storage [21]. Therefore, it is urgent to remove DEHP without compromising the blood component quality [22], a conundrum that has delayed the replacement process for a long time.
Although no other plasticizer has, so far, shown identical RBC storage quality to DEHP, there are a few promising candidates. In a previous study [23], we demonstrated that a structural isomer to DEHP, di(2-ethylhexyl) terephthalate (DEHT), provides adequate RBC quality during seven weeks of storage in both saline-adenine-glucose-mannitol (SAGM; primary AS in Europe) and phosphate-adenine-glucose-guanosine-saline-mannitol (PAGGSM). Satisfying quality of DEHT combined with AS-1 has also been confirmed [24].
It is important that a new plasticizer is versatile enough to maintain acceptable RBC quality not only at ideal storage conditions, but also during exposure to common blood bank stressors. Therefore, we wanted to subject RBCs stored in PVC-DEHT bags, combined with SAGM or PAGGSM, respectively, to X-ray irradiation and compare the results to corresponding DEHP storage. This study sheds light on the characteristics of DEHT for RBC storage during the condition of irradiation cell stress.
MATERIALS AND METHODS
Blood collection and component processing
A total of 59 RCCs were produced through collection and separation of whole blood (WB; 450 ml ± 10% in 63 ml citrate-phosphate-dextrose) from regular, voluntary, non-remunerated blood donors, following Karolinska standard operating procedures [23, 25]. Briefly, these include separation with Macospin (3130 × g, 11 min) and MacoPress Smart Revo (both Macopharma, Mouvaux, France) on the collection day (no overnight WB hold). Blood bag systems corresponding to the commercially available bottom-and-top NPT reference (Macopharma), including identical needle and leucoreduction filters, were manufactured completely of PVC-DEHT or PVC-DEHP, respectively. These bag systems contained either 100 ml SAGM or PAGGSM, respectively, for RBC resuspension. This set-up allowed four study arms to be created: DEHT/SAGM, DEHT/PAGGSM, DEHP/SAGM and DEHP/PAGGSM (blood type A; DEHP/SAGM n = 14, all other arms n = 15). The donors were chosen randomly for each arm; no pool-and-split strategy was applied. The RCCs were cold stored (2–6°C) within 8 h of donation. Tube sealers from the Qseal range (Conroy Medical AB, Upplands Väsby, Sweden) and sterile docking device TSCDII (TerumoBCT, Lakewood, CO) were used for both the DEHT and DEHP material.
On day (d) 2 post-collection, the RCCs were X-ray irradiated (canister center dose 37.85 Gy, maximum 38.53 Gy, minimum 26.42 Gy; Raycell Mk2, Best Theratronics, Ottawa, Canada) following guideline specifications [3]. The RCCs were sampled on d2 pre-irradiation for baseline values, then on d14 and d28 post-irradiation. Sampling was performed through sterile connection of 40 ml sampling bags (also made of PVC-DEHT or PVC-DEHP, respectively) to the RCCs, very gentle mixing during sampling, followed by immediate analysis.
The RBCs were exposed to the same plasticizer (DEHT or DEHP, respectively) at all time points, from collection until study end. Furthermore, the RCCs of different plasticizers were handled and stored separately to avoid accidental cross-contamination, as were the bag systems during the manufacturing process.
Assessment of RBC quality
The impact of X-ray irradiation on the different plasticizer/AS combinations was determined through collective assessment of membrane effects and metabolic effects during storage. The RCCs were analysed for haemoglobin (HbRCC; g/l), haematocrit (Hct) and mean corpuscular volume (MCV; fl) through Swelab Alfa Plus Basic hematology analyzer (Boule Diagnostics AB, Spånga, Sweden). After centrifugation of the samples twice (1450 × g, 10 min, 20°C), free supernatant haemoglobin (Hbsupernatant; g/l) was measured with HemoCue plasma/low haemoglobin photometer (Radiometer Medical ApS). Haemolysis (%) was calculated as (100-Hct) × Hbsupernatant (g/l) / HbRCC (g/l). Extracellular K+ (mmol/l), pH, glucose (mmol/l) and lactate (mmol/l) were assessed with ABL 800 Flex blood gas analyser (Radiometer Medical ApS, Brønshøj, Denmark). Measurements of RMV count and RMV phosphatidylserine externalization (Annexin V positive RMVs) were performed by flow cytometry (CytoFLEX, Beckman Coulter, Brea, CA), protocols identical to previous study [23].
In addition, residual WBCs were counted with cell counter ADAM-rWBC (NanoEnTek, Seoul, South Korea) at storage start. All donors were screened for required serological markers [3], and all RCCs were bacteriologically tested at storage end (Karolinska University Laboratory, Clinical Chemistry and Clinical Microbiology departments).
Statistical analysis
After verifying a Gaussian distribution (D'Agostino-Pearson normality test), mean ± standard deviation (SD) was computed (GraphPad Prism v.8.2 for Windows; GraphPad Software Inc., La Jolla, CA). To test statistical significance between the study arms, two-way analysis of variance (ANOVA) was applied with Holm-Sidak's correction as post-hoc test, while differences between time points within a single study arm were compared using repeated measures one-way ANOVA.
Ethics statement
As all used material was fully anonymised, the Stockholm Regional Ethical Review Board did not consider an ethical application necessary.
RESULTS
All RBC units fulfilled European and national quality criteria in HbRCC, Hct and WBC count [3, 17]. Screenings for bacteria and infectious markers were negative.
Haemolysis was similar for DEHT/PAGGSM and DEHP/SAGM at d14 post-irradiation. However, during the second half of storage, it increased faster in DEHT, especially in combination with SAGM, and at d28, all four study arms differed (p < 0.001; Figure 1a, Table 1). The highest individual unit (DEHT/SAGM) reached 0.65% haemolysis, meaning all units remained safely below 0.8% at storage end.
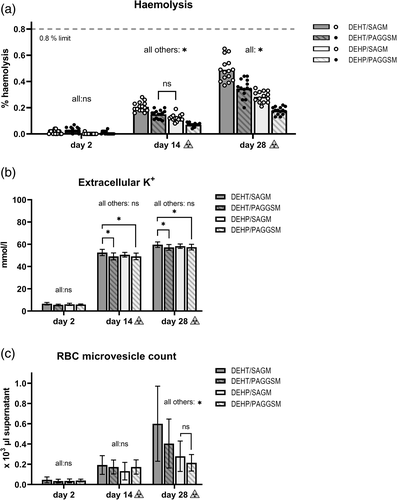
| Analysis parameter | Day 2 (pre irradiation) | Day 14 (post irradiation) | Day 28 (post irradiation) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DEHT/SAGM | DEHT/PAGGSM | DEHP/SAGM | DEHP/PAGGSM | DEHT/SAGM | DEHT/PAGGSM | DEHP/SAGM | DEHP/PAGGSM | DEHT/SAGM | DEHT/PAGGSM | DEHP/SAGM | DEHP/PAGGSM | |
| Haemolysis (%) | 0.01 ± 0.01 | 0.03 ± 0.02 | <0.01 | 0.01 ± 0.01 | 0.21 ± 0.04a,b,c | 0.15 ± 0.03a,e | 0.12 ± 0.03b,f | 0.07 ± 0.01c,e, f | 0.49 ± 0.09a,b,c | 0.35 ± 0.07a,d,e | 0.28 ± 0.04b,d,f | 0.18 ± 0.03c,e, f |
| RMV, count (×103/μl supernatant) | 0.05 ± 0.03 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.19 ± 0.09 | 0.17 ± 0.07 | 0.13 ± 0.09 | 0.17 ± 0.07 | 0.60 ± 0.37a,b,c | 0.40 ± 0.24a,d,e | 0.28 ± 0.15b,d | 0.21 ± 0.08c,e |
| RMV, externalized phosphatidylserine (%) | 57.0 ± 18.8c | 51.3 ± 25.1e | 48.3 ± 22.4f | 32.4 ± 14.7c,e,f | 56.7 ± 11.8 | 47.0 ± 10.9 | 53.8 ± 13.8 | 47.1 ± 10.9 | 71.7 ± 7.6 | 63.1 ± 10.7 | 66.4 ± 12.7 | 58.3 ± 9.7 |
| Extracellular K+ (mmol/l) | 6.6 ± 1.1 | 5.6 ± 0.5 | 6.0 ± 1.0 | 5.9 ± 0.5 | 52.6 ± 2.8a,c | 49.1 ± 3.1a | 50.6 ± 2.1 | 49.1 ± 3.0c | 59.6 ± 2.5a,c | 57.2 ± 2.6a | 58.2 ± 2.0 | 57.4 ± 2.5c |
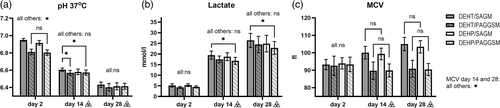
| pH 37°C | 6.950 ± 0.017a,b,c | 6.811 ± 0.032a,d | 6.916 ± 0.028b,d,f | 6.804 ± 0.031c,f | 6.606 ± 0.019a,c | 6.569 ± 0.028a | 6.580 ± 0.032 | 6.573 ± 0.030c | 6.432 ± 0.031 | 6.403 ± 0.044 | 6.414 ± 0.041 | 6.415 ± 0.034 |
| Glucose (mmol/l) | 29.1 ± 1.5a,c | 27.7 ± 1.7a,d | 29.0 ± 0.8d,f | 27.0 ± 1.3c,f | 22.0 ± 0.8 | 21.3 ± 1.2 | 21.8 ± 1.5 | 21.3 ± 1.1 | 18.0 ± 1.5 | 17.1 ± 1.9d | 18.7 ± 1.9d | 17.7 ± 1.4 |
| Lactate (mmol/l) | 5.2 ± 0.8 | 4.3 ± 0.4 | 5.3 ± 0.7 | 4.5 ± 0.5 | 19.5 ± 1.9c | 17.4 ± 1.6 | 18.7 ± 1.9 | 16.8 ± 1.7c | 26.4 ± 3.3c | 24.5 ± 3.9 | 24.9 ± 3.9 | 22.9 ± 3.0c |
| MCV (fl) | 93.3 ± 3.0 | 92.6 ± 5.5 | 94.0 ± 3.3 | 93.4 ± 4.2 | 100.0 ± 3.6a,c | 89.7 ± 5.0a,d | 99.3 ± 3.3d,f | 89.9 ± 3.7c,f | 105.0 ± 3.8a,c | 90.9 ± 4.8a,d | 103.0 ± 3.5d,f | 90.4 ± 3.5c,f |
- Note: Data are presented as mean ± standard deviation. DEHT/SAGM, DEHT/PAGGSM, DEHP/PAGGSM: n = 15, DEHP/SAGM: n = 14. Significant differences (p < 0.05) are shown by letters a-f.
- Abbreviations: DEHT, di(2-ethylhexyl) terephthalate; DEHP, di(2-ethylhexyl) phthalate; SAGM, saline-adenine-glucose-mannitol; PAGGSM, phosphate-adenine-glucose-guanosine-saline-mannitol; RMV, red blood cell microvesicles; MCV, mean corpuscular volume.
- a DEHT/SAGM versus DEHT/PAGGSM.
- b DEHT/SAGM versus DEHP/SAGM.
- c DEHT/SAGM versus DEHP/PAGGSM.
- d DEHT/PAGGSM versus DEHP/SAGM.
- e DEHT/PAGGSM versus DEHP/PAGGSM.
- f DEHP/SAGM versus DEHP/PAGGSM.
Elevated extracellular K+ signifies membrane leakage and/or suppressed metabolism. Extracellular K+ increased sharply after irradiation in all study arms, independent of plasticizer/AS combination. DEHT/PAGGSM stayed similar to both DEHP arms throughout storage, whereas storage in DEHT/SAGM resulted in slightly higher concentration than both PAGGSM arms (d14: p < 0.001, d28: p < 0.05; Figure 1b, Table 1). The two SAGM arms (DEHT/SAGM vs. DEHP/SAGM) remained similar throughout storage. The difference in concentration between the study arms stayed within 2.4 mmol/l (min-max range of the d28 means: 57.2–59.6 mmol/l).
Increased RMV count signifies loss of membrane integrity. The RMV count was similar for all study arms at d14 post-irradiation. However, at d28, DEHT/SAGM had the highest observed count, followed by DEHT/PAGGSM (Figure 1c, Table 1). Although the overall percentage of RMVs exposing the apoptosis marker phosphatidylserine increased after irradiation (p < 0.05), there was no difference between the study arms at the separate time points (Table 1).
Glucose, lactate and pH are markers of metabolic capacity. The glucose concentration and pH were both initially higher in SAGM storage than in PAGGSM (glucose: p < 0.05, pH: p < 0.001), but the differences ceased with storage time. Correspondingly, the lactate generation was faster and indicated an overall higher metabolism during SAGM storage. MCV is sensitive to osmolality. A distinct increase in MCV was detectable from d14 onwards in both SAGM arms, whereas in PAGGSM, MCV decreased compared to d2 (p < 0.001; Figures 2a–c, Table 1). No distinct differences could be related to plasticizer for glucose, lactate, pH or MCV.
DISCUSSION
This study demonstrates that DEHT is a suitable plasticizer for storage of RBCs after applying severe cell stress in the form of X-ray irradiation.
The observed haemolysis in irradiated RBCs stored in DEHT in combination with either SAGM or PAGGSM (Figure 1(a)) was in accordance with the previously shown pattern of non-irradiated RBC units stored in DEHT [23], however, expectedly amplified. The non-irradiated d49 haemolysis values were exceeded somewhere between d14 and d28 post-irradiation. This similarity suggests that irradiation itself does not interfere with the DEHP removal and/or DEHT addition in other ways than the accelerated speed in haemolysis generation observed also after irradiation of conventional DEHP-stored RBCs [6, 11, 13, 14]. Even with DEHP, the possibility of individual units exceeding 0.8% haemolysis during post-irradiation storage remains a health risk, in particular to the more fragile patient categories such as, for instance, paediatric patients. The literature for irradiation of non-DEHP storage containers is scarce, likely because removal of DEHP has been linked to critical haemolysis levels in non-irradiated units [26]. This study indicates that PVC-DEHT containers provide sufficient RBC storage environment with existing commercial ASs for at least 14 days, but even up to 28 days post-irradiation, when irradiation is carried out early after donation.
The extracellular K+ results were also encouraging. The minor concentration differences between the four plasticizer/AS combinations were likely not of clinical significance and suggest that the irradiation itself had substantially more impact than the possible theoretical influence of plasticizer or AS (Figure 1b).
RMVs are shed from the RBC membrane as part of the storage lesion process, as ROS increases and antioxidants and ATP are depleted [2]. In this study, the generation of RMVs had similar but earlier onset than in non-irradiated units [23]. This is likely attributed to increased ROS generation caused by irradiation, both directly and through the positive feedback loop provided by the haemolysis-induced increase of free haemoglobin [11, 12]. A relationship was demonstrated between RMV count and both plasticizer and AS, the latter likely related to the ATP dependency of the RMV generation; the lower RMV count in PAGGSM-stored units suggests a slower reduction of ATP. Though ATP was not analysed in this study, the associated markers pH, lactate and glucose all trended towards a higher metabolic rate in SAGM storage (Figure 2a,b).
Phosphatidylserine is a phospholipid normally located in the inner leaflet of the RBC bilayer membrane. Membrane proteins of ATPase character control the asymmetry between the inner and outer leaflet, and when ATP levels decrease, there is a progressive translocation of phosphatidylserine to the outer leaflet [27], where it plays a signalling role for phagocytosis [28]. The literature is ambiguous as to whether the percentage of phosphatidylserine positive RMVs from non-irradiated RBCs increases during storage or not; several studies, our own previous study included, seem to suggest that it does not [23, 29, 30]. However, as irradiation induces increased oxidizing damage to membrane-bound proteins [11, 12], it may be hypothesized that the observed increase in the frequency of RMVs exposing phosphatidylserine may be a consequence of irradiation damage. Possibly, irradiation targets ATPase the same way as for extracellular K+. Phosphatidylserine externalization could not be related to AS the way it could for non-irradiated units. The irradiation damage to the ATPase may have outweighed the potential later effects of AS- and storage time-dependent metabolic depletion, especially as the RBCs were irradiated as early as d2 post-collection.
Irradiation appears to primarily impact the storage lesion parameters connected to the stability of the RBC membrane. Expectedly, the same parameters were affected when the membrane-stabilizing plasticizer DEHP was exchanged for DEHT. However, there seems to be no negative physical impact by irradiation on the DEHT plasticizer itself, as parameters that are unaffected by choice of plasticizer in non-irradiated RBCs remained unaffected also in this study. The AS-dependent differences for glucose, lactate, pH and MCV were well in accordance with previous findings for non-irradiated units [23] without any detectable dissimilarities that could be ascribed to potential irradiation damage to the plasticizer itself. Furthermore, sealing, sterile docking and general bag handling were performed without remark throughout the study.
This study has both strengths and limitations. One limitation is that irradiation was performed on d2, while it is allowed up until d28 post-collection in many countries [3], and there is evidence connecting irradiation of older RCCs to higher haemolysis rates [6, 13]. This study gives a good first indication, but a similar follow-up study with older units would be a necessary complement for further conclusions. Moreover, the irradiation process was performed by X-ray technique. Although proven to be equal to gamma technique for RBCs stored in DEHP [7], gamma irradiation of DEHT should be verified. Furthermore, it was not possible to directly measure ATP. Instead, indirect markers such as pH, glucose, lactate and K+ had to be used for conclusions about the metabolism. Yet another limitation is the impact of donor variability as, for logistical reasons, no pool-and-split strategy was applied during processing. This is a weakness when comparing different study arms; however, it is simultaneously an important asset for demonstrating that despite donor variation, no individual units exceeded the limit for haemolysis, independent of the plasticizer or AS they were stored in (Figure 1a).
Numerous processing conditions affect the RBC storage lesion, which complicates inter-blood establishment comparisons [31, 32]. A strength of this study is that the irradiated RCCs were processed in parallel with the non-irradiated RCCs of our previous study about DEHT storage, using identical processing specifications, disposable materials, equipment, sampling procedure, laboratory staff and facilities. This optimizes the use of that study for comparison and reference [23].
This study demonstrates an encouraging quality of irradiated RBCs stored in PVC-DEHT blood bags combined with the European primary AS choice SAGM. The membrane integrity loss caused by DEHP removal can, however, be counteracted to some extent by instead choosing PAGGSM. DEHT/PAGGSM differed very little, if at all, to the current DEHP/SAGM storage. It is well established that SAGM is a suboptimal storage solution for RBCs and that, quality-wise, more favourable options exist [26, 33-35]. However, it does remain the first-hand choice in most European countries, likely due to its long-standing regulatory approval, cost, and the extensive evaluation efforts a transition would require. Perhaps, when the new medical device regulation is taken into effect and a new plasticizer will be mandatory, it may be a good opportunity to simultaneously consider a change of AS.
It could also be a good time to revise the guidelines for irradiation, as has been previously suggested [13], and opt for a more conservative post-irradiation timeframe. An important observation of this study was that neither the haemolysis levels nor the RMV count of RBCs stored in DEHT/PAGGSM differed statistically to DEHP/SAGM at 14 days post-irradiation. A maximum of 14 days post-irradiation storage time is already routine practice in Sweden and the UK [17, 18]. Such a revision would further facilitate the transition to DEHP-free materials.
In summary, this work investigates the additional stress of irradiation on red blood cells stored in PVC bags plasticized with DEHT. A satisfactory post-irradiation storage capacity of DEHT with either SAGM or PAGGSM additive solution is demonstrated. This adds further weight to the suitability of DEHT as a non-phthalate replacement option for DEHP.
ACKNOWLEDGEMENTS
The authors want to acknowledge Dr Hans Gulliksson for eminent mentorship; Blenda Westeson, Felicia Daggert, Tove Friis-Christensen, Jenny Eriksson, Alexandra Sabo, Erik Dahl, Andreas Eriksson, Sandra Valencia and Hanna-Stina Ahlzén for valuable contributions at blood collection and component production; Macopharma (especially Stefan Reichenberg, Naïma Frizi and former employees Sonia Chatellier and Åse Tolf) for supporting the research idea and providing material; and the volunteer blood donors whose participation made this study possible.
L.L. provided the research idea, study design, laboratory work, data analysis and manuscript composition; S.O. and J.D. assisted on laborations; B.D. managed the blood collection; P.S. and S.L. provided technical and medical expertise; M.U. supervised the study. All authors revised the manuscript and contributed with valuable input.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




