Splenic vasculitis, thrombosis, and infarction in a febrile dog infected with Bartonella henselae
Supported in part by the state of North Carolina and a monetary donation from Bayer Animal Health. Dr. Nandhakumar Balakrishnan is funded as the Bayer Fellow in Vector Borne Infectious Diseases Research at the College of Veterinary Medicine, North Carolina State University.
In conjunction with Dr. Sushama Sontakke and North Carolina State University, Dr. Breitschwerdt holds U.S. Patent No. 7,115,385; Media and Methods for cultivation of microorganisms, which was issued October 3, 2006. He is the chief scientific officer for Galaxy Diagnostics, a company that provides diagnostic testing for the detection of Bartonella species infection in animals and human patients. All other authors have no potential conflicts.
The funding agencies were not involved in the decision to pursue or provide testing for evidence of Bartonella spp. infection in this dog.
Abstract
Objective
To describe the clinical course and successful management of a febrile dog with polyarthritis, splenic vasculitis, thrombosis, and infarction that was infected with Bartonella henselae.
Case Summary
An 8-year-old female spayed Labrador Retriever was referred to The Ohio State University Veterinary Medical Center Emergency Service for evaluation of limping, fever, vomiting, and malaise of 4 days’ duration. Physical examination abnormalities included generalized weakness, diminished conscious proprioception, bilateral temporalis muscle atrophy, and diarrhea. Peripheral lymph nodes were normal, and there were no signs of abdominal organomegaly, joint effusion, or spinal pain. Abdominal ultrasound identified a nonocclusive splenic vein thrombus. Fine-needle aspirates of the spleen revealed pyogranulomatous inflammation, mild reactive lymphoid hyperplasia, and mild extramedullary hematopoiesis. Splenic histopathology found marked, multifocal to coalescing acute coagulation necrosis (splenic infarctions) and fibrinoid necrotizing vasculitis. Bartonella henselae DNA was amplified by polymerase chain reaction and sequenced from the splenic tissue. The dog responded favorably to antimicrobials and was healthy at the time of follow-up evaluation.
New and Unique Information Provided
Bartonella henselae is an incompletely characterized emerging canine pathogen. This case report establishes a potential role for this bacterium as a cause of vasculitis and thromboembolism, which have not been previously reported in association with B. henselae infection in dogs.
Abbreviations
-
- PCR
-
- polymerase chain reaction
Case Description
An 8-year-old female spayed Labrador Retriever was referred to The Ohio State University Veterinary Medical Center Emergency Service for evaluation of limping, fever, vomiting, and malaise of 4 days’ duration. When evaluated by a veterinarian 2 days prior to presentation, there was neck pain and temporalis muscle wasting, and the dog had a rectal temperature of 40.9°C (105.7°F). Complete blood count (CBC) abnormalities at that time included leukocytosis (25.0 × 109/L [25.0 × 103/μL], reference interval 5.0 − 16.8 × 109/L [5.0 – 16.8 × 103/μL]), neutrophilia (20.3 × 109/L [20.3 × 103/μL], reference interval 3.0 − 11.6 × 109/L [3.0 – 11.6 × 103/μL]), monocytosis (2.8 × 109/L [2.8 × 103/μL], reference interval 0.2−1.1 × 109/L [0.2 – 1.1 × 103/μL]), and eosinopenia (0.01 × 109/L [0.01 × 103/μL], reference interval 0.06 − 1.2 × 109/L [0.06 – 1.2 × 103/μL]). A serum biochemical profile and cervical spinal radiographs were unremarkable. During hospitalization, penicillin (dose and route not recorded) and dexamethasone (0.35 mg/kg SQ) were administered, after which rectal temperature decreased to 38.5°C (101.3°F). The dog was discharged the same day with instructions to administer doxycycline (8.6 mg/kg PO q 12 h), metronidazole (7.1 mg/kg PO q 12 h), and prednisone (0.9 mg/kg PO q 12 h). Two days later, the owner reported refractory vomiting and a rectal temperature of 40.9°C (105.7°F), after which the dog was referred to OSU for further evaluation.
At admission in the emergency room, the dog was quiet, alert, and responsive, and weighed 34.0 kg. Physical examination findings included 5% dehydration, rectal temperature 40.9°C (105.6°F), pulse rate of 104/min, respiratory rate of 36/min, generalized weakness, diminished conscious proprioception in the hind limbs, bilateral temporalis muscle atrophy, and diarrhea. Peripheral lymph nodes were normal, and there were no signs of abdominal organomegaly, joint effusion, or spinal pain. CBC abnormalities included a leukocytosis (35.0 × 109/L [3.5 × 103/μL], reference interval 4.1 − 15.2 × 109/L [4.1 – 15.2 × 103/μL]), neutrophilia (30.1 × 109/L [30.1 × 103/μL], reference interval 3.0 − 10.4 × 109/L [3.0 – 10.4 × 103/μL]) with 9% band neutrophils, monocytosis (1.8 × 109/L [1.8 × 103/μL], reference interval 0.0 − 1.2 × 109/L [0.0 – 1.2 × 103/μL), and an increased plasma protein concentration (76 g/L [7.6 g/dL], reference interval 55 − 72 g/L [5.5 – 7.2 g/dL]). Serum biochemical abnormalities included mild hyponatremia (140 mmol/L [140 mEq/L], reference interval 143 − 153 mmol/L [143 – 153 mEq/L]), hypochloremia (98 mmol/L [98 mEq/L], reference interval 109 − 120 mmol/L [109 – 120 mEq/L]), hypophosphatemia (0.8 mmol/L [2.5mg/dL], reference interval 1.0 − 2.6 mmol/L [3.1 – 8.0 mg/dL]), and increased alkaline phosphatase activity (232 U/L, reference interval 15 − 120 U/L). Urinalysis obtained by cystocentesis revealed a specific gravity of 1.030, pH 8.0, and proteinuria (2+ dipstick value), with hematuria (15 – 20 RBC/high powered field) with leukocytes (8 – 12/high powered field) by sediment examination. Thoracic radiographs did not reveal any abnormalities. A commercial ELISA assay1 was negative for Dirofilaria immitis antigen, and Anaplasma sp., Borrelia burgdorferi, and Ehrlichia sp. antibodies. Upon ICU admission, intravenous fluids, ondansetron2 (0.4 mg/kg IV q 12 h), pantoprazole3 (1 mg/kg IV q 24 h), ampicillin/sulbactam4 (30 mg/kg IV q h), and prednisone5 (1 mg/kg PO q 12 h) were administered. Following this therapy, the dog remained febrile (40.1°C /104.2°F) and had multiple episodes of diarrhea. Diagnostic testing performed the following day included abdominal ultrasonography, arthrocentesis of both stifle joints, and echocardiography.
Abdominal ultrasonography identified hypoechoic splenic parenchyma and a nonocclusive splenic vein thrombus (Figure 1). No other ultrasonographic abnormalities were identified. Fine-needle aspirates of the spleen revealed pyogranulomatous inflammation, mild reactive lymphoid hyperplasia, and mild extramedullary hematopoiesis. Fluid obtained by arthrocentesis from both stifle joints was straw-colored and cloudy. Synovial fluid analysis from the right stifle revealed total protein concentration of > 25 g/L (2.5 g/dL) and white cell count of 3,500/μL consisting of 83% neutrophils, 15% monocytes, and 2% lymphocytes; the white blood cell differential was similar for the left stifle. No neoplastic cells were noted. Echocardiography, performed by a board-certified cardiologist, revealed mild right atrial and right ventricular dilation, normal left atrial and left ventricular size in diastole, and a moderate decrease in left ventricular systolic function (fractional shortening 17.9%, normal > 25%). There were no endocardial or valvular echocardiographic lesions.
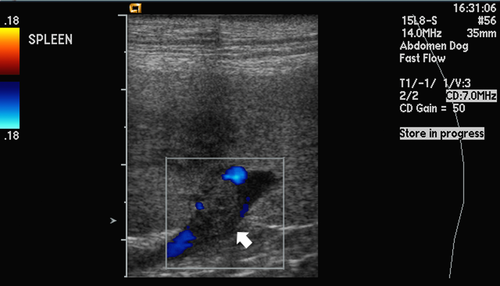
Because of the splenic vein thrombus and pyogranulomatous inflammation, an exploratory laparotomy was performed. The spleen contained multifocal nodules throughout the parenchyma; the rest of the abdomen was unremarkable. A splenectomy was performed and liver biopsies were taken during the laparotomy. Following splenectomy, the dog's rectal temperature decreased to 37.7°C (99.8°F; preoperative temperature 40.7°C [105.2°F]); however, over the ensuing 48 hours, the rectal temperature increased to 40.9°C (105.6°F) and the dog became tachycardic (heart rate 160 beats/min) with harsh bilateral lung sounds. A CBC, arterial blood gas analysis, and thoracic radiographs were performed. CBC abnormalities included a progressive leukocytosis (47.6 × 109/L [47.6 × 103/μL]), neutrophilia (45.7 × 109/L [45.7 × 103/μL]) with no band neutrophils, monocytosis (1.9 × 109/L [1.9 × 103/μL]), and a post-operative decrease in plasma protein concentration (55 g/L [5.5 g/dL]). Thoracic radiographs were unremarkable. Arterial blood gas performed on room air showed pH 7.381 (reference interval 7.38 – 7.48), PaCO2 31.5 mm Hg (reference interval 28 – 49 mm Hg), HCO3 18.8 mmol/L (reference interval 16.9 – 24.2 mmol/L), and a mild hypoxemia with PaO2 67.8 mm Hg (reference interval 80 – 100 mm Hg) and A – a gradient 39.4 mm Hg. Intranasal oxygen was administered at 85 mL/kg/min, after which the dog's respiratory rate dropped to 24/min and pulse oximetry reading was 97%.
Additional diagnostic tests performed in the 2 days following hospital admission included microbiological cultures of urine, blood, and joint fluid; Bartonella henselae and Bartonella vinsonii subsp. berkhoffii serology; and quantitative urine enzyme immunoassay for Blastomyces dermatitidis that cross-reacts with Histoplasma capsulatum. All of these tests were negative. On day 5 of hospitalization, due to persistent fever, the antimicrobial treatment regimen was changed to doxycycline6 (5 mg/kg PO q 12 h for 4 weeks), trimethoprim-sulfamethoxazole7 (23 mg/kg, PO q 12 h for 6 weeks), fluconazole8 (5.7 mg/kg PO q 12 h for 8 weeks), and prednisone9 (0.5 mg/kg PO q 12 h). Within 2 days of initiating this therapy, signs of systemic inflammation began to resolve, the dog regained strength, and the diarrhea ceased; however, inappetence persisted. Due to financial constraints, the dog was discharged on hospital day 9 with instructions to administer the oral antimicrobial regimen for 1 month. Prednisone therapy was weaned and stopped by 30 days following discharge.
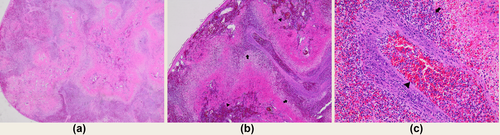
Splenic histopathology found marked, multifocal to coalescing acute coagulation necrosis (splenic infarctions) that in some sections affected over 80% of the splenic parenchyma. Necrotic areas were surrounded by degenerate neutrophils and the intervening parenchyma contained mild to moderate neutrophilic to pyogranulomatous inflammation and moderate extramedullary hematopoiesis. Intermediate-sized muscular arteries within the splenic trabeculae and hilar adipose often contained fibrin thrombi. The tunica media of many vessels contained fibrinoid material and degenerate neutrophils, consistent with fibrinoid necrotizing vasculitis (Figures 2a–2c). Liver histopathology revealed random multifocal neutrophilic hepatitis with sinusoidal fibrin thrombi, suggestive of hematogenously derived emboli. The duodenum, jejunum, and ileum contained mild, multifocal, transmural neutrophilic infiltrates, with neutrophilic serositis involving the stomach and jejunum. While the changes were consistent with infectious agents, such as fungi, fastidious bacteria, rickettsia, protozoa, or amoeba, no organisms were identified by hematoxylin and eosin staining.
After 1 month of therapy, the dog was alert and responsive and physical examination was unremarkable. Appetite had normalized and the dog had gained 2 kg since discharge. Complete blood count abnormalities included a mild normocytic, normochromic, nonregenerative anemia (hematocrit 32%, reference interval 36−54%) and a thrombocytosis (753 × 109/L [753 × 103/μL], reference interval 106 − 424 × 109/L [106 – 424 × 103/μL]), which was attributed to the prior splenectomy. The leukogram was within normal limits (leukocytes 7.2 × 109/L [7.2 × 103/μL], neutrophils 4.8 × 109/L [4.8 × 103/μL], monocytes 0.4 × 109/L [0.4 × 103/μL]). The owner was advised to discontinue doxycycline immediately, followed by trimethoprim-sulfamethoxazole 2 weeks later and fluconazole 8 weeks later. According to the owners, the dog was still alive and doing well 6 months following discharge from the hospital.
Following successful antimicrobial treatment of a presumed infection, formalin-fixed paraffin-embedded splenic tissue was submitted to the Intracellular Pathogens Research Laboratory at North Carolina State University for Bartonella species polymerase chain reaction (PCR). As Bartonella spp. DNA carryover during animal necropsy and subsequent tissue processing has been previously described,1 special precautions were taken to minimize any additional possibility of DNA cross contamination during specimen testing. Using a sterile scalpel, sections were sliced from paraffin embedded splenic tissues, after which DNA was extracted using Qiagen DNeasy Blood and Tissue Kiti according to the manufacturer's instructions. For each PCR reaction, a negative control was processed to ensure that extraction buffers and reagents were not contaminated with Bartonella DNA. PCR targeting the 16S-23S ribosomal RNA intergenic transcribed spacer region of the Bartonella genome was performed as previously described.2 B. henselae San Antonio 2 strain type DNA (99.9% sequence identity, 466/468 base pairs with B. henselae Gen Bank accession number AF369529) was amplified and successfully sequenced from paraffin-embedded splenic tissue sections.
Discussion
In dogs, splenic infarctions are associated with hypercoagulable conditions, including cardiovascular, hepatic, and renal diseases; hyperadrenocorticism; and neoplasia.3 The increased diagnostic use of abdominal ultrasound has resulted in the more frequent ante-mortem identification of focal or diffuse hypoechoic lesions indicative of splenic infarctions in dogs.4 Notably, the dog in this report had a splenic thrombus diagnosed by abdominal ultrasonography and splenic and hepatic thrombi by histopathology supporting the possibility of acute or chronic vascular injury. As erythrocytic/endotheliotropic bacteria, Bartonella spp. could logically contribute to vasculitis and thrombosis. The extent to which this occurs in clinical practice is unknown. In this case, we considered heparin therapy as an alternative to splenectomy for treatment of the dog's splenic vein thrombus. But given the persistent fever and pyogranulomatous inflammation observed on cytology, after consultation with the dog's owner, splenectomy was pursued to facilitate further diagnostic evaluation and to address the splenic thrombosis.
Vasculitis and thrombosis were prominent histopathological features of the splenic lesions observed in this dog. Although vasculitis is not a well-recognized pathology in dogs, B. henselae has been implicated in a number of human vasculitis case reports, of which 2 examples are cited.5, 6 Based upon the numerous splenic infarcts, it is unclear as to whether the granulomatous inflammation observed in the preoperative cytology and postoperative histopathology preceded the splenic vasculitis and thrombosis, or whether the inflammatory response caused by the vasculitis contributed to thrombosis and subsequent infarction in this dog. Although unproven, clinical observations from dogs and human patients suggest that the spleen may play an important immune modulatory role or provide a permissive environment for the growth and perpetuation of Bartonella sp., ultimately contributing to chronic low-grade inflammation and potentially diverse splenic pathology. For example, splenic rupture and granulomatous splenitis have been associated with Bartonella sp. infection in people.7-9 Also, infection with B. henselae was reported in a man with hypergammaglobulinemia, splenomegaly, and polyclonal plasmacytosis who developed fever and other complications postsplenectomy.10 These bacteria can induce in vitro and in vivo proliferation of endothelial cells and pericytes, potentially leading to vascular injury or vasoproliferative lesions in both dogs and humans.5, 6, 11, 12 Although the clinical and pathophysiological relevance is yet to be determined, a higher prevalence of Bartonella spp. DNA was recently reported in dogs with splenic fibrohistiocytic nodules and splenic hemangiosarcoma, as compared to dogs with splenic lymphoid hyperplasia.13 Therefore, long-term intracellular persistence of Bartonella spp., in conjunction with an endotheliotropism, and the ability to modulate cytokine production may ultimately cause or contribute to the development of various forms of splenic pathology including vasculitis, thrombosis, vascular rupture, granulomatous inflammation, plasmacytosis, and potentially neoplasia.
In people, fever often accompanies an acute infection with Bartonella spp.14 In 1 recent human case series, hepatosplenic B. henselae infection was diagnosed in 4 patients with fever of unknown origin.9 In addition, cat scratch disease is characterized by a classical triad including a history of a cat scratch, fever, and lymphadenopathy. Also, B. henselae infection is considered a frequent cause of fever of unknown origin, particularly in children.15 However, persistently infected immunocompetent, bacteremic dogs are rarely febrile,16 and Bartonella spp. bacteremic people are often afebrile and report nonspecific symptoms, including malaise, fatigue, myalgia, joint pain, and neurocognitive abnormalities.17 Whether the fever in this dog was caused by B. henselae, coinfection with another fastidious microorganism, a concurrent undiagnosed fungal infection, or was associated with tissue necrosis secondary to infarction was not determined. However, based upon evolving evidence, Bartonella spp. are stealth intravascular bacteria10 that can induce persistent bacteremia, nonspecific clinical manifestations, and ultimately gross pathology including endocarditis, granulomatous inflammation, and vasoproliferative lesions in both dogs and people.11, 15, 18, 19 Based upon this case report, it is possible that vasculitis, hypercoagulability, and thromboembolic disease should be added to the list of pathological lesions associated with bartonellosis.11, 12, 18
Although diagnostically challenging,17-21 ruling out Bartonella sp. infections through the use of serology, Bartonella alpha proteobacteria growth medium enrichment blood or tissue culture/PCR, and PCR of organism-specific DNA sequences from pathological lesions in tissues may better direct therapeutic decisions and improve patient outcomes. For reasons that remain unclear, and as was true for the dog in this report, approximately half of Bartonella sp. bacteremic dogs do not have positive diagnostic indirect fluorescent antibody titers.22 Advantages and limitations associated with these diagnostic modalities have been discussed previously.23
Although a definitive treatment regimen has not been established, antimicrobials are considered the mainstay of treatment for bartonellosis in dogs. Doxycycline alone is not an effective treatment to eliminate infections associated with any Bartonella species. Experimental evidence from cats24 and clinical evidence in treating dogs16 support the lack of efficacy for this antimicrobial, particularly when given for periods of 4 weeks or less. Because of the rapid development of resistance,25 when compared to several other antimicrobial classes, azithromycin is not recommended as a first line antimicrobial. An optimal protocol has not been established for treating canine bartonellosis. Currently, we treat documented Bartonella spp. infections with concurrent administration of doxycycline and enrofloxacin, or doxycylcine and rifampin if the dog has neurological or ocular involvement. When treating bartonellosis, the clinician should anticipate a potential deterioration in the patient's clinical status (increased lethargy, anorexia, vomiting, and inactivity), potentially due to achieving intracellular antimicrobial concentrations that injure or kill the bacteria, that generally occurs several days after starting antimicrobials. Deterioration is often short-lived, requires only supportive or symptomatic therapy, and the combination antimicrobial therapy should not be stopped due to a presumed adverse drug effect. Because antimicrobial treatment needs to be long in duration (6 weeks to 3 months), because more than 1 antimicrobial is needed (expense), and because of antimicrobial resistance concerns associated with the indiscriminate use of antimicrobials, diagnostic confirmation of bartonellosis, or a very high index of suspicion for this infection, is an important clinical consideration before embarking upon treatment. As all initial testing failed to provide a definitive microbiological diagnosis, anti-fungal therapy was administered concurrently and in retrospect may not have been necessary. Concurrent administration of immunosuppressive drugs as used in this dog may decrease antimicrobial efficacy, resulting in an incomplete treatment response. Also, therapeutic suppression of the innate immune response may contribute to the development of pathology, such as endocarditis or bacillary angiomatosis in dogs or people that are occultly infected with a Bartonella sp.26, 27 Based upon a growing body of case-based evidence, infection with a Bartonella spp. and other fastidious bacteria should be included in the differential diagnostic considerations for dogs with unexplained splenomegaly, splenic vasculitis and infarction, pyogranulomatous splenitis, and vasoproliferative or vaso-occlusive splenic disease.




