Retrospective evaluation of methionine intoxication associated with urinary acidifying products in dogs: 1,525 cases (2001–2012)
The authors declare no conflict of interest.
Abstract
Objective
To describe the signalment, clinical findings, timing of signs, outcome, and prognosis in a population of dogs exposed to methionine through the ingestion of urine acidifying products.
Design
Retrospective observational study from January 1, 2001 to December 31, 2012.
Setting
Animal Poison Control Center.
Animals
A total of 1,197 case calls yielding 1,525 dogs identified with presumed methionine ingestion.
Interventions
None.
Measurements and Main Results
Records of dogs with presumptive methionine ingestion were reviewed from the American Society for the Prevention of Cruelty to Animals Animal Poison Control Center database. Ingested methionine doses ranged from 3.9 mg/kg to 23,462 mg/kg. Clinical signs developed in 47% of dogs. The most common clinical signs were gastrointestinal (GI) and neurologic. The mean onset of GI signs was 2.8 hours following ingestion. The mean onset of neurologic signs was 6.8 hours following ingestion. GI signs were identified with ingested doses ≥22.5 mg/kg. Vomiting was the most common GI sign. Neurologic signs were identified with ingested doses ≥94.6 mg/kg. Ataxia was the most common neurologic sign. Resolution of clinical signs occurred within 48 hours of ingestion, and no fatalities were reported.
Conclusions
Prognosis for dogs with methionine intoxication is excellent. Vomiting and ataxia were the most common clinical signs associated with methionine toxicosis.
Abbreviations
-
- APCC
-
- Animal Poison Control Center
-
- GI
-
- gastrointestinal
-
- NMDA
-
- N-methyl-D-aspartate
Introduction
DL-methionine is a sulfur-containing amino acid1 and is the primary ingredient in products designed to prevent urine-induced damage to lawns by acidifying dog urine.2 Lawn saver products contain 133–500 mg of methionine per pill, biscuit, or chew. Other methionine containing products (eg, multivitamins, joint care) range between 1 to 7.5 mg per pill, biscuit, or chew.
Different forms of methionine have been used in animals to treat diseases and provide nutritional supplementation. DL-methionine is used as a feed supplement for several species of production animals.1 S-adenosyl-L-methionine is used in veterinary medicine as a treatment for hepatopathies such as acetaminophen toxicosis, chronic hepatitis, and cholangiohepatitis through initiation of metabolic pathways and antioxidant activity.3 DL-methionine has also been administered to cats with a history of feline urologic syndrome with the goal of changing urine pH,4 though this treatment is no longer commonly recommended.
While methionine can be beneficial in animals, it is also considered the most toxic of amino acids in feed supplements due to its narrow dosing range, negative effects on growth, and potential to cause organ damage.1 The effects of long-term excessive methionine ingestion or acute methionine overdoses have been reported in dogs, cats, rats, chickens, and people.1, 4-7 Reports of acute or chronic methionine intoxication are rare in dogs. In 2001, 21 dogs developed anorexia and vomiting when fed a commercial diet that contained excessive methionine.8 A case report identified neurologic and gastrointestinal (GI) effects from ingestion of multiple chewable DL-methionine tablets in a dog.2
Single toxic doses of methionine have been used in feline experimental studies because methionine toxicosis can cause recurrent grand mal seizures in cats consistent with those seen in epileptic people.9 Doses of 0.5–1.0 g/kg of methionine per day for multiple days were found to induce Heinz body anemia and methemoglobinemia in cats.4 Rats receiving 2% of their diet from methionine over a 2-year period developed decreased food intake, lower total body fat, and diffuse thickening of their aortic walls.6 Vomiting and dizziness are common adverse effects in people taking methionine at recommended doses.7
To the authors’ knowledge, there has been no comprehensive review of methionine intoxication in dogs. The purpose of this study was to describe the signalment, clinical findings, onset of signs, outcome, and prognosis in a population of dogs exposed to methionine through the ingestion of urine acidifying products.
Materials and Methods
The American Society for the Prevention of Cruelty to Animals Animal Poison Control Center (APCC) database was searched for all cases of dogs with known or suspected methionine ingestion. Information including species; breed; age; sex; weight; number of animals at risk; estimated dose and time of ingestion; the onset, severity, and duration of clinical signs; and any treatments was collected. Because methionine is a common ingredient in multivitamins, joint care products, and liver “detoxifying” agents, case selection was limited to urine acidification and lawn saver products that contain high concentrations of methionine without other active ingredients. Case selection was limited to situations in which only 1 product was ingested.
High suspicion of intoxication was initially recorded when the clinical signs were consistent with methionine toxicosis. Medium suspicion of intoxication was initially assigned when the clinical signs were consistent with methionine toxicosis but some clinical signs were lacking. Dogs that did not have abnormal clinical signs at the time of the call were initially assigned a low suspicion of intoxication. If dogs initially assigned a low suspicion of intoxication were found to have developed clinical signs based on follow-up phone calls, they were reassigned to a high suspicion or medium suspicion level as appropriate. Dogs were identified as juvenile if less than 1 year of age, adult if between the ages of 1 year and 9 years, and geriatric if aged ≥10 years.
Mean and median dosage of methionine for dogs with a medium or high suspicion of intoxication were calculated using a computer-based data spreadsheet.1 Dogs with a low suspicion of intoxication after follow-up call were not included in the analysis.
Results
From January 2001 to December 2012, there were 1,197 case calls with 1,525 animals (mean 1.27 dogs per case call) reported to the APCC for potential methionine intoxication. Ingested methionine dosage ranged from 3.9 to 23,462 mg/kg. In 305 (26%) cases, callers were unable to estimate the amount ingested. The mean dose ingested was 1,274.3 mg/kg and the median dose 857.4 mg/kg. Chewable tablets were the most frequently ingested formulation (1,155 case calls), followed by biscuit products (147 case calls), soft chews (86 case calls), and tablets (84 case calls); fewer than 20 case calls were received for each of the other formulations (gel, liquid, paste, powder).
Female dogs were slightly overrepresented (intact 4%, neutered 51%) when compared to male dogs (intact 6%, neutered 38%). Gender information was not available for 1% of cases. Most cases involved adult dogs (1,110, 73%) or juveniles (119, 13%). Geriatric dogs made up 6% of cases. The most common breed was the Labrador Retriever, followed by Golden Retrievers and German Shepherd Dogs (Table 1). There was a high or medium suspicion of intoxication in 717 dogs (47%).
| Number | Percentage | Breed ranking | |
|---|---|---|---|
| of | of methionine | for other | |
| Breed | dogs | cases | reported toxins |
| Labrador Retriever | 403 | 26.4 | 1 |
| Golden Retriever | 143 | 9.4 | 4 |
| German Shepherd Dog | 95 | 6.2 | 6 |
| Mixed breed dog | 84 | 5.5 | 2 |
| Boxer | 46 | 3.0 | 10 |
| Weimaraner | 40 | 2.6 | 37 |
| Cocker Spaniel | 40 | 2.6 | 11 |
| Beagle | 32 | 2.1 | 9 |
| Dachshund (miniature) | 30 | 2.0 | 13 |
| Border Collie | 29 | 1.9 | 19 |
| Australian Shepherd | 29 | 1.9 | 20 |
Signs were seen involving both the GI and neurologic systems (Table 2). GI signs included vomiting, diarrhea, and anorexia. Neurologic signs included ataxia, lethargy, abnormal posture, weakness, disorientation, hypermetria, vocalization, and tremors. The lowest dose at which vomiting and lethargy were seen was 22.5 mg/kg. Ataxia was reported with doses starting at 94.6 mg/kg. Tremors, hypermetria, disorientation, and vocalization were not seen until dosage exceeded 300 mg/kg. No deaths were reported following exposure to methionine.
| Sign | Number of dogs | Percentage of dogs |
|---|---|---|
| Vomiting | 623 | 31.6 |
| Ataxia | 386 | 19.6 |
| Lethargy | 94 | 4.8 |
| Diarrhea | 63 | 3.2 |
| Abnormal posture | 53 | 2.7 |
| Weakness | 46 | 2.4 |
| Polydipsia | 40 | 2.0 |
| Disorientation | 28 | 1.4 |
| Hypermetria | 20 | 1.0 |
| Vocalization | 20 | 1.0 |
| Tremors | 20 | 1.0 |
| Anorexia | 20 | 1.0 |
Vomiting without induction of emesis occurred at a mean of 2.8 hours (range 5 min to 9 h) after ingestion. Ataxia occurred a mean of 6.8 hours (range 1 to 18 h) after ingestion. Most (92%) cases were resolved in 18–24 hours, and all were resolved by 48 hours.
Approximately 1/3 of case calls were managed at home, 1/3 were treated by a veterinarian on an outpatient basis, and 1/3 were hospitalized. Limited data regarding clinicopathologic abnormalities were reported to APCC by treating veterinarians. Acidosis, hypokalemia, and hyperglycemia were reported in 9, 8, and 7 individual dogs, respectively. Numerical values for these abnormalities were not reported for all patients, so range and mean values could not be determined. Five dogs had at least 1 of the following abnormalities reported: azotemia, discolored urine, increased alkaline phosphatase activity, increased alanine aminotransferase activity, increased ammonia concentration, increased aspartate aminotransferase activity, increased creatine kinase concentration, increased lactate concentration, hematuria, hemoconcentration, hypercholesterolemia, hypernatremia, hyperproteinemia, hypocapnia, hypochloremia, hypocholesterolemia, hypoglobulinemia, hypoglycemia, decreased hemoglobin concentration, melanuria, metabolic alkalosis, decreased serum bicarbonate concentration, and hyponatremia.
Treatment goals included decontamination via induction of emesis if ingestion was within 2–4 hours prior to presentation, intravenous crystalloid fluid therapy, and correction of both electrolyte and acid/base abnormalities. Supportive treatment for GI and neurologic abnormalities was administered as needed and included antiemetics, GI protectants, confinement, and housing in dark, quiet areas. Original recommendations had also included administration of activated charcoal, oral lactulose, oral neomycin, and cleansing enemas. These recommendations were discontinued in 2008.
Discussion
Methionine is metabolized through 2 different pathways (Figure 1): the transamination pathway and the transsulfuration-transmethylation pathway, both of which occur in the liver.10-12 Transsulfuration-transmethylation is the primary pathway in animals,11 leading to the formation of homocysteine. Homocysteine can be converted back to methionine by either betaine-homocysteine methyltransferase or 5-methyltetrahydrofolate-homocysteine methyltransferase, or homocysteine can be acted upon by cystathionine synthase in the presence of serine to start the transsulfuration pathway, which leads to the formation of cysteine.10-13
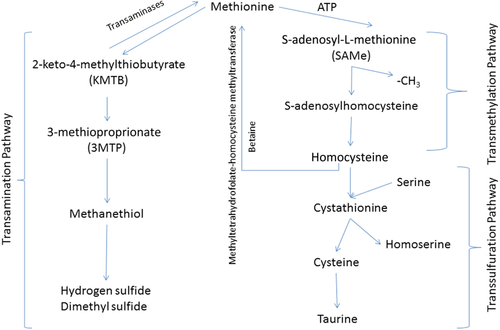
Transmethylation is ATP-dependent, and in the event of ATP depletion or excessive methionine, a second metabolic pathway, transamination, will be used.11 The first step of transamination of methionine is reversible, and in the forward direction leads to the formation of 2-keto-4-methylthiobutyrate.11, 14 Via rate-limiting oxidative decarboxylation, 2-keto-4-methylthiobutyrate converts to 3-methiopropionate, which in turn is converted to methanethiol.11, 14, 15 The final products from this metabolic pathway include hydrogen sulfide and dimethyl sulfide.11, 14, 15
Homocysteine and methanethiol are the 2 metabolites of methionine that appear to cause the majority of toxic effects.2, 7, 8, 13-17 Body systems affected by methionine and its metabolites include hematologic,4, 13, 16 hepatic,11, 13, 14, 16 vascular,6, 7 GI4, 5, 7-9, 13 and neurologic.1, 2, 4, 7, 9, 17
Chronically increased levels of homocysteine and other products of methionine transmethylation-transsulfuration have been extensively studied in both people and veterinary species.1, 4-7 Mildly increased homocysteine concentrations are associated with decreased food intake, low body weight, low adipose tissue deposits, splenomegaly, and mild anemia.1, 4-6 Hepatic changes including decreased intracellular glutathione levels, decreased cytochrome c oxidase activity, and necrotic cell death have been identified secondary to excessive lipid peroxidation and hepatocyte ATP depletion.14, 16 The exact mechanism for this damage is not fully understood. Increased methionine leading to increased homocysteine has been associated with aortic wall hypertrophy, arteriosclerosis, and an increased risk of stroke in people.7
Neurologic effects of homocysteine may be more acute in nature. Homocysteine is both a partial agonist and a partial antagonist of N-methyl-D-aspartate (NMDA) receptors in the brain.17 By competing with glycine at the NMDA receptor to reduce its activity, homocysteine leads to increased calcium concentrations in brain tissue.17 When high levels of glycine are present, homocysteine becomes a high affinity NMDA receptor agonist at the glutamate site, which can lead to neuroexcitation.17 Neurologic damage in rats is suspected to occur due to generation of reactive oxygen species from both excessive NMDA stimulation and increased extracellular levels of homocysteine.17
Methanethiol, a methionine metabolite produced by the transamination pathway, may have primary neurologic effects. Methanethiol can inhibit sodium-potassium-ATPase activity within brain tissue.1 It also leads to decreased oxygen consumption by brain mitochrondria.1 Methanethiol concentrations are increased in patients with hepatic encephalopathy, though it is unclear whether this increase causes clinical signs, or if it is simply a result of decreased hepatic metabolism.15 Symptoms of acute methanethiol exposure in people may include fever, cough, dyspnea, dizziness, headache, loss of sense of smell, nausea, vomiting, and diarrhea.18
The majority of studies reporting methionine toxicity in animals have focused on laboratory animals, primarily rodents.1, 6, 11, 13, 14, 16, 17 Three studies have focused on cats.4, 5, 9 One study described the neurologic response to a single intraperitoneal injection of 12–15 mg/kg of methionine sulfoximine in 50 cats.9 Early behavior changes including depression, apprehension, or hostility were identified within 5–8 hours after administration. Focal facial seizures progressing to generalized tonic-colonic movements were described within 4–48 hours after drug administration. GI signs were occasionally witnessed early after drug administration. Truncal ataxia, intention tremors, and paresis are also described within the first 5–24 hours after administration. Death occurred in an unspecified number of cats following progression to status epilepticus. A separate study evaluated longer-term administration of methionine in cats.4 Cats fed 0.5 g/kg/d for 52 days had no significant clinical signs with the exception of mild transient anorexia. Cats fed 1 g/kg/d for 10 days had anorexia and mild ataxia. Cats fed the higher dose of methionine had a moderate increase in methemoglobin concentration with Heinz body formation. The lower dose of methionine caused a mild, transient increase in methemoglobin concentration. A final study evaluated effects of long-term administration of excessive methionine on weight gain in kittens, and found that administration of 2% methionine for 6 weeks had a negative effect on both food intake and weight gain, and that this effect was partially ameliorated by administration of a glycine supplement.5
Reports of methionine toxicosis in dogs are limited to descriptive case reports.2, 8 In 2001, 21 dogs were identified as suffering from methionine toxicosis after ingesting adulterated dog food for unspecified lengths of times.8 Dogs ingested food that contained 1.60–2.75% methionine, while the recommended maximum is 0.43%. Clinical signs associated with this ingestion were anorexia and vomiting. No neurologic effects were reported. A single case report of a dog ingesting 52.9 g of methionine (1,346 mg/kg) from lawn saver supplements developed both GI and neurologic signs that improved within 48 hours after ingestion.2
Lawn saver products come in many forms including chewable tablets, biscuits, and a liquid. These products usually contain 133 mg of DL-methionine per tablet or biscuit, although some products may contain a greater or lesser amount depending on their formulation. Suggested dosage listed on the label for a chewable tablet2 ranges between 14.8 mg/kg and 266 mg/kg, with smaller animals generally getting larger doses of methionine. Following the manufacturer's labeled dosing for dogs larger than 50 lbs (22.7 kg), the highest recommended dose calculated was 23.4 mg/kg. The median dose of methionine in cases presented to APCC was 857.4 mg/kg, a more than 3-fold increase in dose for small breed dogs and 58-fold increase in the recommended dose for a 50 lb dog.
Dogs in the current study were predominantly large breed, and many households contained more than 1 large breed dog. While the association between large breed dogs, multi-dog homes, and risk of methionine ingestion was not fully explored, it is possible that households with 1 or more large breed dog will have lawns with increased urine scalding, and so would be more likely to use a lawn saver product. Mild neurologic abnormalities were identified at doses greater than 94 mg/kg, indicating that at least 50% of all dog cases reported to APCC were at risk for development of ataxia. However, only a total of 33.9% of dogs developed neurologic abnormalities following ingestion of methionine. The mean ingested dose of 1,274.3 mg/kg with the median ingested dose being 857.4 mg/kg suggests that while some dogs ingested a very large amount of methionine, more dogs ingested a much smaller dose. This greater proportion of dogs ingesting a smaller dose may explain why fewer than expected number of dogs developed clinical signs given the mean ingested doses reported in this population.
GI distress was reported in 35.8% of cases, with vomiting being most common. Vomiting has been reported as an adverse effect of methionine ingestion in people at recommended doses. GI effects have been reported in veterinary patients with both acute and chronic exposure. To the authors’ knowledge, no reports have identified the pathophysiology of GI effects. Possible mechanisms to consider include lipid peroxidation and ATP depletion in enterocytes. Further studies would be needed to determine the mechanism of the toxic effect of methionine on the GI tract.
The early onset of GI signs compared to neurologic abnormalities in dogs suggests that relatively small doses of methionine or its metabolites are enough to affect the GI tract, while higher doses are required to develop effects on the neurologic system. This may be due to localized GI effects as opposed to systemic effects on the nervous system, or a threshold which must be surpassed to overcome the blood-brain barrier. Additionally, since homocysteine antagonizes NMDA receptors at glycine sites but agonizes NMDA receptors’ glutamate sites once glycine sites are fully bound, the delay in neurologic effects seen in this study have occurred because all glycine receptors must become fully bound before NMDA agonism develops, and this binding may take an unspecified length of time. It is uncertain whether 2 molecules of homocysteine can be bound to 2 separate sites on 1 NMDA receptor simultaneously.
Due to potential adverse effects of repeated activated charcoal administration19 and new understanding that ammonia-like substances are not produced during methionine metabolism,2 recommendations for administration of activated charcoal, oral lactulose, oral neomycin, and cleansing enemas were discontinued in 2008.
All clinical signs resolved in this study within 48 hours, and no deaths were reported. Resolution of clinical signs most likely was due to continued metabolism along the transsulfuration pathway. The majority of patients with clinical signs were treated with either emesis alone or with emesis combined with activated charcoal administration, and received supportive care. All had an excellent prognosis. Rapid decontamination via emesis alone in dogs recently ingesting a neurotoxic dose should be considered to prevent prolonged hospitalization and increased costs for clients. However, because induction of emesis is not a completely benign treatment, each case should be evaluated individually.
Limitations of this study include its retrospective nature, which prevents homogeneous data collection. A prospective study may have allowed a more definitive understanding of the onset of clinical signs and response to treatment. In addition, short-term and long-term follow-up on individual cases were not complete, as the APCC depended on information provided by clients or treating veterinarians for data. Finally, the lack of accurate ingested dose measurement may lead to false mean and median values associated with clinical signs.
Conclusion
Methionine intoxication can affect both the GI tract and the nervous system. Signs can be mild to moderate in severity, and generally resolve within 48 hours with decontamination and supportive care. The clinician should consider methionine toxicosis as a potential etiology for any patient exhibiting neurologic abnormalities with access to lawn saver products.




