Presumptive intraperitoneal envenomation resulting in hemoperitoneum and acute abdominal pain in a dog
The authors declare no conflict of interests.
Abstract
Objective
To describe the clinical features, diagnostic findings, treatment, and outcome of a dog with acute abdominal pain and hemoperitoneum secondary to a presumptive intraperitoneal (IP) snakebite.
Case Summary
A 10-month-old castrated male mixed-breed dog was evaluated for suspected snake envenomation. The dog presented recumbent and tachycardic with signs of severe abdominal pain. Two cutaneous puncture wounds and hemoperitoneum were discovered during evaluation. Ultrasonographic examination revealed communication of the wounds with the peritoneal cavity. The dog was treated with supportive care, parenteral analgesia, packed red blood cell and fresh frozen plasma transfusions, crotalid antivenom, and placement of an IP catheter to provide local analgesia. The dog recovered fully and was discharged 5 days after initial presentation.
New or Unique Information Provided
To our knowledge, this is the first report of IP envenomation accompanied by hemorrhage treated with continuous IP analgesia in the veterinary literature.
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- aPTT
-
- activated partial thromboplastin time
-
- CRI
-
- continuous rate infusion
-
- FFP
-
- fresh frozen plasma
-
- HR
-
- heart rate
-
- IP
-
- intraperitoneal
-
- PCV
-
- packed cell volume
-
- PO
-
- per os
-
- PT
-
- prothrombin time
-
- TPP
-
- total plasma protein
Introduction
Pit viper snake envenomation in dogs is well documented in the United States with the highest incidence in the southeast, western, and gulf coast states. There are an estimated 150,000 reported pit viper snakebites in dogs and cats each year.1 Pit vipers belong to the family Crotalidae and include rattlesnakes (Crotalus spp.), copperheads (Agkistrodon contortrix), water moccasins (Agkistrodon piscivorus), and pigmy rattlesnakes (Sistrurus miliaris).2 Envenomation can cause both local and multisystemic effects including local tissue swelling, tissue necrosis, pain, edema, tachypnea, tachycardia, coagulation abnormalities, acute kidney injury, and neurologic impairment.1, 3, 4 The effects of envenomation depend on variables such as time since last feeding, volume of venom injected, size of fangs, age and size of snake, time of year, and location of bite.5, 6
Hemoperitoneum, or extravasated blood within the peritoneal space, can result from several factors, including organ bleeding secondary to malignant or benign masses, organ torsion, coagulopathies such as anticoagulant rodenticide intoxication and disseminated intravascular coagulation.7 In addition, traumatic injury to abdominal organs or vasculature may result in significant intraperitoneal (IP) bleeding.8 This case report describes the successful management of a dog with hemoperitoneum and abdominal pain secondary to a presumptive IP snake envenomation. Snakebites in domestic animals or people have not been previously reported to result in hemoperitoneum.
Case Summary
A 10-month-old castrated male, mixed-breed dog weighing 6.2 kg was referred for further treatment of an acute episode of vocalization and abdominal pain suspected to be caused by snake envenomation. Immediately prior to the episode the dog had been left unsupervised outside in a wooded area where pit vipers, including copperheads (A. contortrix), are indigenous to the area and frequently seen by the owners. Initial examination findings by the primary care veterinarian within 30 minutes (time 0.5 h [T 0.5h]) of discovery included recumbency, depressed mentation, increased rectal temperature (39.3°C [102.7°F]), pale pink mucous membranes with prolonged capillary refill time (3 s), tachycardia (heart rate [HR]: 180/min) with weak femoral pulse quality, and severe abdominal pain. A peripheral IV catheter was inserted into the right cephalic vein and used to rapidly administer lactated Ringer's (16 mL/kg), dexamethasone sodium phosphatea (0.25 mg/kg), and cefazolinb (16 mg/kg). Butorphanolc (0.15 mg/kg) was administered subcutaneously to provide analgesia. A biochemical panel was obtained, demonstrating hyperglycemia (14.0 mmol/L [253 mg/dL]; reference interval (4.1–7.9 mmol/L [74–143 mg/dL])) and hypoproteinemia (50 g/L [5.0 g/dL]; reference interval (52–82 g/L [5.2–8.2 g/dL])) with all other parameters falling within reference interval. After 30 minutes of hospitalization, HR was 160/min and second and third IV crystalloid fluid boluses were administered (100 mL and 50 mL, respectively) with no further change in HR. Hydromorphoned (0.1 mg/kg, subcutaneous and IV) was administered prior to referral.
At admission, 2 hours after the onset of clinical signs (T 2h), the dog remained laterally recumbent, stuporous, and had an increased rectal temperature (39.1°C [102.4°F]). Oral mucous membranes were pale pink and tacky with a prolonged capillary refill time (>3 s) and both the HR and respiratory rate were increased (210/min and 44/min, respectively). Femoral pulses were symmetric in both pelvic limbs, but pulse quality was weak. Pulmonary and cardiac auscultation were unremarkable. The dog exhibited severe, diffuse abdominal pain displayed by vocalization and abdominal splinting in response to palpation. Two small (1–2 mm) puncture wounds were present on the right caudodorsal abdomen surrounded by an area of ecchymosis and edema. The electrocardiogram was consistent with sinus tachycardia, and the arterial blood pressure could not be measured despite repeated attempts with an ultrasonic probe.e
Initial diagnostic tests included a complete blood count (CBC), serum biochemical profile, from which abnormal findings are listed in Tables 1 and 2, and coagulation profile. The CBC abnormalities included leukocytosis, consisting of neutrophilia with regenerative left shift, and monocytosis. Thrombocytopenia and mild anemia were also present. Abnormal results on the biochemistry profile included hyperglycemia, hypocalcemia, severe hypoproteinemia including hypoalbuminemia and hypoglobulinemia, hypocholesterolemia, increased alanine aminotransferase (ALT) activity, increased creatine kinase concentration, and hypokalemia. The coagulation profile showed prolonged prothrombin time (PT; 9.7 s; reference interval 5.5–8.3 s), prolonged activated partial thromboplastin time (aPTT) (14.4 s; reference interval 7.5–13.8 s), and normal fibrinogen concentration (2.94 μmol/L [100 mg/dL]; reference interval (2.9–8.9 μmol/L [100–300 mg/dL])). The patient's geographical location, history, presence of puncture wounds with local edema, thrombocytopenia, prolonged clotting times, and absence of echinocytosis were consistent with A. contortrix envenomation.9
| Time after envenomation | ||||
|---|---|---|---|---|
| 2 hours | 16 hours | 88 hours | Reference interval | |
| WBC | 17.39 × 109 cells/L | 20.83 × 109 cells/L | 24.49 × 109 cells/L | 4.39–11.61 × 109 cells/L |
| 17,390 cells/μL | 20,830 cells/μL | 24,490 cells/μL | 4,390–11,610 cells/μL | |
| Segmented neutrophils | 12.347 × 109 cells/L | 14.889 × 109 cells/L | 19.837 × 109 cells/L | 2.841–9.112 × 109 cells/L |
| 12,347 cells/μL | 14,889 cells/μL | 19,837 cells/μL | 2,841–9,112 cells/μL | |
| Band neutrophils | 1.043 × 109 cells/L | 2.500 × 109 cells/L | 0.735 × 109 cells/L | 0 × 109 cells/L |
| 1,043 cells/μL | 2,500 cells/μL | 735 cells/μL | 0 cells/μL | |
| Monocytes | 1.391 × 109 cells/L | 1.458 × 109 cells/L | 1.959 × 109 cells/L | 0.075–0.850 × 109 cells/L |
| 1,391 cells/μL | 1,458 cells/μL | 1,959 cells/μL | 75–850 cells/μL | |
| Platelets | 109 × 109 cells/L | 196 × 109 cells/L | 114 × 109 cells/La | 190–468 × 109 cells/L |
| 109,000 cells/μL | 196,000 cells/μL | 114,000 cells/μL | 190,000–468,000 cells/μL | |
| PCV | 36% | 33% | 27% | 39–58% |
| Reticulocytes | NA | 1.59% | NA | 0.11–1.26% |
| Plasma protein | 22 g/L | 44 g/L | 59 g/L | 61–75 g/L |
| 2.2 g/dL | 4.4 g/dL | 5.9 g/dL | 6.1–7.5 g/dL | |
- a Platelet clumping noted on microscopic slide evaluation. NA, result not available. SI units (top) and US units (bottom).
- PCV, packed cell volume; WBC, white blood cell count.
| Time after envenomation | ||||
|---|---|---|---|---|
| 2 hours | 16 hours | 88 hours | Reference interval | |
| Glucose | 10.7 mmol/L | 6.6 mmol/L | 5.9 mmol/L | 3.9–7.3 mmol/L |
| 192 mg/dL | 118 mg/dL | 106 mg/dL | 70–131 mg/dL | |
| Total calcium | 2.0 mmol/L | 2.3 mmol/L | 2.6 mmol/L | 2.3–2.9 mmol/L |
| 8.0 mg/dL | 9.0 mg/dL | 10.5 mg/dL | 9.3–11.5 mg/dL | |
| Total protein | 24 g/L | 39 g/L | 52 g/L | 52–73 g/L |
| 2.4 g/dL | 3.9 g/dL | 5.2 g/dL | 5.2–7.3 g/dL | |
| Albumin | 15 g/L | 23 g/L | 30 g/L | 30–39 g/L |
| 1.5 g/dL | 2.3 g/dL | 3.0 g/dL | 3.0–3.9 g/dL | |
| Globulin | 9 g/L | 16 g/L | 22 g/L | 17–38 g/L |
| 0.9 g/dL | 1.6 g/dL | 2.2 g/dL | 1.7–3.8 g/dL | |
| Cholesterol | 1.3 mmol/L | 1.7 mmol/L | 1.8 mmol/L | 3.2–8.9 mmol/L |
| 49 mg/dL | 66 mg/dL | 69 mg/dL | 124–344 mg/dL | |
| ALT | 64 U/L | 51 U/L | 124 U/L | 12–54 U/L |
| ALP | 36 U/L | 105 U/L | 289 U/L | 16–140 U/L |
| CK | 383 U/L | 3,698 U/L | 5,175 U/L | 43–234 U/L |
| Sodium | 144 mmol/L | 138 mmol/L | 144 mmol/L | 140–156 mmol/L |
| 144 mEq/L | 138 mEq/L | 144 mEq/L | 140–156 mEq/L | |
| Potassium | 3.0 mmol/L | 3.5 mmol/L | 4.4 mmol/L | 4.0–5.3 mmol/L |
| 3.0 mEq/L | 3.5 mEq/L | 4.4 mEq/L | 4.0–5.3 mEq/L | |
| Total bilirubin | 3.4 μmol/L | 8.6 μmol/L | 17.1 μmol/L | 0–3.4 μmolL |
| 0.2 mg/dL | 0.5 mg/dL | 1.0 mg/dL | 0–0.2 mg/dL | |
- ALT, alanine aminotransferase; ALP, alkaline phosphatase; CK, creatine kinase. SI units (top) and US units (bottom).
Fluid therapy was initiated and a second IV catheter was inserted in the left cephalic vein immediately after presentation. Two 10 mL/kg aliquots of lactated Ringer's were administered IV within 5 minutes; although this did not reduce the HR, a Doppler estimate of systolic blood pressure (90 mm Hg) was successfully obtained. Additional fluid therapy with 2 rapidly administered IV doses of hydroxyethyl starch (6%, 130/0.4f) solution (5 mL/kg) increased blood pressure to 145 mm Hg without appreciable change in HR (210/min) or pulse quality. Analgesia was administered concurrently in the form of fentanyl citrateg (3 m μg/kg IV) followed by continuous rate infusion (CRI; 5 μg/kg/h, IV). The dog remained laterally recumbent and stuporous, although he would vocalize and guard his abdomen in response to gentle palpation. Shortly after additional fluid therapy, the systolic arterial blood pressure was re-evaluated (95 mm Hg). Norepinephrine bitartrateh (0.05 μg/kg/min CRI, IV) was then administered with dose adjustments, up to 0.15 μg/kg/min, based on pulse quality, with the goal of maintaining the Doppler estimate of systolic blood pressure >100 mm Hg. At T 3h, general anesthesia was induced with propofol9 (3.2 mg/kg, IV) and fentanyl citrate (3 μg/kg, IV) in an effort to relieve intractable abdominal pain and facilitate additional diagnostics. Anesthesia was maintained with isofluranej in 100% oxygen and fentanyl citrate (10 μg/kg/h, IV). A single vial of polyvalent antivenomk was administered IV over 30 minutes beginning immediately after induction.
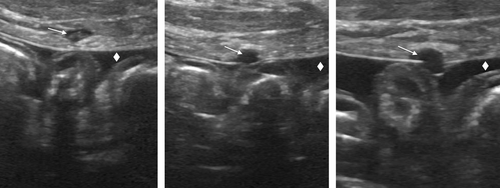
Additional diagnostics performed under anesthesia included abdominal ultrasound and diagnostic abdominocentesis. Ultrasound findings included marked peritoneal effusion, hyperechoic mesentery, and corrugated small intestines consistent with peritonitis and 2 round, hypoechoic areas extending from the visible cutaneous puncture wounds on the right body wall into the peritoneal cavity (Figure 1). Diagnostic abdominocentesis revealed hemorrhagic fluid (packed cell volume (PCV) 22%, total plasma protein [TPP] 40.0 g/L [4.0 g/dL]) with suppurative inflammation, but no cytologic indication of either neoplasia or evidence of infectious organisms. Repeat peripheral PCV and TPP were 14% (reference interval 39–58%) and 22 g/L [2.2 g/dL] (reference interval 61–75 g/L [6.1–7.5 g/dL]). A transfusion with packed RBC (9.7 mL/kg) was administered within 5 minutes and followed by an additional 9.7 mL/kg over the following 30 minutes. Following transfusion, the PCV and TPP were 28% (reference interval 39–58%) and 40 g/L [4.0 g/dL] (reference interval 61–75 g/L [6.1–7.5 g/dL]). Following the transfusion, the HR decreased to 170/min.
A second vial of antivenom was administered IV over 30 minutes after the ultrasound was completed by 5.5 h after onset of clinical signs. In an effort to remedy the severe abdominal pain, a 14-g multifenestrated catheterl was inserted into the cranioventral abdominal cavity using the modified Seldinger technique to facilitate removal and sampling of abdominal fluid and administration of lidocainem (4.8 mg/kg followed by 50 μg/kg/min CRI, IP). A single lumen central venous cathetern was inserted into the external jugular vein to provide additional venous access. Re-evaluation before extubation revealed improved HR (160–170/min), mucous membrane color and pulse quality. Following the second vial of antivenom and extubation, the patient received a fresh frozen plasma (FFP) transfusion (120 mL, IV) and an additional packed RBC transfusion (9.7 mL/kg). Post-transfusion PCV and TPP were 38% (reference interval 39–58%) and 44 g/L [4.4 g/dL] (reference interval 61–75 g/L [6.1–7.5 g/dL]). Following therapy, HR (100/min), pulse quality (strong), respiratory rate (36/min), systolic blood pressure measured by Doppler (100 mm Hg) were improved and the norepinephrine CRI was discontinued without complication. The dog was responsive and did not vocalize or splint his abdomen in response to abdominal palpation. Fluid therapy consisting of 0.45% sodium chloride solution (54 mL/kg/d) with supplemental potassium chloride was started and antibiotic therapy was continued with ampicillin/sulbactam (22 mg/kg, IV, q 8 h).
Sixteen hours after envenomation, the patient was alert, responsive, and able to stand with all vital parameters returning to normal for the remainder of hospitalization. Abdominal bruising was stable with minimal abdominal discomfort during physical examination. Repeat CBC documented progressive leukocytosis characterized by neutrophilia and increased band neutrophils (Table 1). The platelet count normalized and PCV remained stable with an increased reticulocyte count. Biochemistry results revealed hyperbilirubinemia and improved hypoalbuminemia, hypoglobulinemia, hypocholesterolemia and resolved ALT elevation (Table 2). Electrolyte changes included mild hyponatremia and improved hypokalemia. Coagulation panel showed a stable, mild prolongation of PT (9.2; reference interval 5.5–8.3 s) and aPTT (14.6 s; reference interval 7.5–13.8 s).
Pain management with fentanyl citrate CRI (7 μg/kg/h, IV) and IP lidocaine CRI (50 μg/kg/min, IP) were continued. Repeated PCV/TPP measurement of abdominal fluid via IP catheter was improved (12%/36 g/L [3.6 g/dL]). At 30 hours after envenomation, cytology of abdominal fluid obtained via IP catheter showed mild suppurative inflammation with few degenerative neutrophils and no infectious organisms. There were no behavioral signs of abdominal discomfort, and the fentanyl citrate CRI was reduced to 3 μg/kg/h. An additional dose of lidocaine (2 mg/kg, IP) was administered and the IP catheter was removed 36 hours after placement.
At 88 h after envenomation repeat CBC showed leukocytosis with increased neutrophilia and decreased band neutrophils (Table 1). The platelet count had mildly decreased although platelet clumping was noted on blood smear evaluation and PCV and TPP remained stable: 27% (reference interval 39–58%) and 59 g/L [5.9 g/dL] (reference interval 61–75 g/L [6.1–7.5 g/dL]). Biochemistry revealed albumin and globulin values within the reference interval, increased alkaline phosphatase and ALT activities, and progression of hyperbilirubinemia (Table 2). Coagulation profile values were within normal reference intervals. At this time, IV medications were discontinued and amoxicillin with clavulanic acido (12 mg/kg, per os [PO], q 12 h), tramadolp (4 mg/kg, PO, q 8 h), carprofenq (2 mg/kg, PO, q 12 h), and gabapentinr (8 mg/kg, PO, q 8 h) were initiated. The patient was subsequently discharged and these oral medications were continued for 7 days. One week after discharge, the patient was evaluated by a primary care veterinarian and no physical examination or clinicopathologic abnormalities were noted.
Discussion
To our knowledge, ours is the first report of hemoperitoneum secondary to a presumptive snakebite and of the use of a continuous infusion of IP lidocaine to provide analgesia for severe diffuse abdominal pain. Hemoperitoneum due to other causes has been previously described by retrospective studies showing that the most common etiology for a traumatic hemoperitoneum is motor vehicle accidents.10 A similar clinical course of the dog described in the current report has not been described in the traumatic hemoperitoneum literature. With identifiable external fang marks and ultrasonographic evidence of wound communication into the peritoneal space (Figure 1), the inciting cause of this patient's hemoperitoneum was suspected to be fang puncture of a blood vessel or highly vascularized organ since only mild abnormalities in platelet count and coagulation times were noted. Without abdominal radiographs, the authors were unable to rule out the presence of other materials that could have caused these puncture wounds (eg, metal BBs, pellets, or wire).
Anticoagulant properties of crotalid venom were likely perpetuating factors in the patient's intraabdominal bleeding and inflammation.6, 11 Although the bite was not witnessed, the county in which the patient lives is reported to be home to only 1 venomous snake, the copperhead.12 Copperhead venom contains the anticoagulant fibrolase, a metalloproteinase with fibrinolytic enzyme activity.13 This enzyme also acts to inhibit platelet aggregation at the platelet fibrinogen receptor. In addition to the reported affects on PT, aPTT, platelet count and fibrinogen concentration, the authors recommend performing a d-dimer concentration. The d-dimer test can be utilized to differentiate between true disseminated intravascular coagulation and venom-induced coagulopathy; however, this test was temporarily unavailable at the institution's clinical pathology laboratory. Thromboelastography may have also provided additional information about venom-induced changes in hemostasis such as platelet dysfunction and hyperfibrinolysis.14
Based on currently published literature, crotalid venom contains multiple enzymes that contribute to its deleterious effects. Enzymes including phospholipase A2, proteases, amino acid ester hydrolases, phosphatases, and fibrolases present in venom contribute to the most prominent characteristics of snake bite wounds: local tissue damage, inflammation, and hemorrhage.2, 6, 11 Although this dog was in hemorrhagic shock at the time of admission, the rectal temperature was mildly increased (39.1°C [102.4°F]). The additional findings of an inflammatory leukogram, inflammatory cells in the peritoneal effusion, and ultrasonographic evidence of mesenteric hyperechogenicity and small intestinal corrugation suggest that the venom may have led to intraabdominal inflammation and exacerbated the hemorrhagic effects of the traumatic envenomation. It is plausible that presence of venom within the peritoneal cavity and resulting peritonitis were responsible for the refractory abdominal pain.
In a retrospective study of copperhead envenomation in dogs,9 clinicopathologic abnormalities were uncommon and mild when present, consistent with the findings in this case report. Pritchard reported that CBC abnormalities included mild thrombocytopenia (24%), leukocytosis (26%), hemoconcentration (5%), and echinocytosis (3%). Only 3.5% of the dogs with measured coagulation times had prolonged aPTT while none of the patients had prolonged PT. Although our primary differential diagnosis for this patient's anemia and hypoproteinemia was blood loss, other considered causes include hemodilution due to intravenous fluid administration and hemolysis or extravasation of red blood cells and proteins resulting from venom-induced inflammation. Additionally, a component of anaphylactic shock could not be excluded as explanation for the patient's hemodynamic instability upon initial presentation.
In the management of this case, a hydroxyethyl starch solution (6%, 130/0.4f) was administered to achieve rapid volume expansion in an effort to improve tissue perfusion and delivery of oxygen in the face of severe hypoproteinemia. The authors recognize the contraindications of using hydroxyethyl starch in coagulopathic patients, namely due to concern for causing reduction in factor VIII and von Willebrand factor concentrations, impairment of platelet function, increased fibrin clot susceptibility to fibrinolysis, and induction of dilutional coagulopathy.15 However, due to the need for further intravascular volume expansion, a low molecular weight product (HES 130/0.4) was selected as the best available option until FFP could be administered after the antivenom infusion. Rather than using a high molecular weight hydroxyethyl starch solution (eg, HES 670/0.75), this low molecular weight product was selected as these products are thought to induce less-severe changes in coagulation.16
The administration of FFP in crotalid envenomation victims is controversial. Clinicians must consider both the need to replenish clotting factors lost to consumption and the concern that the additional clotting factors may result in acceleration of fibrinolysis and exacerbate hemorrhage until all venom is neutralized.17, 18 A study evaluating the efficacy of FFP to reverse venom-induced coagulopathy in dogs envenomated with brown snake venom showed that most dogs receiving plasma were afibrinogenemic and death only occurred in dogs that received plasma.18 Alternatively, a retrospective study in human medicine showed early FFP administration was associated with earlier improvement of coagulation function within 4 hours and was not associated with hemorrhage or death.17 In this case, the authors administered FFP after antivenom was administered to combat ongoing hemorrhage and volume depletion while first addressing the need to bind the circulating venom proteins. As stated above, had the d-dimer assay been available at our institution, this test may have helped to differentiate true disseminated intravascular coagulation and venom-induced coagulopathy.
The dog exhibited clinical evidence of severe abdominal pain that was refractory to initial single-agent opioid analgesia, necessitating an alternate or multimodal approach. Administration of antivenom was used to neutralize venom and reduce pain associated with its presence. Considering the classes of available analgesics, several other therapies were contraindicated in this dog due to comorbidities at time of presentation. Nonsteroidal anti-inflammatory medications were contraindicated due to the dog's hemodynamic instability and alterations in hemostasis19, 20 and alpha-2 agonists are contraindicated in the presence of hemorrhagic shock.21 The need for immediate pain relief led the authors to cautiously proceed with general anesthesia in this unstable patient, rather than to start infusions of other intravenous analgesics such as ketamine and lidocaine. General anesthesia afforded the authors the ability to obtain diagnostics, administer higher doses of opioid analgesia, and place the IP catheter without causing patient distress and discomfort.
Infiltration of a local anesthetic agent such as lidocaine, bupivacaine, or ropivacaine is often a safe addition to pain management regimens, and the majority of reports describing their use focus on treating abdominal pain immediately following abdominal surgery. Local anesthetics have been administered to veterinary patients by various routes including IV, local wound infiltration, or central neural blockade.22 Infiltration with local medications can reduce the need for systemically administered opioids that are associated with the unwanted side effects of postoperative sedation, respiratory depression, nausea, and gastrointestinal paralysis. In addition to direct analgesic properties, local anesthetic agents may also reduce pain by mitigating inflammation. IP administration of local anesthetics in rats with hydrochloric acid induced peritonitis reduced the histopathologic evidence of peritoneal inflammation.23
Several authors have described the use of one-time peritoneal injections or sprays of aerosolized lidocaine or bupivacaine for adjunct pain control in dogs undergoing laparotomy or laparoscopy for ovariohysterectomy.24-26 Campagnol24 found that a single IP injection of bupivacaine (5 mg/kg) resulted in lower pain scores for 1 hour after ovariohysterectomy in dogs. Others have demonstrated that human patients undergoing various laparoscopic or surgical procedures such as cholecystectomy, gynecological procedures, gastric bypass, or banding have an overall reduction in abdominal pain and postoperative opioid use when given IP injections of local anesthetics.27-30 Although others have reported no benefit of IP local anesthetic administration,31, 32 there are no reports of significant complications of injection.
A single prospective, randomized, double-blind trial evaluated the effect of continuous IP local anesthetic infusion on postoperative pain following laparoscopic surgery in adults.30 Although sample size was small, the authors found that continuous IP infusion of bupivacaine, compared to a saline placebo, resulted in reduced pain from 6 to 48 hours postoperatively when combined with traditional parenteral morphine administration. Single bolus administration of a local anesthetic has a limited effect due to its short duration of action but prolonged administration through a catheter increased duration of action. Lidocaine was found to absorb rapidly from the peritoneal cavity after IP administration in patients who underwent surgical ovariohysterectomy, with serum concentrations reaching plateau by 5 minutes.30 Previous clinical research has favored bupivacaine because of its long duration of action (240–360 min), but its latency to onset is 20–30 minutes compared to 10–15 min for lidocaine.24, 33 We felt that lidocaine's rapid onset of action (10–15 min) and intermediate duration of effect made this the preferred drug for this dog.
IP local anesthetics administered as a continuous infusion should be prescribed by veterinarians on a case-by-case basis and monitored closely. Local anesthetics are absorbed rapidly following IP administration27 and present risk for dose-dependent systemic toxicity in dogs including sedation, nausea, and seizures.24 The same precautions regarding intravenous administration to cats likely apply to IP administration and the drug might not be tolerated by this route in that species. The use of intraabdominal local anesthetics in cases with peritoneal effusion should be administered with caution and close monitoring as the presence of fluid may alter the pH, dilute the anesthetic, and interfere with its absorption. Clinical references34 recommend adding lidocaine to dialysate solutions for patients demonstrating abdominal discomfort during peritoneal dialysis, operating on the notion that IP lidocaine may be effective even when diluted by fluid. Despite these recommendations, the authors could identify no published studies evaluating the efficacy of lidocaine administration in this setting or in conditions of peritoneal effusion. Other possible adverse events include catheter site infection and ascending infection into the peritoneal cavity.
Conclusions
Although we could not confirm the presence of venom within the abdominal cavity and the snake was not captured for identification, the clinical findings in this case were highly suggestive of IP crotalid envenomation that responded well to supportive care which included IP administration of lidocaine. This case demonstrates that IP envenomation may produce severe abdominal pain that requires a multimodal approach to analgesic therapy.




