Unlocking the potential of lipidomic analysis in canine atopic dermatitis research: Insights from a pilot study
Abstract
enBackground
Canine atopic dermatitis (cAD) is a complex skin disease characterised by barrier dysfunction. Studies regarding the role of skin surface lipids (SSL) in cAD are needed.
Objectives/Hypothesis
Evaluate the feasibility of using D-squame tape-stripping for SSL collection and ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry (UHPLC-HRMS) for untargeted lipidomic analysis. A secondary objective was to identify significant differences in SSL between atopic and healthy dogs, and between different body sites.
Animals
Sixteen client-owned Labrador retrievers, eight atopic and eight healthy were recruited through vaccination or dermatology appointments.
Materials and Methods
Skin samples were collected from three body sites (thigh, interdigital and inguinal) using D-Squame tapes. Untargeted lipidomic analysis was conducted using UHPLC-HRMS, and data were processed with MS-DIAL and LipidSearch software.
Results
This study identified 114 SSLs, predominantly ceramides (66.2%) and diacylglycerols (30.5%). The percentage of lipid classes and ceramide subclasses did not significantly differ between healthy and atopic dogs. Two ceramide and two triacylglycerol species were significantly higher in atopic dogs, while another two ceramide species were significantly lower. Additionally, notable regional differences in lipid profiles were observed.
Conclusions and Clinical Relevance
Our findings suggest that D-squame tape-stripping combined with UHPLC-HRMS is a feasible method for SSL research in cAD. Lipid species-specific differences and significant regional variations were found, emphasising the importance of considering body sites in future studies. This study underscores the need for further research to understand the role of SSL in cAD and the insights that untargeted lipidomic analysis can provide.
Zusammenfassung
deHintergrund
Die canine atopische Dermatitis (cAD) ist eine komplexe Hauterkrankung, die durch eine Dysfunktion der Hautbarriere charakterisiert ist. Es sind Studien nötig, die die Rolle der oberflächlichen Hautlipide (SSL) bei cAD klären.
Ziele/Hypothese
Das Ziel war eine Evaluierung der Einsatzmöglichkeit des D-Squame Tape-Stripping für die SSL-Sammlung sowie der Ultrahochperformance- Flüssigkeitschromatographie verbunden mit einer hochauflösenden Massenspektrometrie (UHPLC-HRMS) für eine unspezifische Lipidanalyse. Ein zweites Ziel bestand darin, signifikante Unterschiede bei den SSL zwischen atopischen und gesunden Hunden, sowie zwischen den verschiedenen Körperstellen festzustellen.
Tiere
Es wurden sechzehn Labrador Retriever in Privatbesitz, acht davon atopisch und acht gesund, bei Impfterminen oder bei dermatologischen Untersuchungen rekrutiert.
Materialien und Methoden
Es wurden Hautproben mittels D-Squame Tape von drei Körperstellen (Schenkel, Zwischenzehen und Inguinalgegend) genommen. Es wurde eine ungezielte lipidomische Analyse mittels UHPLC-HRMS durchgeführt und die Daten mittels MS-DIAL und LipidSearch Software analysiert.
Ergebnisse
Diese Studie identifizierte 114 SSLs, vor allem Ceramide (66,2%) und Diacylglycerole (30,5%). Der Prozentanteil der Lipidklassen und der Ceramid Unterklassen unterschied sich zwischen den gesunden und atopischen Hunden nicht signifikant. Zwei Ceramide und zwei Triacylglycerol Spezies waren bei atopischen Hunden signifikant höher, während zwei weitere Ceramid Spezies signifikant niedriger waren. Zusätzlich bestanden deutliche regionale Unterschiede zwischen den beobachteten Lipidprofilen.
Schlussfolgerungen und klinische Bedeutung
Unsere Ergebnisse weisen darauf hin, dass D-Squame Tape-Stripping kombiniert mit UHPLC-HRMS eine machbare Methode für die SSL-Erforschung bei cAD darstellt. Es wurden Lipid Spezies-spezifische und signifikante regionale Unterschiede gefunden, was die Bedeutung der Beachtung der Körperstellen bei zukünftigen Studien hervorhebt. Diese Studie unterstreicht den Bedarf für weitere Studien, um die Rolle der SSL bei der cAD zu verstehen und die Einblicke, die eine ungezielte Lipidomische Analyse gewähren kann.
摘要
zh背景
犬特应性皮炎 (cAD) 是一种以屏障功能障碍为特征的复杂皮肤病。需要研究皮肤表面脂质 (SSL) 在 cAD 中的作用。
目标/假设
评估使用 D-squame胶带剥离法收集 SSL的可行性,并使用超高效液相色谱-高分辨率质谱法 (UHPLC-HRMS) 进行非靶向脂质组学分析。次要目标是确定特应性犬与健康犬之间,以及不同身体部位SSL 的显著差异。
动物
通过疫苗接种或皮肤科门诊招募了 16 只客户饲养的拉布拉多猎犬,其中 8 只为特应性犬,8 只为健康犬。
材料与方法
使用 D-squame胶带从三个身体部位(大腿、趾间和腹股沟)采集皮肤样本。使用UHPLC-HRMS进行非靶向脂质组学分析,并使用MS-DIAL和LIPIDSEARCH软件处理数据。
结果
本研究鉴定出114种SSL,主要为神经酰胺(66.2%)和二酰甘油(30.5%)。健康犬和过敏性犬的脂质类别和神经酰胺亚类的百分比差异不显著。两种神经酰胺和两种三酰甘油在过敏性犬中显著较高,而另外两种神经酰胺在过敏性犬中显著较低。此外, , 还观察到脂质谱存在显著的区域差异。
结论与临床意义
我们的研究结果表明,D-squame胶带剥离法结合UHPLC-HRMS是研究cAD中SSL的可行方法。研究发现特定脂质种类的差异和显著的区域变异,强调了在未来研究中考虑身体采样部位的重要性。这项研究强调了进一步研究的必要性,以了解 SSL 在 cAD 中的作用,以及非靶向脂质组学分析可以提供的潜在洞察。
Résumé
frContexte
La dermatite atopique canine (DAC) est une maladie cutanée complexe caractérisée par un dysfonctionnement de la barrière cutanée. Des études sur le rôle des lipides de surface cutanée (LSC) dans la DAC sont nécessaires.
Objectifs/Hypothèse
Évaluer la faisabilité de l'utilisation du ruban adhésif D-squame pour le prélèvement de LSC et de la chromatographie liquide ultra-haute performance couplée à la spectrométrie de masse haute résolution (UHPLC-HRMS) pour l'analyse lipidomique non ciblée. Un objectif secondaire était d'identifier les différences significatives entre les LSC des chiens atopiques et des chiens sains, ainsi qu'entre différents sites corporels.
Animaux
Seize labradors retrievers appartenant à des clients, huit atopiques et huit sains, ont été recrutés lors de rendez-vous de vaccination ou de dermatologie.
Matériaux et méthodes
Des échantillons de peau ont été prélevés sur trois sites corporels (cuisse, interdigital et inguinal) à l'aide de bandes D-Squame. Une analyse lipidomique non ciblée a été réalisée à l'aide de l'UHPLC-HRMS, et les données ont été traitées à l'aide des logiciels MS-DIAL et LipidSearch.
Résultats
Cette étude a identifié 114 LSC, principalement des céramides (66,2 %) et des diacylglycérols (30,5 %). Le pourcentage des classes de lipides et des sous-classes de céramides ne différait pas de manière significative entre les chiens sains et les chiens atopiques. Deux espèces de céramides et deux espèces de triacylglycérols étaient significativement plus élevées chez les chiens atopiques, tandis que deux autres espèces de céramides étaient significativement plus faibles. De plus, des différences régionales notables dans les profils lipidiques ont été observées.
Conclusions et pertinence clinique
Nos résultats suggèrent que le prélèvement de squames à l'aide d'un ruban adhésif combiné à l'UHPLC-HRMS est une méthode viable pour la recherche sur les LSC dans le cadre de la DAC. Des différences spécifiques aux espèces lipidiques et des variations régionales significatives ont été observées, soulignant l'importance de prendre en compte les zones du corps dans les études futures. Cette étude souligne la nécessité de poursuivre les recherches afin de comprendre le rôle des LSC dans la DAC et les informations que l'analyse lipidomique non ciblée peut fournir.
要約
ja背景
犬アトピー性皮膚炎(cAD)は、バリア機能障害を特徴とする複雑な皮膚疾患である。cADにおける皮膚表面脂質(SSL)の役割に雑する研究が求められている。
目的/雑雑
本研究の目的は、SSL採取にD-squameテープストリッピングを用い、非標的リピドミクス解析に超高速液体クロマトグラフィーおよび高分解能質量分析計(UHPLC-HRMS)を用いることの実現可能性を評価することであった。二次的な目的は、アトピー犬と健常犬の間、および異なる身体部位間のSSLの有意差を同定することであった。
対象動物
オーナー所有のラブラドール・レトリーバー16頭(アトピー犬8頭、健常犬8頭)をワクチン接種または皮膚科の予約を通じて募集した。
材料と方法
皮膚サンプルは、D-Squameテープを用いて3つの部位(大腿部、趾間部、鼠径部)から採取した。UHPLC-HRMSを用いて非標的リピドミクス解析を行い、MS-DIALおよびLIPIDSEARCHソフトウェアでデータを処理した。
結果
本研究では、セラミド(66.2%)とジアシルグリセロール(30.5%)を主成分とする114種類のSSLを同定した。脂質クラスおよびセラミドサブクラスの割合は、健常犬とアトピー犬で有意差はなかった。2種のセラミドと2種のトリアシルグリセロールはアトピー犬で有意に高く、別の2種のセラミドは有意に低かった。さらに、脂質プロファイルに顕著な地域差が観察された。
結論と臨床的意義
我々の知見は、D-squameテープストリッピングおよびUHPLC-HRMSの組み合わせた手法が、cADにおけるSSL研究の実現可能な方法であることを示唆していた。脂質種特異的な差異と有意な地域差が認められたことから、今後の研究において身体部位を考慮することの重要性が強調された。本研究は、cADにおけるSSLの役割を理解するためのさらなる研究の必要性と、非標的リピドミクス解析が提供できる知見を強調するものであった。
RESUMO
ptIntrodução
A dermatite atópica canina (DAC) é uma dermatopatia complexa caracterizada por disfunção da barreira cutânea. Estudos sobre o papel dos lipídios da superfície cutânea (LSC) na DAC são necessários.
Objetivos/Hipóteses
Avaliar a viabilidade do uso de fitas adesivas D-Squame para coleta de LSC e cromatografia líquida de ultra-alta eficiência acoplada à espectrometria de massas de alta resolução (UHPLC-HRMS) para análise lipidômica não direcionada. Um objetivo secundário foi identificar diferenças significativas na LSC entre cães atópicos e saudáveis, e entre diferentes regiões corporais.
Animais
Dezesseis Labrador retrievers de propriedade de clientes, oito atópicos e oito saudáveis, foram recrutados em consultas dermatológicas e de vacinação.
Materiais e Métodos
Amostras de pele foram coletadas de três regiões corporais (coxa, região interdigital e inguinal) usando fitas D-Squame. A análise lipidômica não direcionada foi conduzida utilizando UHPLC-HRMS, e os dados foram processados com os softwares MS-DIAL e LIPIDSEARCH.
Resultados
Este estudo identificou 114 LSCs, predominantemente ceramidas (66,2%) e diacilgliceróis (30,5%). A porcentagem de classes lipídicas e subclasses de ceramidas não diferiu significativamente entre cães saudáveis e atópicos. Duas espécies de ceramidas e duas de triacilgliceróis foram significativamente maiores em cães atópicos, enquanto outras duas espécies de ceramidas foram significativamente menores. Além disso, diferenças regionais notáveis nos perfis lipídicos foram observadas.
Conclusões e Relevância Clínica
Nossos achados sugerem que a técnica de remoção por fita adesiva D-squame combinada com UHPLC-HRMS é um método viável para a pesquisa de LSC na DAC. Diferenças específicas entre espécies de lipídios e variações regionais significativas foram encontradas, enfatizando a importância de considerar os locais do corpo em estudos futuros. Este estudo ressalta a necessidade de mais pesquisas para compreender o papel do LSC na DAC e os insights que a análise lipidômica não direcionada pode fornecer.
RESUMEN
esIntroducción
La dermatitis atópica canina (cAD) es una enfermedad cutánea compleja que se caracteriza por una disfunción de la barrera cutánea. Se necesitan estudios sobre el papel de los lípidos de la superficie cutánea (SSL) en la cAD.
Objetivos/Hipótesis
Evaluar la viabilidad del uso de cinta adhesiva D-squame para la obtención de SSL y de la cromatografía líquida de ultra alta resolución acoplada a espectrometría de masas de alta resolución (UHPLC-HRMS) para el análisis lipidómico no dirigido. Un objetivo secundario fue identificar diferencias significativas en LSC entre perros atópicos y sanos, y entre diferentes zonas corporales.
Animales
Se reclutaron dieciséis labradores retrievers de propietarios particulares, ocho atópicos y ocho sanos, tras vacunación o tras consultas dermatológicas.
Materiales y métodos
Se recogieron muestras de piel de tres zonas corporales (muslo, zona interdigital e inguinal) utilizando cintas adhesivas D-Squame. El análisis lipidómico no dirigido se realizó mediante UHPLC-HRMS y los datos se procesaron con los programas informáticos MS-DIAL y LIPIDSEARCH
Resultados
Este estudio identificó 114 SSL, predominantemente ceramidas (66,2%) y diacilgliceroles (30,5%). El porcentaje de clases lipídicas y subclases de ceramidas no mostró diferencias significativas entre perros sanos y atópicos. Dos especies de ceramidas y dos de triacilglicéridos presentaron concentraciones significativamente mayores en perros atópicos, mientras que otras dos especies de ceramidas presentaron concentraciones significativamente menores. Además, se observaron diferencias regionales significativas en los perfiles lipídicos.
Conclusiones y relevancia clínica
Nuestros hallazgos sugieren que la técnica de extracción con cinta adhesiva D-squame combinada con UHPLC-HRMS es un método viable para la investigación de SSL en la cAD. Se encontraron diferencias específicas entre las especies lipídicas y variaciones regionales significativas, lo que subraya la importancia de considerar las localizaciones corporales en futuros estudios. Este estudio subraya la necesidad de realizar más investigaciones para comprender el papel de los SSL en la cAD y la información que puede proporcionar el análisis lipidómico no dirigido.
INTRODUCTION
Canine atopic dermatitis (cAD) is a complex, hereditary and pruritic skin disease involving an intricate interplay between allergen sensitisation, immunological alterations, microbial dysbiosis and skin barrier dysfunction.1 Its striking similarities with human atopic dermatitis (hAD) make them interchangeable models.2, 3 One of the critical features of atopic dermatitis pathogenesis is skin barrier dysfunction, which has gathered significant interest in both veterinary and human dermatology.4, 5
The stratum corneum (SC) plays a crucial role in maintaining skin barrier integrity.6 This layer consists of corneocytes embedded in a complex lipid matrix,7 containing various species of lipids with different structural and regulatory roles. Besides forming an impermeable barrier to protect the body's internal environment,7, 8 SC lipids have antimicrobial activity, contribute directly to shaping commensal microbiota and are involved in the events that modulate the immune response to environmental stress and keratinocyte differentiation.7, 8 Additionally, sebaceous lipids, which are part of the skin surface lipids (SSL),7, 8 have also been associated with hAD9-11 and have been found to have barrier-repairing, antimicrobial and immune-modulation effects as well as keratinocyte differentiation modulation effects.7, 8, 12, 13 Given the potential significance of these lipids in cutaneous health and disease, further studies are warranted to explore the role of sebaceous lipids in cAD.
A lipidomic analysis is a large-scale analysis of the complete set of lipids in any biological system.14 Skin lipidomics is increasingly of interest to dermatological research. Various sampling techniques and analytical approaches exist, including both targeted and untargeted lipidomics.15, 16
Numerous studies have already employed lipidomic analysis in cAD,17-24 yet most have focused on targeted approaches for SC lipids. Only two studies utilised untargeted methods, enabling a more comprehensive investigation of sebaceous lipids and the entire SSL ecosystem.17, 18
Sampling lipids from the SC can be effectively achieved using noninvasive tape-stripping21, 23, 25-27 with a variety of tapes available, as for example, the D-squame.15, 19 This noninvasive D-squame tape has been reliably used in hAD research.28-31 To the best of the authors' knowledge, Marsella et al.18 were the first to use this D-squame tape coupled with targeted lipidomic analysis to evaluate the impact of a topical product on SC lipids. The advantages of using tape-stripping are that it is a noninvasive, painless and repeatable method, making it particularly valuable for longitudinal studies and clinical trials, especially in identifying predictors of treatment response, disease progression and comorbidities.28, 32 A recent pilot study by Mosca et al.32 demonstrated the innovative potential of the D-squame tape to extract the SC in dogs. However, D-squame has not been used previously for sampling other SSL, such as sebaceous lipids, as the studies employing untargeted lipidomics utilised different sampling techniques.17, 18
Therefore, this pilot study aimed to assess the feasibility of a lipidomic analysis methodology using D-squame for SSL sampling and ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry (UHPLC-HRMS) for untargeted lipid analysis in cAD clinical research.
The secondary objective was to evaluate whether there were significant differences in the skin-surface lipidome between body sites (interdigital, inguinal and lateral aspect of the thigh) and condition (atopic and nonatopic) in Labrador retriever dogs.
MATERIALS AND METHODS
Ethics
This study was approved by the Ethics Committee for Research and Teaching of the Faculty of Veterinary Medicine, University of Lisbon (no. 09/2023). Healthy Labrador retriever dogs were enrolled from client-owned dogs from vaccination appointments in the Veterinary Teaching Hospital of the Faculty of Veterinary Medicine, University of Lisbon. Atopic Labrador retriever dogs were client-owned and recruited in the Dermatology Service of the Veterinary Teaching Hospital of the Faculty of Veterinary Medicine, University of Lisbon. Clients gave informed written consent to enrol in the study and were free to withdraw at any time.
Study design and population
Sixteen dogs were recruited (eight atopic, eight nonatopic), ranging in age from 1 to 11 years old, in a randomised and unblinded study. Owners of dogs of any age diagnosed with cAD according to published criteria33 attending the Veterinary Teaching Hospital dermatology service were invited to participate. Dogs with food allergies or other cutaneous diseases were not included. At the time of recruitment, most atopic dogs were under therapeutic regimens, including both systemic and topical treatments, as detailed in Table S1 in Supporting Information. Owners of healthy dogs attending vaccination appointments in the Veterinary Teaching Hospital were invited to participate if the inclusion criteria were satisfied. These were the exclusion of dermatological or allergic alterations, exclusion of other systemic diseases and absence of administration of systemic or topical medication, excluding antiparasitic prophylaxis. Throughout the study period, dogs were kept in their usual environment, had no diet change and received routine antiparasitic prophylaxis.
Sample collection
Three samples were collected from each dog at three specific body sites: two cAD-typical lesion sites (inguinal and dorsal interdigital) and one nontypical (lateral aspect of thigh). Skin-surface lipid samples were obtained using D-Squame adhesive tapes (22 mm diameter; Clinical & Derm). These were applied with uniform pressure using the D-Squame Pressure Instrument (Clinical & Derm), carefully removed and then re-applied on the same spot, until the skin had a shiny appearance (30–55 times). The tapes were then stored in 2 mL Lysing Matrix homogenising tubes (MP Biomedicals) and then stored at −80°C until further analysis.
Lipid extraction
Lipids were extracted using the solvent proportions established by Folch et al.34 yet with dichloromethane replacing chloroform. Briefly, 800 μL of dichloromethane/methanol (2:1, v/v) and 10 μL of an isotope labelled internal standard mixture (EQUISPLASH LIPIDOMIX; Avanti Polar Lipids) were added and underwent homogenisation using a FastPrep 24-5G homogeniser (MP Biomedicals) for 10 min. Then, the samples were centrifuged for 10 min at 4°C and 13,000 g. The supernatant was collected and dried under nitrogen gas. The dried lipid extracts were reconstituted in 500 μL of acetonitrile/isopropanol (1:1, v/v) and transferred to an HPLC vial for further analysis. Extraction blank samples also were prepared using the same procedure. Quality control (QC) samples were prepared by mixing 100 μL of each final lipid extract into a pooled sample.
Untargeted lipidomics
Untargeted lipidomic analysis was achieved by HRMS using an UHPLC Vanquish Core coupled with an electron spray ionisation (ESI) and orbitrap mass analyser (Exactive Plus; Thermo Fisher Scientific). Lipid separation was done on a C18 Hypersyl Gold (100 × 2.1 mm, 1.9 nm particle size; Thermo Fisher Scientific) kept at 50°C and at a constant flow rate of 200 μL/min. The binary solvent system included a gradient A (Milli-Q Water with 10 mm ammonium formate and 20 mm formic acid) and gradient B (Isopropanol:acetonitrile [9:1, v/v] with 10 mm ammonium formate and 20 mm formic acid). The gradient elution was as follows: 0 min (35% B), 0.5 min (35% B), 4 min (65% B), 22 min (100% B), 24 min (100% B), 25 min (35% B) and 35 min (35% B). The total run time was 35 min. The mass spectra were acquired in the m/z range 250–2000, in both positive ESI and negative ESI modes, with a capillary voltage of 3.2 and 3.5 kV, respectively. The gas flow was 10 U, the auxiliary gas was 10 U, the capillary temperature was 320°C, the S-lenses RF was 50 U, and the probe temperature was 250°C. Data acquisition was performed in full scan mode with a high resolution of 140,000 and an automatic gain control (AGC) target of 1 × 106, in an m/z range of 250–2000, with 1 micro scan, and a maximum injection time (IT) of 200 ms. The mass spectra were acquired for samples, QC and extraction blank samples in one sequence. A system suitability blank sample was analysed at the start of the analytical batch, followed by eight pooled QC samples to condition the platform. The QC samples were also analysed at every fifth sample through the sequence and at the end of the batch. Additionally, at least two blank extraction samples were analysed at the very beginning of the run and the very end of the run. A standard reference (LightSplash LIPIDOMIX standard; Avanti Polar Lipids, Inc.) was analysed three times during the sequence.
In order to assist with the lipid identification, high-energy collisional dissociation (HCD) analysis was performed in at least five pooled samples. For that, a cycle of full-scan mass spectrum and MS/MS scans was repeated continuously throughout the analysis with a high resolution of 70,000 and AGC target of 3 × 106, in an m/z range of 100–1000, with 1 micro scan, an IT of 100 ms and a collision energy (CE) of 35 eV. Data acquisition was performed using the Xcalibur data system (v4.4.16.14, 2020; Thermo Fisher Scientific).
Data processing and statistical analysis
Raw data were processed using MS-DIAL v4.8.35 A QC sample file was used as the reference file for peak alignment with a retention time tolerance of 1.5 min and MS1 tolerance of 0.05 Da. Peak picking, deconvolution and annotation were completed using the MS-DIAL internal lipid library. Accurate mass tolerances for lipid identification were 0.01 and 0.05 Da for MS1 and MS2, respectively. The cut-off value of the identification score was 80% to avoid false positives. For adduct ions selection, [M + H]+, [M + NH4]+, [M + Na]+ and [M + H–H2O]+ were selected in positive ionisation mode, whereas [M–H]−, [M + Na–2H]−, [M + TFA-H]− and [M + HCOO]− were selected in negative ionisation mode. Finally, data were normalised according to the total ion chromatograms. The data matrix containing information on lipid molecular species and corresponding peak areas was exported from MS-DIAL software. Only peaks with a putative identification were maintained in the peak table. Additionally, data were filtered using blank and QC by removing features where the contribution from blanks was >5%, removing features where CV was >20% in QC, and removing features present in only a small proportion of the samples. The peak areas of each lipid species were then normalised to the total area of the identified lipid species, and quantification was done by calculating the ratio against the area of the isotope labelled internal standard and their concentration in the standard.
Statistical analyses were conducted using IBM Spss Statistics v27.0 (IBM Corp.). Lipid classes, ceramide subclasses, ceramide species and other lipid species relative concentrations were analysed using a two-way ANOVA followed by Tukey's post hoc test for multiple comparisons. Results were reported using mean ± standard error of the mean. A p-value of <0.05 was considered statistically significant.
RESULTS
A total of 2507 lipids were detected in the 48 analysed samples. Of these, only 114 lipids were putatively annotated through the MS-DIAL database and used in the data analysis. Ten classes of SSL were identified: ceramides (CER), acyl alpha-hydroxy fatty acids (AAHFA), diacylglycerols (DG), triacylglycerols (TG), sphingomyelin (SM), sphinganine (SPB), N-acyl ethanolamine (NAE), phosphatidylethanolamine (PE), phosphatidylinositol (PI) and sulfonolipids (SL). CER was the most prevalent, accounting for approximately 66.2% of the total identified lipids, followed by diacylglycerols (30.5%). The remaining SSL classes represented approximately 1% or less. Owing to their low individual percentages, PEs, PIs and SLs were grouped under the category ‘Others’. The relative concentrations and brief descriptions of each class are provided in Table 1.
| Lipid classes | Relative concentration (%) | Brief description |
|---|---|---|
| Ceramides (CER) | 66.2 |
CER are sphingolipids and crucial components of the SC lipid matrix CER exhibit extreme complexity and heterogeneity owing to the many possible combinations between their substructures: sphingoid bases and acyl chains.8 Additionally, CER play important roles in keratinocyte differentiation and immune modulation. Alterations in ceramide molecular species are linked to skin disorders characterised by barrier dysfunction, such as atopic dermatitis.36 |
| Diacylglycerols (DG) | 30.5 | DG is a significant lipid class in sebum, belonging to the acylglycerols or glycerolipids. They contain two fatty acids esterified to a glycerol backbone.8 DG serves as essential components of cellular membranes, act as building blocks for other glycerolipids such as triacylglycerols, and play a pivotal role in various metabolic processes and signalling pathways.37 |
| Sphinganine (SPB) | 1.2 |
SPBs are percursors and degeneration products of complex sphingolipids and mediators in membrane second-messenger cascades.38 They are lethal for Gram-positive micro-organisms and can affect bacterial adherence on epithelial and mucosal cells.38 Additionally, the ratio between sphinganine and sphingosine, another sphingolipid, showed a significant, inverse correlation with hAD severity in both, lesional and nonlesional skin.39 |
| N-acylethanolamine (NAE) | 0.8 | NEAs belong to the group of fatty amides within the fatty acyls. They are involved in various cutaneous functions, including keratinocyte differentiation and immunomodulation.13 NEAs also can interact with cannabinoid and vanilloid receptors.40, 41 |
| Acyl alpha-hydroxy fatty acids (AAHFA) | 0.7 | AAHFA are popular cosmetic ingredients owing to their exfoliating and rejuvenating effects by promoting apoptosis in skin cells, boosting collagen and elastin synthesis, and improving skin texture and luminosity.41, 42 Examples of lipids in this class are salicylic, glycolic, lactic and citric acids |
| Triacylglycerols (TG) | 0.3 | TG, a key lipid class in sebum, belongs to the glycerolipid group and consists of three fatty acids esterified to a glycerol backbone.8 Lipase hydrolyzes TGs into DGs and nonesterified fatty acids (NEFA), contributing to skin acidification. In humans, sebum production decreases with age, reducing TG levels and NEFA content in the SC and impairing acidification.8 A recent study reported that TG are decreased in hAD patients, and their concentration is inversely correlated with severity.10 |
| Sphingomyelin (SM) | 0.1 | SM belongs to the group of the sphingolipids. It is present in the lamellar bodies8 and can be converted to ceramides in SC's intercellular space.43 It has been found increased in hAD patients' skin.44 |
|
Others: Phosphatidylethanolamine (PE) Phosphatidylinositol (PI) Sulfonolipid (SL) |
0.4 | PEs and PIs belong to the group of ester phospholipids within the phospholipids. PEs, the second most abundant phospholipids in cells, serve as key building blocks for biological membranes and are linked to signalling pathways.45 PIs are the major source of arachidonic acid for synthesising eicosanoids, bioactive lipids with signalling function in allergy and inflammation.46 Sulfonolipids belong to the Sphingolipids class and are lipids that have the sulphur atom linked directly to a carbon atom |
- Abbreviations: hAD, human atopic dermatitis; SC, stratum corneum.
Lipid classes
A two-way ANOVA examined the effects of condition and body site on lipid class relative concentrations. No significant interaction between condition and body site was observed for any lipid class (p > 0.05 for all comparisons), allowing main effects analysis. Lipid concentrations did not differ significantly between healthy and atopic across all classes (p > 0.05 for all comparisons). Significant differences, however, were found between body sites for four lipid classes: DG, SPB, NAE and AAHFA (p < 0.001). The interdigital site showed lower concentrations than the inguinal and thigh sites for DG. Inversely, for SPB, NAE and AAHFA, the interdigital sites showed higher concentration than the other body sites (Table 2).
| Lipid classes | Relative concentrations by condition (mean ± SEM) | Relative concentrations by body site (mean ± SEM) | Interaction between condition and body site (p-value) | |||||
|---|---|---|---|---|---|---|---|---|
| H | A | p-Value | T | ID | IN | p-Value | ||
| CER | 65.74 ± 1.15 | 67.28 ± 0.81 | 0.28 | 66.25 ± 0.82 | 64.86 ± 1.68 | 68.42 ± 0.87 | 0.12 | 0.63 |
| DG | 30.80 ± 1.30 | 29.39 ± 1.08 | 0.34 | 31.66 ± 0.89a | 32.97 ± 1.58a | 25.66 ± 1.17b | <0.001 | 0.87 |
| SPB | 1.31 ± 0.30 | 1.15 ± 0.18 | 0.56 | 0.79 ± 0.14a | 0.62 ± 0.08a | 2.26 ± 0.38b | <0.001 | 0.27 |
| NAE | 0.78 ± 0.17 | 0.72 ± 0.16 | 0.74 | 0.34 ± 0.04a | 0.42 ± 0.07a | 1.50 ± 0.25b | <0.001 | 0.85 |
| AAHFA | 0.78 ± 0.20 | 0.68 ± 0.12 | 0.65 | 0.44 ± 0.09a | 0.34 ± 0.04a | 1.40 ± 0.27b | <0.001 | 0.53 |
| TG | 0.16 ± 0.11 | 0.23 ± 0.13 | 0.68 | 0.04 ± 0.01 | 0.23 ± 0.16 | 0.30 ± 0.20 | 0.43 | 0.15 |
| SM | 0.09 ± 0.05 | 0.12 ± 0.06 | 0.69 | 0.05 ± 0.02 | 0.11 ± 0.07 | 0.17 ± 0.09 | 0.49 | 0.20 |
|
Others: PE, PI and SL |
0.34 ± 0.06 | 0.43 ± 0.10 | 0.45 | 0.42 ± 0.13 | 0.45 ± 0.09 | 0.29 ± 0.07 | 0.49 | 0.47 |
- Abbreviations: AAHFA, acyl alpha-hydroxy fatty acids; CER, ceramides; DG, diacylglycerols; NAE, N-acylethanolamine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; SEM, standard error of the mean; SL, sulfonolipids; SM, sphingomyelin; SPB, sphingonine; TG, triacylglycerols.
Ceramide subclasses
Given the dominance of CER as the most abundant lipid class, we further examined CER subclasses and species. Ten subclasses of CER were identified: alpha-hydroxy fatty acid-dihydrosphingosine (ADS), alpha-hydroxy fatty acid-phytosphingosine (AP), alpha-hydroxy fatty acid-sphingosine (AS), beta-hydroxy fatty acid-dihydrosphingosine (BDS), esterified omega-hydroxy fatty acid-sphingosine (EOS), hydroxy fatty acid-sphingosine (HS), nonhydroxy fatty acid-dihydrosphingosine (NDS), nonhydroxy fatty acid-phytosphingosine (NP), nonhydroxy fatty acid-sphingosine (NS) and ‘Others’. The classification system used was the one available in the MS-DIAL program.47 Broad classification systems can be consulted in a recent review.48 The relative concentrations were as follows: 76.86% ADS, 3.93% AP, 9.75% AS, 0.80% BDS, 0.56% EOS, 3.56% HS, 1.18% NDS, 2.29% NP, 0.36% NS and 0.70% others.
A two-way ANOVA was conducted to assess the effects of condition (healthy versus atopic) and body site (inguinal, interdigital and thigh) on CER subclass relative proportions. No significant interaction between condition and body site was found for any CER subclass (p > 0.05), permitting main effects analysis. No significant differences were observed between healthy and atopic dogs for any of the CER subclasses (p > 0.05). However, significant differences between body sites were observed for all CER subclasses except AS and HS (p = 0.90 and p = 0.68, respectively). For AP, EOS, NDS, NP and NS subclasses, the inguinal site had significantly higher relative concentrations than the other sites. BDS showed significantly higher concentration on thigh and interdigital sites, compared to the inguinal area. The ‘Others’ subclass showed significantly lower concentration on thigh than the other sites (p < 0.001). The inguinal site generally showed the highest concentrations for most subclasses, except for ADS and AS (where it had the lowest values) and BDS (where it had intermediate values). Significant differences were observed for AP, EOS, NDS, NP, NS and ‘Others’, with the inguinal site having the highest relative concentrations compared to the other locations (p < 0.001).
These findings are further detailed in Table 3.
| Ceramide subclasses | Relative concentrations by condition (mean ± SEM) | Relative concentrations by body site (mean ± SEM) | Interaction between condition and body site (p-value) | |||||
|---|---|---|---|---|---|---|---|---|
| H | A | p-Value | T | ID | IN | p-Value | ||
| ADS | 78.44 ± 2.68 | 76.15 ± 2.51 | 0.55 | 85.16 ± 2.03a | 74.61 ± 3.31b | 70.82 ± 3.42b | 0.01 | 0.87 |
| AP | 4.25 ± 0.94 | 3.78 ± 0.54 | 0.55 | 2.20 ± 0.18a | 2.64 ± 0.30a | 6.94 ± 1.02b | <0.001 | 0.61 |
| AS | 9.92 ± 1.41 | 9.68 ± 1.27 | 0.91 | 8.58 ± 1.86 | 12.41 ± 1.71 | 8.27 ± 1.33 | 0.19 | 0.90 |
| BDS | 0.73 ± 0.13 | 0.84 ± 0.11 | 0.50 | 0.42 ± 0.05a | 1.30 ± 0.16b | 0.69 ± 0.13a | <0.001 | 0.93 |
| EOS | 0.59 ± 0.13 | 0.54 ± 0.08 | 0.71 | 0.27 ± 0.03a | 0.38 ± 0.08a | 1.02 ± 0.13b | <0.001 | 0.65 |
| HS | 1.38 ± 0.19 | 4.56 ± 1.75 | 0.24 | 0.90 ± 0.10 | 4.53 ± 2.51 | 5.27 ± 2.62 | 0.54 | 0.68 |
| NDS | 1.14 ± 0.27 | 1.20 ± 0.17 | 0.83 | 0.47 ± 0.06a | 1.03 ± 0.14a | 2.05 ± 0.30b | <0.001 | 0.55 |
| NP | 2.45 ± 0.39 | 2.21 ± 0.23 | 0.53 | 1.54 ± 0.14a | 2.07 ± 0.23a | 3.26 ± 0.45b | <0.001 | 0.39 |
| NS | 0.42 ± 0.08 | 0.34 ± 0.04 | 0.21 | 0.20 ± 0.02a | 0.30 ± 0.04a | 0.59 ± 0.08b | <0.001 | 0.44 |
| Others | 0.67 ± 0.14 | 0.71 ± 0.10 | 0.76 | 0.27 ± 0.04a | 0.74 ± 0.10b | 1.09 ± 0.16b | <0.001 | 0.43 |
- Abbreviations: ADS, alpha-hydroxy fatty acid-dihydrosphingosine; AP, alpha-hydroxy fatty acid phytosphingosine; AS, alpha-hydroxy fatty acid-sphingosine; BDS, beta-hydroxy fatty acid dihydrosphingosine; EOS, esterified omega-hydroxy fatty acid-sphingosine; HS, hydroxy fatty acid sphingosine; NDS, nonhydroxy fatty acid-dihydrosphingosine; NP, nonhydroxy fatty acid dihydrosphingosine; NS, nonhydroxy fatty acid-sphingosine; SEM, standard error of the mean.
Ceramide species
Of the 114 identified lipid species, CER accounted for the largest group, with 63 species putatively annotated (55.26% of the total species).
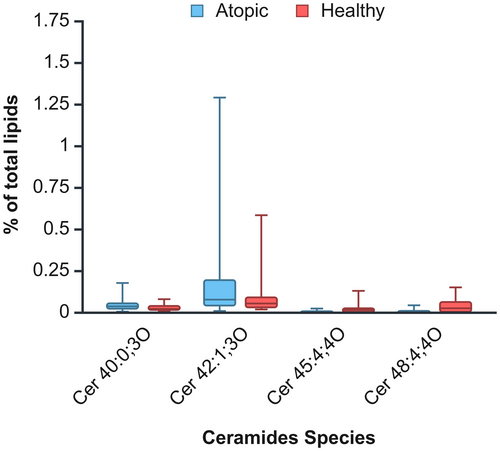
A two-way ANOVA was performed to evaluate the influence of health status (healthy versus atopic) and body site (inguinal, interdigital and thigh) on CER species in proportion to total lipids. No significant interaction between condition and body site was observed for any CER species (p > 0.05), allowing for the analysis of main effects. Four of the 63 CER species (6.35%) showed significant differences between healthy and atopic dogs. Two CER species exhibited significantly increased relative concentration in atopic dogs: Cer 40:0;3O (H = 0.03 ± 0.004; A = 0.05 ± 0.01; p = 0.047) and Cer 42:1;3O (H = 0.10 ± 0.03; A = 0.24 ± 0.07, p = 0.03), while the other two were significantly reduced: Cer 45:4;4O (H = 0.02 ± 0.01; A = 0.01 ± 0.001; p = 0.01) and Cer 48:4;4O (H = 0.04 ± 0.01; A = 0.01 ± 0.002; p = 0.0005). These differences are visualised in Figure 1. Regarding body site differences, significant variations were observed in 47 of the 63 CER species (74.60%). A general trend was noted: the thigh area tended to have the lowest concentrations of CER species, followed by the interdigital area, whereas the inguinal area generally showed the highest concentrations. Detailed results of the two-way ANOVA and post hoc analysis for location can be found in Table S2.
Other lipid species
Among the remaining 51 lipid species, 2 (3.92%) belonged to the AAHFA class, seven (13.73%) to the DG class, four (7.84%) to the NAE class and one species each (1.96%) to the PE, PI and SL classes. Additionally, three species (5.88%) were sphingomyelins (SM), five (9.80%) were SPB and the remaining 27 (52.94%) were TG.
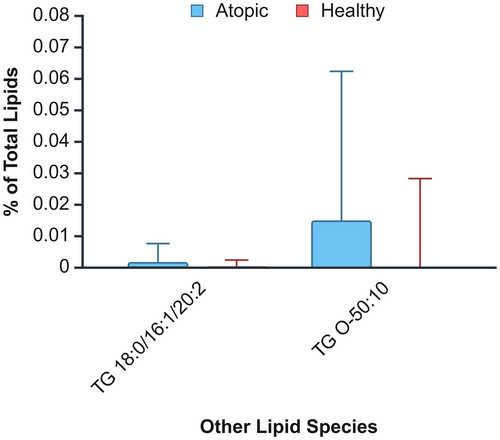
A two-way ANOVA was conducted to examine the effects of health condition and body site on these lipid species concentrations. Like ceramides, no significant interaction between health status and body site was observed, permitting an analysis of main effects. Two of the 51 nonceramide lipid species (3.92%) showed significantly higher concentrations in atopic dogs: TG 18:0/16:1/20:2 (H = 0.0003 ± 0.0001; A = 0.001 ± 0.0004; p = 0.03) and TG O-50:10 (H = 0.002 ± 0.001; A = 0.01 ± 0.003; p = 0.04) (Figure 2). Regarding body sites, significant differences were identified in 11 (21.57%) of the nonceramide lipid species: two AAHFA, two DG, three NAE, three SPB and one TG. Relative concentrations results were mixed, with AAHFA, NAE and SPB having significantly higher values in the inguinal area, and DG and TG having significantly higher values in the thigh. Detailed results of the two-way ANOVA and post hoc analysis for location can be found in Table S3.
DISCUSSION
This pilot study explores the use of D-squame tape-stripping combined with UHPLC-HRMS for untargeted lipidomic analysis of SSL in cAD. The primary objective was to assess the feasibility of this methodology in clinical practice, whereas the secondary aim was to identify differences in SSL profiles between atopic and healthy dogs and across different body sites.
The study successfully demonstrated the feasibility of the sampling method and untargeted lipidomic analysis for cAD clinical research, identifying a total of 2507 lipids, 114 of which were putatively annotated. This is the highest number of SSL detected in dogs, to the best of the authors' knowledge. From the two previous studies that have used untargeted lipidomics, one detected and identified 421 and 10 lipids, respectively,17 and the other did not specify how many lipids were detected and identified.18
One of the key findings of this study was the identification of 10 classes of SSL, with CER being the most abundant lipid class, constituting 66.2% of the total lipids identified. This aligns with previous human and canine studies, highlighting the importance of CER in skin barrier function.36 Still, the proportion of CER found in this study is higher than in previous studies22, 27, 49, 50 and the sebaceous lipids, except for DG (wax ester, squalene, triglycerides) were not identified, contrary to previous studies.50 Several factors, such as different collection techniques, varying identification and quantification methods, and breed variability among study populations, make direct comparisons with previous studies challenging. Establishing a standardised consensus for measuring skin surface lipids in future studies, similar to established outcome measurements in therapeutic clinical trials for canine atopic dermatitis, would be useful.51 However, contrary to expectations, CER class percentage did differ significantly between healthy and atopic dogs. This finding contrasts with earlier studies where CER depletion was commonly observed in canine atopic skin.18, 20-25, 27, 52
Several factors might explain this discrepancy. First, given that this was a pilot study to ascertain the feasibility of the methodology, the sample size was small. Secondly, most atopic dogs in this study were under treatment, which could have influenced lipid restoration18, 27; two dogs were treated with oclacitinib and the other two with lokivetmab, which have shown to impact the skin lipidome.18, 53 For comparing CER classes between healthy and cAD dogs, further studies with quantitative analysis of ceramide classes should be conducted in the future. Thirdly, three dogs from the healthy group were under 3 years of age, so it is possible that they could already have some lipidic alterations before cAD onset. There also was no distinction between age and sex, which are factors that could influence the SSL composition.18, 54 Finally, breed-specific lipid profiles in Labrador retrievers could play a role as the lipidome may vary across breeds.17, 18, 25
No significant differences between healthy and atopic dogs were found in the other lipid classes. The same reasons presented previously can be applied to this finding.
However, four CER species exhibited significant differences between the groups, indicating that while overall class percentage was similar, species-specific changes were detectable. Although some were reduced, others were increased in atopic skin. This mirrors findings in hAD, which is a consequence of lipid metabolic dysfunction and possibly affects SC structure.8, 36
A noteworthy result was the high abundance of DG, constituting 30.5% of SSL. This sebaceous lipid class has been reported only once in dogs.17 These compounds can result from TG hydrolysis, which could occur naturally on the skin surface after sebaceous secretion or during sample processing or being present in hair contaminated with vegetable oils. Given that no dog was undergoing treatment with vegetable oils, this origin is not probable. Interestingly, other sebaceous lipids (wax esters, squalene, triglycerides) were barely detected in our samples. This could be attributed to our untargeted lipidomic approach, where these lipids might have been present below detection thresholds or among the unidentified lipid features. Sebaceous lipids are an area of growing interest in hAD research owing to their role in epidermal homeostasis and skin barrier function.7, 10, 12 Given the species-specific nature of sebaceous lipid composition,7 more focused research on sebaceous lipids in cAD is needed.
Additionally, two TG species (TG 18:0/16:1/20:2 and TG O-50:10) had significantly higher percentages in atopic dogs, suggesting a broader involvement of sebaceous lipids in cAD pathogenesis. These findings further support the need for more studies focused on the whole SSL in cAD.
Finally, significant regional differences in lipid profiles were observed, irrespective of the dog's condition. These findings partially agree with a previous study that found body site to be a significant factor in lipid variation.24 However, contrary to the present results, there were no differences between body sites in healthy dogs. However, that study focused only on SC lipids and not the entire SSL lipid ecosystem. Also, the regional variation in sebaceous lipid density may contribute to the significant differences observed across locations in the present study.
Comparing results between studies is challenging, as lipid variation can be influenced by factors such as age, sex, body site, disease status and breed.17, 18, 24, 25, 54 Methodological differences in lipidomic analysis can also affect outcomes.18 Therefore, establishing a standardised SSL lipid collection and analysis methodology would improve comparability across studies.51
Several limitations should be noted. The small sample size reduced the statistical power, and the lack of trichotomy before tape-stripping may have affected lipid collection. We are currently conducting a study comparing lipid collection before and after trichotomy to determine whether this step is necessary. Furthermore, because differences between lesional and nonlesional skin have been observed in previous studies, the absence of lesion severity data for atopic sites limits the interpretation of results in the context of lesion-specific lipid alterations. It would be interesting to explore whether specific lipid species correlate with lesion severity and/or pruritus.
In conclusion, our results suggest that D-squame tape-stripping combined with untargeted lipidomic analysis is both feasible and effective for SSL research in cAD, and could be used for future longitudinal studies. Species-specific lipid differences were detected between healthy and atopic dogs, indicating the potential for identifying biomarkers for cAD diagnosis or monitoring. The significant regional variations highlight the importance of considering body site in future studies, particularly when comparing lesional and nonlesional skin, as location may confound results. This pilot study highlights the need for further research to clarify the role of SSL in cAD and the valuable insights that untargeted lipidomic analysis can offer.
AUTHOR CONTRIBUTIONS
Beatriz Fernandes: Conceptualization; methodology; investigation; formal analysis; visualization; writing – original draft; funding acquisition. Ana Mafalda Lourenço: Conceptualization; methodology; writing – review and editing; project administration; supervision. Joana Marto: Writing – review and editing. Hugo Pereira: Investigation. Susana Paula Alves: Conceptualization; methodology; investigation; formal analysis; writing – review and editing. Margarida Silva: Investigation; formal analysis. Vanessa Merta Schmidt: Writing – review and editing. Marta Pinto: Writing – review and editing. Ana Filipa Bizarro: Writing – review and editing.
ACKNOWLEDGEMENTS
The authors sincerely thank the institutions where this work was conducted for their invaluable support and contributions. We also greatly appreciate the insightful comments and suggestions provided by the reviewers, which have significantly enhanced the quality of this manuscript.
FUNDING INFORMATION
This study was funded by the Centre for Interdisciplinary Research in Animal Health (CIISA) and Associate Laboratory for Animal and Veterinary Sciences (AL4Animals) (UIDB/00276/2020, LA/P/0059/2020), under the project MSC21Set-11. It was performed in the context of a Doctoral Research Fellowship (2021.05985.BD awarded to BF; 2021-2025) by the Foundation for Science and Technology (FCT, Lisbon, Portugal).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




