Pharmacology of drugs used in autoimmune dermatopathies in cats and dogs: A narrative review
Abstract
enImmunosuppressive drugs are the mainstay of treatment for many feline and canine autoimmune skin diseases, either as monotherapy or in combination with other drugs. Treatment with these drugs is often lifelong and may have long-term consequences on the affected animal's overall quality-of-life. Clinicians need to understand the pharmacology of immunosuppressants in planning and executing the treatment regimen for the best possible clinical outcome, as well as reducing the risk of adverse effects. This review paper will focus on the mechanism of action, pharmacokinetics and pharmacodynamics, clinical uses and adverse effects of immunosuppressive drugs used to treat autoimmune dermatoses in cats and dogs. These include glucocorticoids, ciclosporin A, azathioprine, chlorambucil, mycophenolate mofetil, oclacitinib and Bruton's tyrosine kinase inhibitors.
摘要
zh免疫抑制药物是治疗许多猫和犬自身免疫性皮肤病的主要药物,无论是单药治疗还是与其他药物联合治疗。这些药物的治疗通常是终身的,可能会对患病动物的整体生活质量产生长期影响。临床医生在规划和执行治疗方案时需要了解免疫抑制剂的药理学,以获得尽可能好的临床结果,并降低不良反应的风险。本文将重点介绍免疫抑制药物治疗猫犬自身免疫性皮肤病的作用机制、药代动力学和药效学、临床应用和不良反应。这些药物包括糖皮质激素、环孢素、硫唑嘌呤、苯丁酸氮芥、吗替麦考酚酯、奥拉替尼和布鲁顿酪氨酸激酶抑制剂。
Résumé
frLes médicaments immunosuppresseurs constituent la base de la thérapeutique de nombreuses dermatoses auto-immunes félines et canines, soit en monothérapie, soit en association avec d'autres médicaments. Le traitement par ces médicaments dure souvent toute la vie et peut avoir des conséquences à long terme sur la qualité de vie globale de l'animal affecté. Les cliniciens doivent comprendre la pharmacologie des immunosuppresseurs afin de planifier et de mettre en place le plan thérapeutique, afin d'obtenir le meilleur résultat clinique possible et de réduire le risque d'effets indésirables. Cet article de synthèse cible le mécanisme d'action, la pharmacocinétique et la pharmacodynamie, les utilisations cliniques et les effets indésirables des médicaments immunosuppresseurs utilisés pour traiter les dermatoses auto-immunes chez les chats et les chiens. Ces médicaments comprennent les glucocorticoïdes, la ciclosporine A, l'azathioprine, le chlorambucil, le mycophénolate mofétil, l'oclacitinib et les inhibiteurs de la tyrosine kinase de Bruton.
Zusammenfassung
deImmunsuppressive Medikamente stellen entweder als Monotherapie oder in Kombination mit anderen Medikamenten den Hauptbestandteil der Therapie vieler Autoimmunerkrankungen von Katzen und Hunden dar. Die Behandlung mit diesen Medikamenten ist oft lebenslang nötig und kann Langzeitfolgen für die gesamte Lebensqualität der betroffenen Tiere haben. KlinikerInnen müssen die Pharmakologie der immunsuppressiven Medikamente bei der Planung und Ausführung des Behandlungsregimes verstehen, um ein bestmögliches klinisches Ergebnis zu erzielen und um die Nebenwirkungen zu minimieren. Diese Review Arbeit wird sich auf den Aktionsmechanismus, die Pharmakokinetik und die Pharmakodynamik, den klinischen Einsatz und die Nebenwirkungen der immunsuppressiven Medikamente konzentrieren, die verwendet werden, um Autoimmundermatosen bei Katzen und Hunden zu behandeln. Dazu zählen Glukokortikoide, Ciclosporin A, Azathioprin, Chlorambucil, Mycophenolate Mofetil, Oclacitinib und Bruton´s Tyrosin Kinasehemmer.
要約
ja免疫抑制剤は、多くのネコやイヌの自己免疫性皮膚疾患の治療において、単独療法または他剤併用療法の主役である。これらの薬剤による治療は多くの場合一生続くものであり、症例のQoLに長期的な影響を及ぼす可能性がある。臨床医は、可能な限り最良の臨床結果を得るために、また副作用のリスクを軽減するために、治療レジメンを計画し実行する際に免疫抑制剤の薬理学を理解する必要がある。本総説では、犬猫の自己免疫性皮膚疾患の治療に用いられる免疫抑制剤の作用機序、薬物動態、薬力学、臨床使用法、副作用に焦点を当てた。グルココルチコイド、シクロスポリンA、アザチオプリン、クロラムブシル、ミコフェノール酸モフェチル、オクラシチニブ、ブルトン型チロシンキナーゼ阻害薬などが含まれる。
Resumo
ptOs medicamentos imunossupressores são a base do tratamento para muitas doenças de pele autoimunes felinas e caninas, seja em monoterapia ou em combinação com outros medicamentos. O tratamento com esses medicamentos costuma durar toda a vida e pode ter consequências a longo prazo na qualidade de vida geral do animal afetado. Os clínicos precisam compreender a farmacologia dos imunossupressores para planejar e executar o protocolo de tratamento para se obter o melhor resultado clínico possível, assim como reduzir o risco de efeitos adversos. Este artigo de revisão será focado no mecanismo de ação, farmacocinética e farmacodinâmica, indicações clínicas e efeitos adversos de medicamentos imunossupressores usados para tratar dermatoses autoimunes em cães e gatos. Estes incluem glucocorticóides, ciclosporina A, azatioprina, clorambucil, micofenolato de mofetila, oclacitinib e inibidores da tirosina quinase de Bruton.
RESUMEN
esLos tratamientos inmunosupresores son la línea de tratamiento principal en muchas enfermedades autoinmunes de la piel de perros y gatos, bien como monoterapia o en combinación con otros fármacos. El tratamiento con estos fármacos es a menudo de larga duración o de por vida y puede tener consecuencias adversas de larga duración en la calidad de vida de los animales. Los veterinarios clínicos tienen que entender la farmacología de los inmunosupresores durante la planificación y ejecución de los tratamientos para obtener los resultados más beneficiosos y reducir los efectos adversos. Este artículo de revisión está enfocado en los mecanismos de acción, farmacocinética, farmacodinámica, usos clínicos y efectos adversos de tratamientos inmunosupresores utilizados en perros y gatos para tratar dermatopatías inmunomediadas de la piel. Se incluyen glucocorticoides, ciclosporina A, azatioprina, clorambucilo, mofetil micofenolato, oclacitinib e inhibidores de la tirosina quinasa de Bruton.
INTRODUCTION
Autoimmune dermatoses are uncommon-to-rare in cats and dogs. For instance, pemphigus foliaceus (PF) was diagnosed in 26 of 9750 dogs between 1975 and 1984 in one institution.1 Autoimmune dermatopathies pose a challenge regarding the choice of treatment for induction and maintenance of clinical remission (CR). This requires the usage of immunosuppressive drugs, either as monotherapy or combination therapy, and lifelong treatment is often required. Clinicians need to understand the pharmacology of immunosuppressants to help with decision-making associated with treatment efficacy and adverse effects. This review will focus on the mechanism of action (MoA), pharmacokinetic and pharmacodynamics of immunosuppressive drugs used to treat feline and canine autoimmune skin diseases, including extra-label uses. Information in this review is based on the published supporting evidence.
GLUCOCORTICOIDS
Glucocorticoids (GC) are synthesised from cholesterol through steroidogenesis. predominantly in the adrenal cortex, although local GC production also has been reported in the lungs, intestine and skin.2 The production of adrenal GCs are controlled by the hypothalamic–pituitary–adrenal (HPA) axis which is activated by physiological (e.g. circadian cycles), physical (e.g. inflammation) and/or psychological (e.g. stress) signals.2, 3
Synthetic GCs (i.e. prednisone, triamcinolone and dexamethasone) are more potent immunosuppressive and immunoregulatory agents than endogenous cortisol with the synthetic GCs having relatively minimal mineralocorticoid activity.3
Mechanism of action
Glucocorticoids are lipophilic and diffuse easily through the cell membranes. Once in the cell cytosol, GCs bind to glucocorticoid receptors (GR) and translocate into the nucleus as GC–GR complexes via binding to proteins known as importins.4, 5 In the nucleus, the GR interacts with DNA and proteins to alter gene expression (Figure 1).
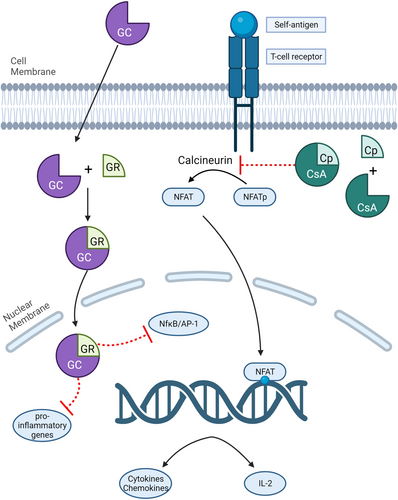
The genomic effects of GCs occur via several mechanisms: direct binding of the ligand-bound GR to DNA via positive glucocorticoid response elements (+GREs) or negative GREs (nGREs) and physical interaction with another transcription factor without direct contact with DNA (called ‘tethering’).3, 5 Direct binding to +GREs induces transcription of genes that have anti-inflammatory and immunomodulatory properties such as annexin-A1 (ANAX1, also known as lipocortin 1), GC-induced leucine zipper (GLIZ) and mitogen-activated protein kinase phosphatase 1 (MPK1).4 By contrast, binding to nGREs inhibits the transcription of genes such as those associated with corticotropin-releasing hormone, melanocyte-stimulating hormone and β-endorphin.5
Tethering interferes with NF-κB and activator protein-1 (AP-1)—two transcriptional activators that produce a repertoire of inflammatory cytokines, chemokines and adhesion molecules involved in most, if not all, of the inflammatory and autoimmune dermatoses. It also interferes with key proinflammatory transcription factors from members of the signal transducer and activator (STAT) and nuclear factor of activated T cells (NFAT).3 Finally, GR may also bind to both DNA and protein (composite GRE binding), resulting in the aforementioned anti-inflammatory and immunosuppressive effects.3
Our understanding of the effects of GCs on B cells and antibody production remain incomplete. It has been suggested that GCs have effects on B-cell selection as B cells express the GR throughout development and immature B cells are more sensitive to apoptosis.3 Chronic use of GC is also associated with the inhibition of antibody production in B cells.6
Glucocorticoids can also exert their effects via a nongenomic mechanism that occurs more rapidly (within minutes) than the genomic mechanism, which may take hours to days. In the nongenomic pathway, interaction of GCs with membrane-specific GR, cytosolic GR (resulting in the release of a variety of proteins without the need to translocate into the nucleus) and nonspecific interactions with cell membranes leads to alteration of transmembrane currents, signal transduction and intracellular calcium levels5, 7—all of which have anti-inflammatory effects.
Pharmacokinetics and pharmacodynamics
The pharmacokinetics (PK) of GCs in dogs and cats are sparsely reported, possibly because the effects of GCs do not necessarily correlate to the time they are detectable in plasma.8 Doses are therefore often derived from human use, or based on the pharmacodynamic (clinical) response in the individual animal. Current literature on prednisone, prednisolone and dexamethasone is summarised below, with an emphasis on their use as immunosuppressive agents. There are no relevant publications on triamcinolone PK currently in the literature.
Prednisone is used clinically in dogs owing to their ability to rapidly absorb the drug and efficiently metabolise it to prednisolone. Following oral administration of prednisone, prednisolone concentrations in the plasma of healthy dogs are approximately sixfold higher than the concentrations of prednisone.9 Over a dose range of 0.5–4.0 mg/kg, the kinetics of prednisone and prednisolone were not linear based on a disproportionate increase in drug exposure at the 1 mg/kg dose using the area under the curve (AUC), which is a measure of overall drug exposure. Relatively proportional increases in maximum concentrations were seen, and the half-life remained consistent. This finding is consistent with data in other species and is thought to be due to concentration-dependent protein binding.
In man and laboratory animals, prednisone is converted to prednisolone in the liver via the hepatic enzyme 11-β-hydroxysteroid dehydrogenase type 1.10 Studies in cats show that this enzyme, while present to a certain degree in the liver, is not capable of dehydrogenase activity when evaluated in the context of the ability to convert cortisol to cortisone.11 This may explain the low conversion of prednisone to prednisolone in cats. Following administration of prednisone, only approximately 20% of drug is converted to prednisolone. The prednisolone AUC following administration of prednisolone is reported as 3230.6 ng/mL/h versus 672.63 ng/mL/h following administration of prednisone.12 These differences may represent an inability to convert the drug to prednisolone in the liver, yet could also be a result of lower oral absorption of prednisone. Because of this, prednisolone is recommended for use in cats over prednisone.
Prednisolone has excellent oral absorption in both dogs and cats and can be used for long-term administration in either species.12, 13 As discussed, it is preferred over prednisone in cats, and should be used in dogs with altered hepatic function, where metabolism of prednisone to prednisolone may be diminished. In over-conditioned cats [body condition score (BCS) of 4–5 of 5], plasma concentrations of prednisolone were, on average, twofold higher than the concentrations in normal cats (BCS 3–3.5 of 5), suggesting that the drug may be better dosed on lean body mass.14
Data on dexamethasone PK in dogs and cats are even scarcer. Dogs administered 1 mg/kg of dexamethasone in alcohol (intravenously) or as isonicotinate (intramuscularly) had drug detectable for 10 h post-administration.15 Using a very sensitive assay, dexamethasone was detected in the plasma of Greyhound dogs for ≤96 h post-administration of approximately 0.05 mg/kg dexamethasone sodium.16 Despite the long detection time, dexamethasone suppression of endogenous cortisol production was not significant after 24 h. The PK of oral dexamethasone in dogs is not published. There are studies available that report the PK of oral dexamethasone in cats following a single dose of injectable dexamethasone sodium phosphate administered at 0.05 or 0.2 mg/kg.17, 18 Oral absorption is adequate, although variable in cats. Willis-Goulet et al. (2003) also examined transdermal absorption of dexamethasone in cats. Following transdermal administration, plasma concentrations were low to undetectable; this route should not be used.18
In general, immunosuppressive doses of GCs are higher and require more frequent administration than anti-inflammatory doses. For example, in dogs, prednisolone plasma concentrations necessary to suppress cortisol, neutrophil and lymphocyte responses are 0.04, 10 and 22.5 ng/mL, respectively.19 At 1 mg/kg prednisolone i.v., plasma neutrophil counts increased and lymphocyte counts decreased, and had returned to baseline within the suggested 24-hour dosing interval.
Species differences in responsiveness to GCs also need to be taken into account. Cats are less responsive to the anti-inflammatory, immunosuppressive and adverse effects of GCs compared to dogs.20 This is due to a reduced number of GR in the skin and liver of cats compared to dogs.21 Those receptors present are also lower-affinity receptors in cats. Therefore, doses of GCs used in cats are higher than those used in dogs.
Glucocorticoid resistance (i.e. absence of clinical response to optimal dosage of GCs) has been reported in humans with conditions such as asthma,22 nephrotic syndrome23 and ulcerative colitis.24 In animals, GC resistance has been recognised in several diseases such as canine mast cell tumour,25 feline lymphoma26 and canine protein-losing enteropathy.27 The exact pathomechanism of GC resistance in animals is not well-understood, yet low expression of GR and alterations in the metabolic pathways have been suggested.28, 29 Although GC resistance in feline and canine autoimmune dermatoses has not been reported, the possibility exists, especially if there is poor response to GC therapy during the induction phase.
Use for autoimmune dermatopathies in dogs and cats
Immunosuppressive doses of systemic GCs, either as monotherapy or in combination with other immunosuppressants/immunomodulators, are the mainstay and often first-line therapy for many autoimmune dermatoses in cats and dogs. GC is considered as the standard first-line treatment for the induction phase of therapy in all untreated patients with active disease, except for those patients for whom GCs are contraindicated. Pemphigus foliaceus, autoimmune subepidermal blistering dermatoses (AISBD) [e.g. mucous membrane pemphigoid (MMP) and epidermolysis bullosa acquisita (EBA)], cutaneous lupus erythematosus (CLE) and pemphigus vulgaris (PV) are examples of autoimmune dermatoses that are treated with systemic GCs. Dosing regimens vary and are likely need to be tailored to individual patients. In one comparative study, dogs with PF treated with oral GC pulse therapy (10 mg/kg once daily for three days, consecutively, followed by a dose of <2 mg/kg/day between pulses) had higher proportions of clinical remission (CR) during the first three months and a lower average maximal oral GC dosage given between pulses.30 The benefit of pulse therapy over conventional daily GC dosing was not apparent in a small case series of 12 cats with PF.31 In a critically appraised topic on the dosage of prednisone or prednisolone for the treatment of canine PF, there was no compelling evidence that oral prednisone or prednisolone at the dose of 2 mg/kg/day was effective in induction and/or maintenance of CR, and the authors suggested that a higher dose is more likely to be associated with CR.32 Likewise, high doses of oral GCs (≥3 mg/kg/day for dogs and ≤3 mg/kg twice daily for cats)—often in combination with another immunosuppressant—have been recommended for the treatment of canine and feline PV,33 and canine uveodermatological syndrome (UDS).34 In cats with PF, GC monotherapy was the most common treatment administered at the time of CR.31
Adverse effects
Owing to the wide distribution of GR in all nucleated cells, the chronic use of GC results in many systemic adverse effects. These include iatrogenic hyperadrenocorticism, gastrointestinal ulceration, cutaneous atrophy, diabetes mellitus resulting from insulin resistance, opportunistic infections and delayed wound healing. Chronic dosing of GCs in cats can result in different dermatological adverse effects to those in dogs; cats tend to develop extreme thinning of the skin with possible tearing and curling of the pinna caused by attenuation of the cartilage, whereas dogs uniquely develop calcinosis cutis. Long-acting methylprednisolone acetate has been associated with the induction of congestive heart failure in cats.35 This is believed to be the consequence of a shift in fluids resulting in an increased plasma volume secondary to glucocorticoid-induced hyperglycaemia; it is noteworthy that this conclusion was from a study that was conducted over 24 days and, therefore, the aforementioned pathomechanism could be a short-term adverse effect.36 Although the anti-inflammatory dose of the intermediate-acting steroid prednisolone (1–2 mg/kg/day, p.o.) given to healthy cats with allergic dermatitis for 14 days did not result in significant haemodynamic and echocardiographic changes,37 these results should not be extrapolated to cats requiring immunosuppressive doses of GC for the long-term treatment of autoimmune dermatoses.
Adverse effects of GCs are related to both the dose used and the duration of therapy. In humans, the cumulative dose administered is often calculated to determine the risk for adverse effects developing. One study of patients being treated with steroids showed that the risk for complications developing increased by 3%–8% for each 1 g of cumulative steroid dosed (most frequently prednisone or prednisolone).38 To the best of the author's knowledge, similar data are not available in veterinary species, yet chronicity of dosing should be considered when determining risk for adverse effects. One strategy to potentially decrease risk for adverse effects is to dose on a mg/m2 basis, rather than a mg/kg basis. Large-breed dogs had higher Cmax and AUC when dosed with prednisolone at 2 mg/kg compared to 40 mg/m2, and to small dogs dosed at 2 mg/kg.39 Optimal dosing regimens still require further study.
CICLOSPORIN A
Ciclosporin A (CsA) is derived from the soil fungus Beauveria nivea. In veterinary medicine, oral ciclosporin is formulated into an ultramicronised (microemulsified) preparation, in which absorption is more consistent and predictable. Although microemulsified oral ciclosporin is only approved for the treatment of canine atopic dermatitis (AD) and feline atopic skin syndrome, it has been used to treat many autoimmune and immune-mediated dermatoses. This is a result of its immunomodulatory and immunosuppressive effects.
Mechanism of action
Ciclosporin A is classified as a calcineurin inhibitor; its primary immunosuppressive effect is the inhibition of T-lymphocyte function. Calcineurin is an intracellular protein that activates gene transcription factors by dephosphorylation. When an antigen (e.g. self-antigen) binds to the T cell receptor (TCR), it triggers calcium ion release from the endoplasmic reticulum. The increase of intracellular calcium ions causes the activation of several downstream signalling effectors such as mitogen-activated protein (MAP) kinases, protein kinase C (PKC) and calcineurin.40 Activated calcineurin dephosphorylates nuclear factor of activated T cells (NFAT), thereby allowing its translocation into the nucleus, which subsequently upregulates transcription of genes important for innate and adaptive immunity such as interleukin (IL)-2, IL-4, tumour necrosis factor (TNF)-α and TNF-γ.41 Interleukin-2 in particular, is a potent T-lymphocyte stimulator: it induces proliferation and differentiation of naïve CD8+ T cells into effector T cells by promoting secretion of granzyme B and perforins; it stimulates the proinflammatory activity of T helper (Th)-dependent B cells, antigen-presenting cells, mast cells, basophils and eosinophils; and it influences the differentiation of CD4+ T cells into Th1 or Th2, and subsequently affects the proliferation and differentiation of natural killer (NK) cells and B cells.42-45
Ciclosporin A acts by binding to intracellular cyclophilin, which creates a complex that has a high affinity for calcineurin.41 The binding of the ciclosporin–cyclophilin complex with calcineurin inhibits its function of dephosphorylating NFAT and thus prevents the translocation of NFAT into the nucleus. Without NFAT in the nucleus, the aforementioned inflammatory cytokines are not transcribed and full T-lymphocyte activation is impaired (Figure 1).
Pharmacokinetics and pharmacodynamics
The pharmacokinetics and pharmacodynamics of CsA have been explored extensively in dogs,41 and to a lesser degree, cats. Formulation is important and the FDA-approved formulations for dogs and cats should be used. The use of generic human formulations in dogs has been shown to result in a threefold variability in drug absorption between formulations41; likewise compounded formulations are not recommended. With a microemulsified formulation (Atopica), the bioavailability of CsA in cats and dogs when administered per os was reported to be 25%–29%46 and 35%,47 respectively. An oral solution (Cyclavance) is also available and has been shown to be bioequivalent to Atopica in dogs with a relative bioequivalence of approximately 101%.48 Administration of food along with CsA reduces the mean bioavailability by 22% in dogs,49 although this did not affect the clinical outcome in 15 dogs treated for AD.50 However, this result cannot be extrapolated when treating cats or dogs with autoimmune dermatoses as no data are currently available. Other routes of administration of CsA include transdermal and subcutaneous. A study involving six healthy cats reported that the absorption of transdermal CsA in the majority of the cats is poor.51 Median concentrations in that study following seven days of oral administration were 2208 ng/mL at two hours post-dosing, compared to 37 ng/mL at two hours after transdermal applications of equivalent doses.
Ciclosporin is primarily metabolised in the liver by the cytochrome P450 3A (CPY3A) family of metabolising enzymes. Several drugs that also are metabolised by this enzyme will affect the blood concentration of CsA if given concurrently. Of these drugs, azole antifungals (e.g. ketoconazole and fluconazole) are often used along with CsA (especially in large dogs) because they decrease the metabolism of CsA, and therefore increase blood and skin CsA concentrations.52 This enables a CsA dose reduction of 50%–70%,45 which reduces the overall cost of therapy. The interaction of CsA with other drugs is reviewed elsewhere.41, 45 Ciclosporin is also a substrate for p-glycoprotein efflux pumps, which may result in other significant drug–drug interactions. This also implies that caution must be used when dosing CsA in dogs with the MDR1 (ABCB1-1Δ) mutation. Animals heterozygous for this mutation may develop excessive immunosuppression at lower-than-expected doses, potentially resulting in severe infections.53 Because a similar mutation has been identified in cats,54 this caution may apply to affected feline patients as well.
Disease states also may affect CsA PK. In an experimental model of diabetic dogs, overall drug exposure (as measured by AUC) was decreased by 52%, clearance was significantly increased and, subsequently, half-life was significantly decreased (9.32 h vs. 22.56 h) compared to healthy dogs.55 The mechanism for this is not fully understood, although it was speculated to be caused by increased clearance secondary to hyperglycaemia or alterations in the lipid profile of these dogs. Similar changes in PK were reported in human diabetic transplant patients placed on CsA,56 and it would be assumed that a similar phenomenon be seen in diabetic cats.
In order to determine the effectiveness of CsA in dogs, there are two types of tests; therapeutic drug monitoring (TDM) and quantitative reverse transcription polymerase chain reaction (qRT-PCR). However, there is a poor correlation between CsA blood concentration and the clinical response in dogs with AD,49 and therefore TDM should only be interpreted in conjunction with clinical assessment of skin lesions and signs. Although data on this correlation in dogs or cats with autoimmune dermatoses are lacking, it is reasonable to suggest that clinical response plus TDM is superior to TDM alone when using oral CsA as a treatment. An alternative way to determine the effect of CsA dosing in a particular animal is through a pharmacodynamic assay that measures cytokine (IL-2) gene expression using qRT-PCR technology to detect the percentage suppression in the animal.57
Use for autoimmune dermatopathies in cats and dogs
Ciclosporin A is used to treat many autoimmune dermatoses in cats and dogs; these include, and are not limited to, PF, CLE, PV, UDS, perianal fistula, sebaceous adenitis and ischaemic dermatopathies.31, 33, 34, 58-60 The efficacy of oral CsA for the treatment of generalised discoid and vesicular variants of CLE has been reported in a comprehensive review.58 In a recent retrospective study, CR of PF was achieved in nine of 11 dogs treated with oral CsA along with oral GC; of these, in five dogs, the disease was maintained in CR with tapered oral CsA monotherapy. The authors of that study proposed that the efficacy of CsA in these cases may be associated with inhibition of B-cell activation (via inhibition of Th cell function), reduction in metalloproteinase-9 expression and blocking of the c-Jun N-terminal kinase (JNK) and p38 signalling pathways that are involved in the pathogenesis of pemphigus.61 Ciclosporin A may be effective as monotherapy for cell-mediated autoimmune dermatoses such as chronic cutaneous lupus erythematosus and sebaceous adenitis as studies showed that they are associated with cell-mediated autoimmunity involving high expression of T lymphocytes in the epidermis and dermis,58 and sebaceous glands,62 respectively. However, for canine and feline PF, the usage of CsA as the sole immunosuppressant for maintenance therapy may not be sufficient for all patients, yet it is valuable as a steroid-sparing agent. Tables 1 and 2 summarise the response to CsA in selected autoimmune dermatoses in cats and dogs. Clinicians need to be aware that treatment of autoimmune dermatoses with CsA is considered an extra-label use in cats and dogs.
| Diseases (number of dogs evaluated) | Type of study | Responses | Concurrent use of oral GC when CR achieved (number of dogs) | Maintenance therapy | References | ||
|---|---|---|---|---|---|---|---|
| Complete remission | Partial remission | Misc | |||||
| Pemphigus foliaceus | |||||||
| n = 11 | Retrospective | 9/11 | 2/11 (poor response) | Yes (n = 11) |
CsA monoRx: 5/9 CsA/GC: 4/9 |
Chong et al. (2022)61 | |
| n = 41 | Retrospective | 28/41 | Not reported | Yes (13/28) | Not reported | Zhou et al. (2021)63 | |
| n = 1 | Case report | 1 (>90% improvement) | No, but the dog was concurrently receiving AZA | CsA/AZAb | Yun et al. (2020)64 | ||
| n = 3 | Case series | 3/3 | 2/3 dogs treated with CsA | Yes (n = 2)c | Similar to induction Rx | Simpson et al. (2019)65 | |
| n = 5 | Prospective open-label | 5/5 dogs: no response | No | NA | Olivry et al. (2003)66 | ||
| VCLE | |||||||
| n = 11 | Retrospective | 8/11 | Yes (6/8) |
CsA monoRx: 4/8 CsA/topical CI: 4/8 |
Banovic et al. (2017)67 | ||
| 3/11 | 2/3 dogs treated with CsA | Yes (1/3) |
CsA monoRx: 2/3 AZA monoRx: 1/3 |
||||
| n = 1 | Case report | 1 | Yes | CsA monoRx | Font et al. (2006)68 | ||
| Diseases (number of dogs evaluated) | Type of study | Responses | Concurrent use of oral GC during the induction phase (number of dogs) | Maintenance therapy | References | ||
|---|---|---|---|---|---|---|---|
| Complete remission | Partial remission | Misc | |||||
| GDLE | |||||||
| n = 10 | Retrospective | 5/10 | 2/5 dogs treated with CsA | Yes (n = 2) | Not reported | Banovic et al. (2016)69 | |
| 5/10 | 4/5 dogs treated with CsA | Yes (n = 4) | Not reported | ||||
| ECLE | |||||||
| n = 4 | Case series | None | CsA monoRx improves clinical signs temporarily | NA | Mauldin et al. (2010)70 | ||
| MMP | |||||||
| n = 11 | Retrospective | 10/11 |
1/11 dogs 1/11 dogs treated with CsA/KTZ |
Yes (n = 1) No (n = 1) |
Not reported | Tham et al. (2016)71 | |
| PF/GDLE | |||||||
| n = 2 | Case report | 2/2 | Yes (n = 2) | CsA monoRx: 2/2 dogs | Levy et al. (2020)72 | ||
- Abbreviations: AZA, azathioprine; CI, calcineurin inhibitor; CsA, ciclosporin A; ECLE, exfoliative cutaneous lupus erythematosus; GC, glucocorticoid; GDLE, generalised discoid lupus erythematosus; KTZ, ketoconazole; Misc, miscellaneous; MMP, mucous membrane pemphigoid; NA, not applicable; PF/GDLE, pemphigus foliaceus-GDLE polyautoimmunity; PSGAG, polysulphated glycosaminoglycan; Rx, therapy; VCLE, vesicular cutaneous lupus erythematosus.
- a Based on published supporting evidence using the following search strategy (PubMed, Web of Science and CAB Abstract):(cat OR cats OR feline OR dog OR dogs OR canine) AND (pemphigus OR pemphigoid OR autoimmune skin OR lupus erythematosus) performed on 27 October 2023; only records in the English language are included; excluded were review publications without specific clinical case information, records not published in the English language, proceedings and abstracts.
- b Clinical remission maintained until Day 99, after which a relapse of PF resulted in the change of treatment to mycophenolate mofetil.
- c In one dog, PSGAG was given concurrently; in the other dog, PSGAG and AZA were given concurrently.
| Diseases (number of dogs evaluated) | Type of study | Responses | Concurrent use of oral GC when CR achieved (number of cats) | Maintenance therapy | References | |
|---|---|---|---|---|---|---|
| Complete remission | Number of cats treated with CsA | |||||
| Pemphigus foliaceus | ||||||
| n = 45 | Retrospective | 33/45 | 4 | Yes (n = 4) | Not reported | Jordan et al. (2019)73 |
| n = 35 | Retrospective | 31/35 | 4 | Yes (n = 4) |
CsA monoRx: 3/4 cats CsA/GC: 1/4 cats |
Bizikova et al. (2019)31 |
| n = 40 | Retrospective | 36/40 | 11; CR achieved in 8/11 cats | Yes (n = 11) |
CsA monoRx: 7/28 cats CsA/GC: 5/28 cats |
Coyner et al. (2018)74 |
| n = 15 | Retrospectiveb | 15/15 | 8 |
Yes (n = 6) No (n = 2) |
CsA monoRx: 6/8 cats CsA/GC: 1/8 cats |
Irwin et al. (2012)75 |
- Abbreviations: CR, complete remission; CsA, ciclosporin A; GC, glucocorticoid; Rx, therapy.
- a Based on published supporting evidence using the following search strategy (Pubmed, Web of Science and CAB Abstract):(cat OR cats OR feline OR dog OR dogs OR canine) AND (pemphigus OR pemphigoid OR autoimmune skin OR lupus erythematosus) performed on 27 October 2023; only records in the English language are included; excluded were review publications without specific clinical case information, records not published in the English language, proceedings and abstracts.
- b Only cases in which the disease achieved complete remission were included.
Owing to the difference in the MoAs, the combination of CsA and azathioprine (AZA) as immunosuppressive agents has been used successfully to treat dogs diagnosed with PF,64, 65 PF with concurrent immune-mediated thrombocytopaenia76 and idiopathic aplastic pancytopenia.77 Because CsA and AZA target different pathways, the risk of myelosuppression from the combination can be expected to be no higher than that from AZA monotherapy, yet there are still no data available to make this conclusion and severe immunosuppression may still occur. Therefore, administration of these combinations should be carefully considered.
Adverse effects
The most common adverse effect reported with CsA is gastrointestinal (GI) upset, with vomiting (28%) and diarrhoea (14%) the most frequently reported clinical signs in dogs.78 Gastrointestinal upset is usually transient and resolves with dose reduction. Anecdotally, storing CsA capsules (Atopica) in the freezer for 30–60 min before oral administration reduces the incidence of vomiting,79 and the author has found this beneficial in preventing vomiting in some dogs. When stored in a –20°C freezer for one month, the stability and absorption of CsA in dogs is not impacted.80 Other adverse effects include gingival hyperplasia, cutaneous papillomatosis and opportunistic infections. Opportunistic bacterial (Nocardia spp., Burkholderia cepacian complex)81-83 and fungal (Alternaria spp., Curvularia spp. and Aspergillus spp.)84-86 infections have been reported in dogs treated with CsA, particularly when used in combination with GC.
Similar to dogs, the most common adverse effects of oral CsA in cats are diarrhoea and vomiting.87 In cats that were previously infected with feline herpesvirus-1 (FHV-1), administration of oral CsA at the dose of 7 mg/kg/day for 42 days could result in reactivation of FHV-1 yet the clinical signs were mild and self-limiting.88 Acute fatal toxoplasmosis secondary to CsA therapy has been reported in cats89, 90 yet is still considered rare. In a study by Lappin et al.,91 cats receiving oral CsA 42 days after experimental infection with Toxoplasma gondii did not show reactivation of toxoplasmosis and oocyst shedding. It is unlikely that toxoplasma-naïve cats would develop clinical toxoplasmosis induced by oral CsA at 7 mg/kg/day, yet measures to reduce the risk of exposure (e.g. prevent hunting and consuming raw meat) should be emphasised to cat owners. Finally, subcutaneous administration of CsA (50 mg/mL injection USP; Sandimmune, Novartis) was administered to 11 cats with nonseasonal allergic dermatitis; although all cats showed a significant decrease in Feline Dermatitis Extent and Severity Index (FeDESI) and pruritus Visual Analog Scale (PVAS) scores, five cats were withdrawn from the study; reasons for withdrawal include behavioural changes and owner-perceived lack of efficacy (n = 1), injection site adverse reaction (n = 2) and owner discomfort in the administration of the subcutaneous injections (n = 2).92 Cats receiving chronic, high-dose CsA following renal transplants had a higher incidence of neoplasia compared to control populations of cats that did not receive transplants.93-95
Azathioprine
Azathioprine is a drug that was first used to reduce the risk of organ transplant rejection.96 It is a pro-drug of 6-mercaptopurine (6-MP) that exerts its immunosuppressive effects by interfering with nucleotide synthesis. In addition to its use as a drug to prevent transplant rejection, AZA is used to treat Crohn's disease, ulcerative colitis and AD in humans; the latter as an extra-label use for moderate to severe AD that is nonresponsive to CsA.97 In dogs, a pilot study on the usage of AZA for canine AD concluded that this drug has insufficient efficacy and carries too high a risk for adverse effects (compared to CsA) to be recommended as a treatment.98
Mechanism of action
After oral ingestion, AZA is absorbed in the intestinal tract. In the intestinal wall, liver and red blood cells (RBCs), AZA is converted to 6-MP.99 Thereafter, 6-MP undergoes further conversion via three different metabolic pathways associated with three different enzymes: xanthine oxidase (XO), thiopurine-S-methyltransferase (TPMT) and hypoxanthine-guanine phosphoribosyltransferase (HPRT).96, 99 The metabolic pathways associated with XO and TPMT result in the formation of 6-thiouric acid and 6-merthymecaptopurine (6-MMP), respectively. These two metabolites have minimal to no immunosuppressive effects.97, 99 However, the metabolic pathway involving HPRT converts 6-MP to 6-thioguanine nucleotide (6-TGN) which has cytotoxic effects (Figure 2).
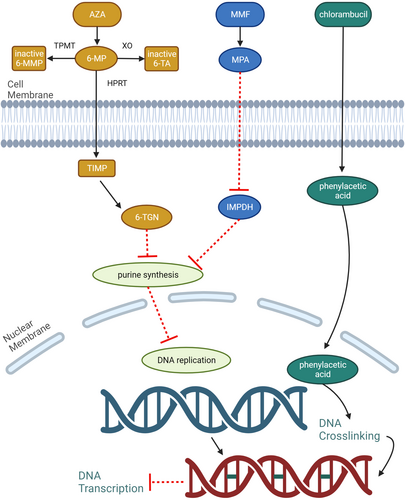
6-TGNs can be considered ‘false’ nucleotides (e.g. nonfunctioning purines). During the normal cell cycle, in the S phase, DNA is synthesised from the pairing of nucleotides. The generation of 6-TGNs ‘provides’ a pool of nonfunctioning purine, which when incorporated into DNA, results in mutation and subsequent cessation of the cell cycle. This effect is most profound in cells that are actively dividing such as lymphocytes (B and T cells) and thrombocytes. Amidotransferase enzymes and purine ribonucleotide interconversion are also inhibited by 6-TGNs, therefore reducing the formation of purine nucleotides.99 Finally, AZA and its toxic (active) metabolites affect T cell migration and adhesion, and reduce survival and proliferation of T cells through inhibition of RAS-related C3 botulinum toxin substrate-1 (RAC1) and/or B-cell lymphoma-extra large (BCL-XL).99 The anti-inflammatory effects of the metabolites from AZA also expand to nonimmune cells such as endothelial cells. 6-MP is also shown to inhibit the function of RAC1, which is important for the formation of ICAM-1 and VCAM-1.100 As such, leucocyte adhesion to endothelial cells is impaired.
Because AZA is a cytotoxic drug, it should be used with caution with other alkylating agents that interfere with DNA synthesis (e.g. cyclophosphamide) as it may lead to profound myelosuppression. There is some evidence that concurrent use of GC and AZA may increase the risk of acute pancreatitis.101, 102 Concurrent administration of allopurinol with AZA is not recommended because the antagonism of xanthine oxidase may interfere with the metabolism of AZA.103
Pharmacokinetics and pharmacodynamics
Despite a relatively long history of use of AZA in veterinary medicine, there are no published PK studies in either dogs or cats. In humans, the beneficial effects of azathioprine may take weeks to months to occur.
Use for autoimmune dermatopathies in cats and dogs
In dogs, AZA is used to treat several autoimmune skin diseases such as PF, PV, pemphigus vegetans, vesicular cutaneous lupus erythematosus (VCLE) and several variants of autoimmune subepidermal dermatoses (Table 3) owing to its effect on both B and T cells. It is often used as a steroid-sparing agent. Interestingly, AZA along with oral GC is the most common therapy at the time of optimal disease control in canine PF,109 UDS34 and canine PV.33 The use of AZA in cats is not recommended owing to the high risk of myelosuppression (see Adverse effects section below).
| Diseases (number of dogs evaluated) | Type of study | Responses | Concurrent use of oral GC when CR achieved (number of dogs) | Maintenance therapy | References | ||
|---|---|---|---|---|---|---|---|
| Complete remission | Partial remission | Misc | |||||
| Pemphigus foliaceus | |||||||
| n = 15 | Retrospective | 5/15 | 8/15 | 2/15 (poor response) | Yes (n = 15) |
AZA monoRx: 1/15 dogs AZA/GC: 14/15 dogs |
Hernandez-Bures et al. (2023)104 |
| n = 1 | Case report | 1b | No | AZA | Yun et al. (2020)64 | ||
| n = 3 | Case series | 3/3 | 1/3 dogs treated with AZA |
Yes (n = 1)c |
Similar to induction Rx | Simpson et al. (2019)65 | |
| n = 1 | Case report | 1 | Yes | AZA/GC | Sung et al. (2017)105 | ||
| n = 3 | Case series | 2/3 |
1 dog euthanized 1/2 treated with AZA |
Yes (n = 1) | Not reported | Bizikova et al. (2015)106 | |
| n = 22 | Retrospective |
Localised: 8/8 Generalised 10/13 |
2 dogs treated with AZA Rx for 10 dogs not reported |
Yes | Not reported | Oberkirchner et al. (2011)107 | |
| n = 37 | Retrospective | 28/37 | 5/37 | 11/28 dogs treated with AZA | Yes | AZA/GC: 7/11 dogs | Vaughan et al. (2010)108 |
| n = 88 | Retrospective | 46/88 | 33/46 dogs treated with AZA | Yes |
AZA/GC: 31/80 dogs AZA monoRx:9/80 dogs |
Mueller et al. (2006)109 | |
| n = 1 | Case reportd | 1 | Yes | AZA/GC | Gonsalves-Hubers et al. (2016)110 | ||
| n = 6 | Case series | 6/6 | 5/6 dogs treated with AZA | Yes (n = 5) | AZA/GC for six months, then stopped | Olivry et al. (2004)111 | |
| n = 1 | Case seriese | 1 | No | AZA stopped after four months | White et al. (2002)112 | ||
| n = 24 | Retrospective | 24/24 |
1/24 dogs treated with AZA/GC 2/24 dogs treated with AZA monoRx |
Yes (n = 1) | Similar to induction Rx | Scott et al. (1987)113 | |
| n = 1 | Case seriese | 1 | Yes | Not reported | White et al. (1987)114 | ||
| n = 34 | Retrospective | 18/34 | 2/18 dogs treated with AZA | Yes (n = 2) | AZA/GC: two dogs | Irhke et al. (1985)115 | |
| Pemphigus vulgaris | |||||||
| n = 1 | Case report | 1 | Yes | AZA/GC stopped after five weeks | Rybnicek et al. (2007)116 | ||
| n = 1 | Case seriese | 1 | Yes | Not reported | White et al. (1987)114 | ||
| n = 10 | Retrospective | 24/24 |
1/24 dogs treated with AZA/GC 2/24 dogs treated with AZA monoRx |
Yes (n = 1) | Similar to induction Rx | Scott et al. (1987)113 | |
| Pemphigus vegetans | |||||||
| n = 1 | Case report | 1 | Yes | AZA/GC | Heimann et al. (1987)117 | ||
| ECLE | |||||||
| n = 25 | Retrospective | 5/25 | 1/5 dogs treated with AZA | Yes | Not reported | Bryden et al. (2005)118 | |
| 2/25 | |||||||
| VCLE | |||||||
| n = 10 | Retrospective | 4/10 | 1/4 dogs treated with AZA | Yes | AZA/GC | Jackson et al. (2004)119 | |
| 4/10 | 4/4 dogs treated with AZA | Yes | AZA/GC | ||||
| EBA | |||||||
| n = 20 | Retrospective | 14/20 | 8/14 dogs treated with AZA | Yes (n = 8) | AZA monoRx: one dog | Bizikova et al. (2015)120 | |
| n = 1 | Case report | 1 | Yes | AZA/GC | Hill et al. (2008)121 | ||
| MMP | |||||||
| n = 11 | Retrospective | 10/11 | 1/10 dogs treated with AZA | Yes | Not reported | Tham et al. (2016)71 | |
| AISBDf | |||||||
| n = 9 | Retrospective | 6/9 | 2/6 dogs treated with AZA | Yes | Not reported | Olivry et al. (2010)122 | |
| 3/9 | 1/3 dogs treated with AZA | Yes | Not reported | ||||
| BP | |||||||
| n = 1 | Case report | 1 | 6-MP used as Rx | Yes | White et al. (1984)123 | ||
- Abbreviations: 6-MP, 6-mercaptopurine; AISBD, autoimmune subepidermal blistering disease; AZA, azathioprine; BP, bullous pemphigoid; CsA, ciclosporin A; EBA, epidermolysis bullosa acquisita; ECLE, exfoliative cutaneous lupus erythematosus; GC, glucocorticoid; MMP, mucous membrane pemphigoid; Rx, therapy; VCLE, vesicular cutaneous lupus erythematosus.
- a Based on published supporting evidence using the following search strategy (Pubmed, Web of Science and CAB Abstract):(cat OR cats OR feline OR dog OR dogs OR canine) AND (pemphigus OR pemphigoid OR autoimmune skin OR lupus erythematosus) performed on 27 October 2023; only records in the English language are included; excluded were review publications without specific clinical case information, records not published in the English language, proceedings and abstracts.
- b Defined as >90% improvement in the amount of crust and erythema.
- c The dog was also receiving PSGAG and CsA concurrently.
- d The dog has pemphigus foliaceus because basal cell involvement was not reported.
- e Only one dog was treated with AZA.
- f Clinical phenotype not determined.
Adverse effects
In humans, TPMT activity is variable and correlates with clinical outcomes and therefore, the measurement of TPMT activity is used to assess therapeutic efficacy and toxicity.124 Low activity of TMPT increases the risk of myelosuppression in people. Likewise, TPMT activity is detected in RBCs of dogs, cats and horses,125 and is highly variable in dogs.126 One study reported that Giant Schnauzers had much lower TPMT activity, whereas Alaskan Malamutes had higher TPMT activity when compared to the other dog breeds.126 Although lower TPMT activity may influence the development of myelosuppression in some dogs, there could be other pathways involved in AZA-induced myelosuppression because some dogs that experience marked myelosuppression did not have a deficiency of TPMT activity.127 More importantly, the measurement of TPMT activity in dogs thus far were from RBCs and therefore it is necessary to investigate further correlation (if any) between the activity of canine RBC TPMT and other organs that are more involved in the metabolism of AZA, such as the liver.
Compared to dogs, blood TPMT activity in cats is much lower.125 Therefore, it is reasonable to assume that the risk of myelosuppression in cats is significantly higher if treated with AZA and this drug should be avoided in cats.
Hepatotoxicity [defined as a twofold increase in alanine aminotransferase (ALT) above the upper limit of the reference interval] is another adverse effect of AZA in dogs; this may be idiosyncratic or dose-dependent. In one study that included 34 dogs, the prevalence of hepatotoxicity was 15%, with the median time to onset of 14 days (range 13–22 days).128 However, in a more recent study, the prevalence of AZA-induced hepatotoxicity was 5% when AZA was administered every other day with tapering doses of GC.129 Therefore, it is recommended that liver enzymes are routinely monitored within 2–3 weeks of initiation of AZA therapy.
CHLORAMBUCIL
Chlorambucil is an anticancer drug from the nitrogen mustard group. Other anticancer drugs in this group include melphalan, cyclophosphamide and ifosphamide.130 In humans, chlorambucil is widely used as a chemotherapy for chronic lymphocytic leukaemia and Hodgkin's lymphoma.131 Likewise, chlorambucil is most commonly used to treat low-grade T-cell lymphoma in cats. However, certain immune-mediated and autoimmune diseases have been treated with chlorambucil, more often in cats than dogs. One example is inflammatory bowel disease in cats.6
Mechanism of action
Chlorambucil is classified as an alkylating agent.132 In the liver, chlorambucil is converted into its active metabolite, phenylacetic and its cytotoxic effects are based on its ability to alkylate the nucleophilic portion of a DNA molecule through the formation of covalent bonds.130 Chlorambucil causes ‘unwanted’ cross-linking of DNA, by forming adducts at the guanine-N7 position.130 These ‘unwanted’ cross-linkages can occur within the same DNA strand (i.e. intrastrand cross-links) or with the opposite DNA strand (i.e. interstrand cross-links). This results in a defect of the DNA (e.g. mutation) which will then lead to cell death. Interstrand cross-links are most cytotoxic as they generate double-strand breaks.130 Chlorambucil has high activity against lymphoid cells (e.g. B cells) and is considered a slow-acting drug (it may take ≤2 weeks for its therapeutic effects).6
Chlorambucil is a cytotoxic agent and therefore will potentiate other immunosuppressive drugs such as vincristine, doxorubicin and cisplastin. This may lead to severe myelosuppression if used together.
Pharmacokinetics and pharmacodynamics
In the literature, there are no PK studies for chlorambucil in dogs, and only one population PK study published for cats. In cats, it is rapidly absorbed from the GI tract, reaching a peak plasma concentration of 170 ng/mL within 15 min of oral administration, although a small secondary peak also was noted at four hours. At a dose of 2 mg/cat (0.28–0.74 mg/kg), the terminal half-life was 1.8 h, with no drug accumulation following two weeks of every-other-day dosing.132 The PK of chlorambucil in cats with immune-mediated dermatoses may differ from those reported in this study, however, as the cats in the population PK analysis were being treated for lymphoproliferative malignancies, including GI involvement, which may have affected absorption. The therapeutic concentration of chlorambucil in cats is unknown and additional information is needed on this drug.
Use for autoimmune dermatopathies in cats and dogs
In the cat, similar to CsA, chlorambucil is most often used as an adjunct or steroid-sparing agent for the treatment of PF. Indeed, in two retrospective studies, combination therapy of chlorambucil with GC is often required for maintenance of CR.31, 73 Chlorambucil is less frequently used in dogs for the treatment of autoimmune dermatoses. There are two case reports of the usage of chlorambucil in dogs for the treatment of vaccine-induced ischaemic dermatopathy133 and canine eosinophilic granuloma,134 both of which required concurrent therapy with ciclosporin and oral prednisolone. Table 4 summarises the usefulness of chlorambucil for selected autoimmune dermatoses in cats and dogs.
| Diseases (number of dogs evaluated) | Type of study | Responses | Concurrent use of oral GC when CR achieved (number of cats) | Maintenance therapy | References | |
|---|---|---|---|---|---|---|
| Complete remission | Number of animals treated with CHL when CR achieved | |||||
| Pemphigus foliaceus | ||||||
| Feline | ||||||
| n = 45 | Retrospective | 33/45 | 3 | Yes (n = 3) | Not reported | Jordan et al. (2019)73 |
| n = 35 | Retrospective | 31/35 | 2 | Yes (n = 1) |
CHL monoRx: two cats CHL/GC: three cats |
Bizikova et al. (2019)31 |
| n = 37 | Retrospective | 37/37 | 1 | Yes |
CHL monoRx: two cats CHL/GC: four cats |
Simpson et al. (2013)135 |
| n = 15 | Retrospectiveb | 15/15 | 8/8 | Yes (n = 8) |
CHL monoRx: 1/8 cats CHL/GC: 6/8 cats Stopped: 1/8 cats |
Irwin et al. (2012)75 |
| n = 44 | Retrospective | 37/44 | 9/13 | Yes |
CHL monoRx: three cats CHL/GC: six cats CHL/topical GC: one cat |
Preziosi et al. (2003)136 |
| Canine | ||||||
| n = 41 | Retrospective | 28/41 | 3 | Yes | Not reported | Zhou et al. (2021)63 |
| Canine AISBD | ||||||
| n = 1 | Case report | 1 | 0c | Yes | CHL/GC | Griffin et al. (1981)137 |
- Abbreviations: AISBD, autoimmune subepidermal blistering dermatosis; CR, complete remission; CsA, ciclosporin A; GC, glucocorticoid; Rx, therapy.
- a Based on published supporting evidence using the following search strategy (Pubmed, Web of Science and CAB Abstract):(cat OR cats OR feline OR dog OR dogs OR canine) AND (pemphigus OR pemphigoid OR autoimmune skin OR lupus erythematosus) performed on 27 October 2023; only records in the English language are included; excluded were review publications without specific clinical case information, records not published in the English language, proceedings and abstracts.
- b Only cases in which the disease achieved complete remission were included.
- c CR was achieved with cyclophosphamide-oral GC therapy but changed to CHL-oral GC as maintenance Rx due to haemorrhagic cystitis.
Adverse effect
Chlorambucil is generally well-tolerated in cats and dogs, although GI effects such as inappetence, vomiting and diarrhoea may be seen. However, cytotoxic myelosuppression can occur 7–14 days after initiation of treatment.6 Other less common adverse effects include reversible myoclonus138 and Fanconi syndrome,139 both of which were reported only in cats.
MYCOPHENOLATE MOFETIL
Mycophenolate mofetil (MMF) is a pro-drug that is converted to mycophenolic acid (MPA), where it exerts its pharmacological activity. Interestingly, MPA was first isolated from Penicillium stonoliferum in 1913 where it was discovered to possess antibiotic, antiviral and anti-inflammatory properties.99 In the early 1970s, MPA's anti-inflammatory properties were utilised to treat moderate-to-severe psoriasis in humans, although it causes undesirable GI upset.99, 140 This led to the creation of MMF which has higher oral bioavailability and fewer GI adverse effects. In veterinary medicine, the use of MMF as an immunosuppressive drug for the treatment of various autoimmune diseases in dogs (and to a lesser extent in cats) has gained momentum for the past decade. Examples of these diseases include immune-mediated haemolytic anaemia, thrombocytopaenia and polyarthritis, immune-complex glomerulonephritis, meningoencephalitis and several autoimmune/immune-mediated dermatoses.
Mechanism of action
Mycophenolate mofetil exerts its immunosuppressive effect by inhibiting the formation of guanine nucleotides. Guanine is one of the four nucleotide bases that is needed for the formation of DNA. Guanine nucleotide is generated through two distinct pathways: the de novo pathway and the salvage pathway.99, 140 In the de novo pathway, the generation of guanine nucleotides requires the enzyme inosine monophosphate dehydrogenase (IMPDH). Mycophenolate mofetil, via its active metabolite MPA, inhibits the function of IMPDH, thereby reducing the number of guanine nucleotide formations available for DNA replication.99 Lymphocytes are particularly affected by this drug because they rely solely on the de novo pathway for guanine nucleotide synthesis.99, 140 The result is decreased DNA production and cessation of the proliferation of both activated T and B lymphocytes, which subsequently stop autoantibody production.
Mycophenolate mofetil also affects other nonlymphocytic cells. It has been shown to inhibit the proliferation of fibroblasts, expression of cytokines and co-stimulatory receptors, and various adhesion molecules required for leucocyte chemotaxis and mobilisation to the target cells.140
Pharmacokinetics and pharmacodynamics
The pharmacokinetics of MPA in dogs has been reported and show that oral absorption is highly variable, with maximum concentrations ranging from 380 to 5040 ng/mL and occurring at 45 min following a dose of 10 mg/kg MMF per os.141 Plasma concentrations of MPA decreased by 80% within eight hours of administration, suggesting a relatively high clearance and short half-life. In humans and, presumably, dogs, the elimination of MPA is mainly as the glucuronide conjugate. As cats are deficient in the glucuronyl transferase enzymes responsible for this reaction, there was concern that the drug could not safely be used in this species. However, there are several reports of MPA PK following MMF administration now published in cats. As with dogs, oral absorption is variable, yet as opposed to other species, the main metabolic route appears to be glucosidation.142, 143 Using a two-hour intravenous infusion model, conversion of MMF to MPA appears to be relatively slow in cats, and peak MPA concentrations do not occur until 0.75–1.5 h after stopping the MMF infusion.142 This is suggested as a potential reason for the high intra- and interindividual variability of MPA PK seen in cats, even with intravenous administration.
Using an ex vivo mitogen stimulation assay in dogs, a proposed target therapeutic concentration of between 600 and 1000 ng/mL would result in a 50% inhibition in T-cell proliferation.141 These effects are not linearly increased at higher plasma MPA concentrations, yet chronic dosing (>14 days) does seem to cause a cumulative effect, with a more prolonged duration of inhibition during the dosing interval. Chronic dosing (every 8–12 h for eight days per os or two-hour infusion i.v. every 12 h for three days) in cats did not alter total peripheral blood mononuclear cell counts, nor alter CD4+ or CD8+ cell counts.143, 144 More information is still needed on the pharmacodynamics of this drug in cats, including the safety and efficacy of long-term dosing. TDM is available at the time of this writing for MMF, MPA and other metabolites using an immunoassay. Recommended sampling times include peak (1–2 h post-dosing) and trough concentrations.
In humans, concurrent administration of CsA, antacid drugs (e.g. omeprazole) and certain antibiotics (e.g. ciprofloxacin and amoxicillin/clavulanic acid) with MMF reduces the oral bioavailability of MMF,145-147 yet this has not been recognised in animals.
Use for autoimmune dermatopathies in cats and dogs
Mycophenolate mofetil has been used to treat canine PF,148, 149 exfoliative cutaneous lupus erythematosus (ECLE),150 epidermolysis bullosa acquisita,149 VCLE149 and MCLE.151 In all but the dog with ECLE,150 MMF was used as an adjunct therapy with GC,148, 149 CsA149 and GC combined with topical tacrolimus.151 Of these autoimmune dermatoses, by far, most dogs that were treated with MMF were dogs with PF. In an earlier retrospective study, six and two of nine dogs with PF achieved a CR and PR, respectively.149 However, in a more recent retrospective study, the treatment outcome utilising combination therapy of MMF with GC for canine PF was not as favourable, as CR and PR were only achieved in two and four of eleven dogs, respectively, and none of the eleven dogs were successfully maintained in CR with MMF monotherapy.148 In the dog with ECLE, monotherapy with MMF resulted in marked improvement within three weeks.150 The variable response to oral MMF in dogs is due to a narrow therapeutic index and high inter- and intrapharmacokinetic variability.141 This further complicates the use of oral MMF to treat dogs with immune-mediated diseases because of the lack of data to define the therapeutic MFF levels in dogs. There are too few data available to make any conclusion on the usefulness of MMF, compared to other immunosuppressants, for the treatment of various autoimmune dermatoses. However, based on the information available (Table 5), MMF may produce a better clinical outcome as a steroid-sparing agent compared to being utilised as a sole therapy.
| Diseases (number of dogs evaluated) | Type of study | Responses | Concurrent use of oral GC when CR/PR is achieved (number of dogs) | Maintenance therapy | References | ||
|---|---|---|---|---|---|---|---|
| Complete remission (CR) | Partial remission (PR) | Misc | |||||
| Pemphigus foliaceus | |||||||
| n = 11 | Retrospective | 2/11 | 4/11 | 4/11 (poor response) | Yes (n = 11) |
CsA monoRx: two dogs GC/CsA or GC/AZA: four dogs |
Putra et al. (2022)148 |
| n = 41 | Retrospective | 28/41 | Not reported | 4/28 dogs treated with MMF | Yes (13/28) | Not reported | Zhou et al. (2021)63 |
| n = 1 | Case report | 1 | Nob | MMF monoRx | Yun et al. (2020)64 | ||
| n = 3 | Case series | 3/3 | 1/3 dogs treated with MMF | Yes |
MMF/GC/PSGAG |
Simpson et al. (2019)65 | |
| n = 1 | Case report | 1 | Yes | MMF/GC | Damari et al. (2018)152 | ||
| n = 9 | Retrospective | 6/9 | 2/9 | 1/9 (poor response) | Yes (n = 8) | MMF/GC: nine dogs | Ackermann et al. (2016)149 |
| MCLE | |||||||
| n = 1 | Case report | 1 | Yes | MMF/topical tacrolimus | Hyun et al. (2021)151 | ||
| Diseases (number of dogs evaluated) | Type of study | Responses | Concurrent use of oral GC during the induction phase (number of dogs) | Maintenance therapy | References | ||
|---|---|---|---|---|---|---|---|
| Complete remission | Partial remission | Misc | |||||
| ECLE | |||||||
| n = 1 | Case report | 1 | Yes | MMF/GC | Eberhardy et al. (2021)153 | ||
| n = 1 | Case report | 1 | No | MMF | Ferrigno et al. (2019)150 | ||
| VCLE | |||||||
| n = 1 | Retrospective | 1 | Yes | MMF/GC | Ackermann et al. (2016)149 | ||
| EBA | |||||||
| n = 1 | Retrospective | 1 | Yes | MMF/GC | Ackermann et al. (2016)149 | ||
| AISBD | |||||||
| n = 2 | Case report | 1 | Yes | MMF/GC | Ginel et al. (2010)154 | ||
- Abbreviations: AISBD, autoimmune subepidermal blistering dermatosis; AZA, azathioprine; CsA, ciclosporin A; EBA, epidermolysis bullosa acquisita; ECLE, exfoliative cutaneous lupus erythematosus; GC, glucocorticoid; MCLE, mucocutaneous lupus erythematosus; Misc, miscellaneous; MMF, mycophenolate mofetil; NA, not applicable; PSGAG, polysulphated glycosaminoglycan; Rx, therapy; VCLE, vesicular cutaneous lupus erythematosus.
- a Based on published supporting evidence using the following search strategy (Pubmed, Web of Science and CAB Abstract):(cat OR cats OR feline OR dog OR dogs OR canine) AND (pemphigus OR pemphigoid OR autoimmune skin OR lupus erythematosus) performed on 27 October 2023; only records in the English language are included; excluded were review publications without specific clinical case information, records not published in the English language, proceedings and abstracts.
- b 90% improvement was reported with the combination of azathioprine, modified ciclosporin and ketoconazole, but these drugs had to be discontinued due to severe neutropenia and moderate increase in alkaline phosphates, and replaced with MMF.
Adverse effects
The most common adverse effect reported in dogs receiving MMF is GI upset, particularly diarrhoea. In one recent retrospective study that included 127 dogs, 24% of dogs (n = 31) experienced GI adverse effects, of which diarrhoea was reported in 23 dogs.155 In most dogs, these GI adverse effects resolve with discontinuation or dose reduction of MMF. In cats, doses of 10 mg/kg given orally every 12 h were well-tolerated, yet 15 mg/kg per os every 12 h caused mild and self-limiting diarrhoea in some cats, while 15 mg/kg given orally every eight hours caused more severe GI upset in all cats treated.143
OCLACITINIB
Oclacitinib is a Janus kinase (JAK) inhibitor (JAKi) that was approved for the treatment of allergic dermatitis in dogs. At the approved dose for allergic dermatitis, oclacitinib has a high affinity for JAK1, which is involved in the pathogenesis of the allergic pathway.156 The human counterpart drug, ruxolitinib, was the first JAKi approved for the treatment of myelofibrosis and polycythaemia vera. Since then, many other JAKis have been approved in humans, mainly for the treatment of rheumatoid arthritis (RA); these include tofacitinib, baricitinib, fedratinib and upadacitinib.157
Mechanism of action
Janus kinases are intracellular, nonreceptor tyrosine kinases. They reside in the cytoplasm of cells and are attached to the intracellular/proximal portion of Type I and Type II cytokine receptors.156, 157 There are four family members of the JAKs—JAK1, JAK2, JAK3 and TYK2—and they play an important role in the transduction of cytokine-mediated signals through the JAK–signal transducers and activators of transcription (JAK–STAT) pathway. Upon binding of a ligand (e.g. cytokines and growth factors) to the receptor, the JAKs are activated and phosphorylate the intracellular domain of the receptor, thereby creating docking sites for the STAT molecules, which are signalling proteins. Once docked, the STAT molecules are further phosphorylated by the JAKs and then released back into the cytoplasm, where they form dimers with other phosphorylated STAT molecules. The dimerised STAT molecules translocate into the nucleus and bind with the DNA to transcript genes that regulate immunity, inflammation and haematopoiesis (Figure 3).156, 157
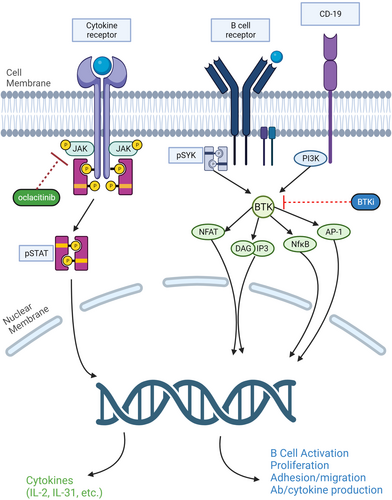
Cytokine receptors can be grouped based on which JAKs are associated with the receptor complex. While cytokines involved in allergy, inflammation and pruritus bind receptor complexes that utilise JAK1, the binding of other cytokines or growth factors involved in haematopoiesis (e.g. erythropoietin) and innate immunity (e.g. IL-12) activate receptors which are associated with the pairing of JAK2/JAK2 or JAK2/TYK2.156
Higher extra-label doses of oclacitinib are associated with immunosuppressive activity. Lymphocyte-enriched cells treated with 10 μM of oclacitinib (equivalent to 3–4 mg/kg twice daily) resulted in reduced secretion of IL-2, IL-15, IL-18 and IFN-γ, which inhibits the proliferation of T cells.158 Higher doses of oclacitinib also have been shown to induce apoptosis of canine CD4+ and CD8+ T cells in vitro.159 In a more recent study, oclacitinib prevented the generation (rather than inducing depletion) of regulatory T cells and the production of IL-10, which are important to maintain immune tolerance.160 Taken together, higher doses of oclacitinib or doses used concurrently with another immunosuppressive drug can have immunosuppressive effects on the immune system.
Pharmacokinetics and pharmacodynamics
The pharmacokinetics of oclacitinib in the dog have been published.161 It is rapidly absorbed following oral administration with 89% bioavailability, reaching a peak concentration of 259 ng/mL within one hour of administration of label doses (0.4–0.6 mg/kg once daily). There are no significant effects of feeding, sex or breed (Beagles versus cross-breeds) on oral absorption. Importantly, PK were linear over a range of 0.6–3.0 mg/kg, with dose-proportional increases in Cmax and AUC being reported. Twice-daily dosing of 0.6 mg/kg or once-daily dosing of 1.8 mg/kg, as used in the treatment of immune-mediated disease noted above, resulted in maximum concentrations of 328 and 1030 ng/mL, respectively, after 21 days of dosing.161 There is one small PK study in cats, which confirmed values similar to those found in the dog, with excellent bioavailability (87%).162 Absorption and elimination were faster in cats, compared to dogs, however, and the concentrations were more variable.
Use for autoimmune dermatopathies in cats and dogs
The first reported use of oclacitinib for the treatment of autoimmune dermatosis in veterinary medicine was in 2017 when a dog with autoimmune subepidermal blistering disease was successfully treated with oclacitinib monotherapy at the dosage of 0.5 mg/kg twice daily; a relapse occurred when the dose was tapered to once daily and resolved when it was increased to twice daily. Other autoimmune skin diseases that have been treated successfully with oclacitinib include PF in a cat,163 a dog with PV,164 and several variants of CLE (ECLE, MCLE and facial DLE) in seven dogs.165 Interestingly, in all of these cases, oclacitinib monotherapy (dosages between 0.5 mg/kg twice daily and 1.8 mg/kg once daily in dogs; 1 mg/kg twice daily in a cat) was successful in inducing and maintaining CR; time-to-CR or significant improvement also was relatively short at between one and three weeks. Oclacitinib was also used successfully to treat immune-mediated dermatoses such as hyperkeratotic erythema multiforme,166 ear tip ulcerative dermatitis167 and ischaemic dermatopathy168; the latter required a combination of oclacitinib (0.4–0.7 mg/kg twice daily) with oral GC.
Recent studies in canine CLE involving skin lesions transcriptomes150, 169, 170 showed strongly activated IFNαβ signalling via JAK–STAT with upregulation of CXCL10, ISG15 and S100, suggesting that these CLE variants represent a form of interferonopathy. Thus, treatment with oclacitinib could be an effective therapeutic option either as monotherapy or as a steroid-sparing agent. It is important to point out that the usage of oclacitinib for the treatment of autoimmune dermatopathies is considered as extra-label use in animals. Table 6 summarises the response to oclacitinib in dogs with selected autoimmune dermatopathies.
| Diseases (number of dogs evaluated) | Type of study | Responses | Concurrent use of oral GC when CR/PR is achieved (number of dogs) | Maintenance therapy: Number of animals | References | ||
|---|---|---|---|---|---|---|---|
| Complete remission (CR) | Partial remission (PR) | Misc | |||||
| CCLE | |||||||
|
FDLE n = 4 |
Retrospective | 3 | 1 (good response) | No | OCL monoRx: four dogs | Harvey et al. (2023)165 | |
|
MCLE n = 2 |
2 | No | OCL monoRx: two dogs | ||||
|
ECLE n = 1 |
1 | No | OCL monoRx: one dog | ||||
| Pemphigus foliaceus | |||||||
| n = 15 | Retrospective | 9/15 | 2/15 | 4/15 (poor response) | Yes |
OCL monoRx: three dogs OCL/GC: 12 dogs |
Hernandez-Bures et al. (2019)104 |
| n = 41 | Retrospective | 28/41 | 1 dog treated with OCL | Yes | Not reported | Zhou et al. 202163 | |
| AISBD | |||||||
| n = 1 | Case report | 1 | No | OCL monoRx | Aymeric et al. (2017)171 | ||
- Abbreviations: AISBD, autoimmune subepidermal blistering dermatosis; CCLE, chronic cutaneous lupus erythematosus; ECLE, exfoliative cutaneous lupus erythematosus; FDLE, facial discoid lupus erythematosus; GC, glucocorticoid; Misc, miscellaneous; MCLE, mucocutaneous lupus erythematosus; OCL, oclacitinib; Rx, therapy.
- a Based on published supporting evidence using the following search strategy (Pubmed, Web of Science and CAB Abstract):(cat OR cats OR feline OR dog OR dogs OR canine) AND (pemphigus OR pemphigoid OR autoimmune skin OR lupus erythematosus) performed on 27 October 2023; only records in the English language are included; excluded were review publications without specific clinical case information, records not published in the English language, proceedings and abstracts.
Adverse effects
Owing to the MoA, higher doses of oclacitinib could lead to impaired T-cell proliferation and increased risk of infection. Indeed, during the initial animal safety study, oclacitinib induced papillomas in 12-month-old dogs, and led to the development of bacterial pneumonia and generalized demodicosis in 6-month-old dogs (package insert; Apoquel, Zoetis). In a retrospective study of 53 dogs with AD treated with prolonged twice-daily administration of oclacitinib (0.4–0.6 mg/kg twice daily), pyoderma, gastrointestinal signs and otitis externa were the most common adverse effect reported; only three dogs developed mild neutropaenia.172 In an feline immunodeficiency virus (FIV)-positive cat, treatment with oclacitinib (1 mg/kg twice daily) for feline atopic skin syndrome resulted in fatal disseminated toxoplasmosis; the clinical signs developed five months after initiation of this therapy.173 In cats, doses of 1–2 mg/kg every 12 h for 28 days were shown to be safe and well-tolerated, although the higher doses did result in gastrointestinal signs in two of 10 cats.174 Long-term studies with a large number of cats have not been performed and therefore, the long-term safety of oclacitinib in cats is as yet unknown. At present, oclacitinib is not licensed for use in cats.
BRUTON'S TYROSINE KINASE INHIBITOR
Bruton's tyrosine kinase (BTK) is an important signalling protein that serves as a link between the B-cell receptor (BCR) and B-cell proliferation and survival. BTK is a Tec family tyrosine kinase that also is expressed in many other cells of haematopoietic origin, including monocytes, macrophages, neutrophils, mast cells, eosinophils and platelets and not in T cells.175, 176 The role of BTK in B-cell proliferation and survival has been associated with several B-cell malignancies in humans such as chronic lymphocytic leukaemia (CLL), mantle cell lymphoma (MCL) and Waldenstrom's macroglobulinaemia.177 More recently, there is evidence that BTK plays a critical role in autoimmunity in animal models and human studies. For instance, transgenic mice overexpress BTK in B cells, which leads to the generation of antinuclear antibodies and results in systemic lupus erythematosus (SLE)-like autoimmune pathological results.178 In humans, enhanced BTK activity is found in peripheral blood B lymphocytes from patients with RA and Sjögren's syndrome.179 Therefore, small-molecule BTK inhibitors (BTKi) have gained greater attention as a therapy for several B-cell malignancies and autoimmune diseases. Ibrutinib is the first class of BTKi that is approved for the treatment of CLL. More recently, the first noncovalent (reversible) BTKi, pirtobrutinib, was approved for the treatment of patients with relapsing or refractory MCL. Many other BTKi are still being studied in clinical trials; these include acalabrutinib (for RA), fenebrutinib (for RA) and rilzabrutinib (for PV).175
Mechanism of action
BTKi can be classified into two types: reversible inhibitors and irreversible inhibitors. Irreversible BTKi have been associated with more adverse effects as they may bind to off-target sites (e.g. endogenous thiols, which are important to protect cells from ionising radiation180, 181), while reversible BTKi may be safer yet less efficacious, depending on the degree of selectivity, clearance and maintenance of target inhibition.182 More recently, a new hybrid BTKi that can reversibly bind to BTK in a covalent manner (i.e. reversible covalent BTKi) has been developed with increased potency, prolonged duration of action and fewer off-targets effects (and, thus, fewer adverse effects).183 One example of this new hybrid BTKi is rilzabrutinib (PRN1008).182
Receptors that activate BTK are antigen receptors that include BCRs, growth factor and cytokines receptors, chemokine receptors and integrins.184 In B cells, attachment of ligands to BCR leads to activation of BTK, which triggers several downstream signalling cascades, including the PI3K-ALT pathway, PLC, PKC and NFκB, which are important for B-cell survival, proliferation and differentiation to plasma cells that produce autoantibodies.175, 184 BTK is also involved in downstream signalling vital for neutrophil recruitment such as macrophage antigen-1 (MAC-1).185
Altogether, BTK inhibition causes a blockade of different downstream signalling pathways related to the development of autoimmunity and B-cell malignancies (Figure 3).
Pharmacokinetics and pharmacodynamics
The PK/PD of these drugs are not available in dogs and cats at the time of writing.
Use for autoimmune dermatopathies in cats and dogs
The use of BTKi for the treatment of autoimmune dermatoses in veterinary medicine is still in its infancy and off-label. In 2020, two studies involving a total of 13 dogs investigated the efficacy and safety of two BTKis, PRN473 and PRN1008 (rilzabrutinib), for the treatment of PF.186, 187 Treatment outcomes were evaluated based on a modified canine version of a validated human pemphigus disease activity index (cPDAI) and classified as good, fair or poor response.187 At the end of the study period (20 weeks), five, three and two dogs had a good, fair and poor response, respectively. In all dogs, lesions reduction could be seen within 2 weeks. Interestingly, in both studies, there was no sustained depletion of B-cell counts over the 20-week period and the authors commented that this does not mean that the studied BTKi does not induce pan-B-cell deficiency.187 There were only two dogs where the frequency of administration of BTKi was able to be reduced to every other day without resulting in a relapse.187
Adverse effects
Adverse effects reported from the study involving dogs with PF treated with PRN473 included immune-mediated polyarthritis (n = 1; Week 12), mast cell tumour (n = 1; Week 4), peripheral lymphadenopathy (n = 2) and pancreatitis (n = 1).187 Of the four dogs that received PRN1008 for canine PF, only one dog had pyometra, and increased ALT and aspartate aminotransferase.186 In tolerability studies using an unrelated BTKi (G278), hepatotoxicity was reported in dogs.188
It is clear that more studies are needed to gather data on the safety profiles of BTKis in dogs, yet it is very important to be aware that the sample sizes for both studies in canine PF were small and it cannot be concluded that all of the adverse effects were a direct result of the BTKis administered. For instance, peripheral lymphadenopathy in two dogs resolved spontaneously while still receiving PRN472,187 while the one dog that developed pyometra was also an eight-year-old intact female dog.186
TOPICAL GLUCOCORTICOID AND TACROLIMUS
Topical GCs are effective adjunct therapy for many autoimmune dermatoses. Topical GCs can be used as the first-line adjunct therapy for the induction of disease remission along with other systemic immunosuppressants. They are also a valuable therapy to treat localised relapses of skin lesions without the need to increase the existing dose of systemic immunosuppressive drugs. Although the adverse effects of topical GC are less severe than systemic GC, prolonged use of topical GC could lead to cutaneous atrophy. Adrenocortical suppression as a result of chronic use of topical GC has been reported in the veterinary literature and involved the usage of potent GC such as dexamethasone, betamethasone, fluocinonide and triamcinolone.189-191 It is reasonable to use a high-potency topical GC (e.g. dexamethasone) for a short period to rapidly induce CR, after which the topical GC should either be used sparingly (e.g. twice weekly) or a nonsteroidal immunosuppressant (e.g. topical tacrolimus) can be considered. If long-term usage of topical GC is needed as an adjunct therapy to maintain CR, topical mometasone furoate may be a safer long-term option as it has minimal effect on the adrenocortical function and low systemic availability.192, 193
Tacrolimus is a calcineurin inhibitor that inhibits T-cell activation. However, unlike CsA, tacrolimus binds to FK506 binding protein, which then suppresses the activation of the NFAT pathway and inhibits early activation of T cells.99 This results in a broad range of anti-inflammatory and immunomodulatory effects similar to CsA. Topical tacrolimus is poorly absorbed into the systemic circulation and therefore, the risk of systemic immunosuppression is low.194 It also does not cause cutaneous atrophy.195 In dogs, topical tacrolimus has been effectively used as an adjunct therapy for variants of CCLE,67, 69, 151, 194, 196 pemphigus erythematosus194 and canine immune-mediated perianal fistula197, 198; most of the cases used a 0.1% concentration. In humans, the most common adverse effect of topical tacrolimus is a localised burning sensation at the site of application.194, 199 Although localised adverse effects have not been proven in animals, it is safe to assume that this may occur and the pet owner should be informed.
CONCLUSIONS
The use of immunosuppressants for the treatment of canine and feline autoimmune dermatoses requires an understanding of the MoA of these drugs and where they will be beneficial in relation to the disease pathogeneses. Glucocorticoids are almost always used as the first-line therapy to induce clinical remission. Ciclosporin and oclacitinib may be effective as monotherapy for cell-mediated autoimmune dermatoses and interferonopathic dermatopathies, respectively. Azathioprine and chlorambucil are often used as a steroid-sparing agent. Compared to the human counterparts, data on the optimum treatment and outcome for autoimmune skin diseases in cats and dogs are lacking, and therefore, treatment algorithms, consensus or guidelines remain unavailable in veterinary medicine. As such, it is important that clinicians evaluate their patients frequently and tailor the treatment regimen based on the clinical response. Finally, the usefulness of immune checkpoint inhibitors for autoimmune dermatoses warrants further studies.
AUTHOR CONTRIBUTIONS
Heng L. Tham: Conceptualization; writing – original draft; writing – review and editing; methodology. Jennifer L. Davis: Writing – original draft; writing – review and editing; software; conceptualization.
ACKNOWLEDGEMENTS
The authors would like to thank BioRender (biorender.com) for allowing the use of the software for the creation of the figures in this review article. [Correction added on 14 May 2024, after first online publication: The second sentence in Adverse Effects of OCLACITINI was corrected].
FUNDING INFORMATION
Self-funded.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.




