Longitudinal evaluation of the cutaneous and rectal microbiota of German shepherd dogs with perianal fistulas undergoing therapy with ciclosporin and ketoconazole
Christine L. Cain and Charles W. Bradley II should be considered joint senior authors.
Study presentation: Results of this study were presented, in part, in September 2019 at the 31st European Veterinary Dermatology Congress, Liverpool, UK.
Abstract
enBackground
Perianal fistulas are painful ulcers or sinus tracts that disproportionately affect German shepherd dogs and are proposed as a spontaneous animal model of fistulising Crohn's disease.
Objectives
To characterise the rectal and cutaneous microbiota in German shepherd dogs with perianal fistulas and to investigate longitudinal shifts with lesion resolution during immunomodulatory therapy.
Animals
Eleven German shepherd dogs with perianal fistulas and 15 healthy German shepherd dogs.
Materials and Methods
Affected dogs were evaluated and swabbed at three visits, 30 days apart, while undergoing treatment with ciclosporin and ketoconazole. Healthy German shepherd dogs were contemporaneously sampled. Sites included the rectum, perianal skin and axilla. The microbiome was evaluated following sequencing of the V4 hypervariable region of the 16S ribosomal RNA (rRNA) gene.
Results
Alpha diversity was not significantly different between healthy and affected dogs at each of the three body sites (p > 0.5), yet rectal and perianal beta diversities from affected dogs differed significantly from those of healthy dogs at Day 0 (p = 0.004). Rectal and perianal relative abundance of Prevotella spp. increased and perianal Staphylococcus spp. relative abundance decreased in affected dogs over time, coincident with lesion resolution.
Conclusions and Clinical Relevance
Changes in lesional cutaneous and rectal microbiota occur in German shepherd dogs with perianal fistulas and shift over time with lesion resolution during immunomodulatory therapy. Further investigations of the role of cutaneous and enteric microbiota in the pathogenesis of perianal fistulas, and whether manipulation of microbial populations may ameliorate disease, are needed.
Zusammenfassung
deHintergrund
Perianale Fisteln sind schmerzhafte Ulzera oder Sinustrakte, die Deutsche Schäferhunde unproportional häufig betreffen und als spontanes Tiermodell für die fistelbildende Morbus Crohn Erkrankung vorgeschlagen wurden.
Ziele
Eine Beschreibung der rektalen und kutanen Mikrobiome beim Deutschen Schäferhund mit perianalen Fisteln und die Untersuchung des longitudinalen Verlaufs mit einer Resolution der Veränderungen während einer immunmodulatorischen Therapie.
Tiere
Elf Deutsche Schäferhunde mit perianalen Fisteln und 15 gesunde Deutsche Schäferhunde.
Materialien und Methoden
Betroffene Hunde wurden bei drei Vorstellungen jeweils im Abstand von 30 Tagen evaluiert und jeweils ein Tupfer genommen, während die Hunde eine Behandlung mit Ciclosporin und Ketokonazol erhielten. Von den gesunden Deutschen Schäferhunden wurden gleichzeitig Proben genommen. Die Probenahmen erfolgten vom Rektum, der perianalen Haut und der Achsel. Das Mikrobiom wurde nach der Sequenzierung der V4 hypervariablen Region des 16S ribosomalen RNA (rRNA) Gens evaluiert.
Ergebnisse
Die Alpha Diversität war zwischen den gesunden und betroffenen Hunden bei keiner der drei Körperstellen signifikant verschieden (p > 0,5), trotzdem unterschieden sich die rektalen und perianalen beta Varianten bei den betroffenen Hunden signifikant von jenen der gesunden Hunde am Tag 0 (p = 0,004). Das relative Auftreten von Prevotella spp. nahm rektal und perianal zu, während das relative Auftreten von Staphylococcus spp. bei den betroffenen Hunden mit der Zeit abnahm, was mit einer Verbesserung der Läsionen einherging.
Schlussfolgerungen und klinische Bedeutung
Veränderungen der Mikrobiome der läsionalen Haut und des Rektums treten bei Deutschen Schäferhunden mit perianalen Fisteln auf und verändern sich mit der Zeit, während die Läsionen während einer immunmodulatorischen Therapie abheilen. Weitere Untersuchungen der Rolle der kutanen und enterischen Mikrobiome bei der Pathogenese der perianalen Fisteln sind nötig, sowie eine Abklärung, ob die Manipulation der mikrobiellen Populationen die Krankheit verbessern könnte.
摘要
zh背景
肛周瘘是一种疼痛性溃疡或窦道,对德国牧羊犬的影响尤为突出,被提议作为自发瘘道性克罗恩病的动物模型。
目的
表征患有肛周瘘的德国牧羊犬的直肠和皮肤微生物群,并研究免疫调节治疗期间病变消退的纵向变化。
动物
患有肛周瘘的德国牧羊犬11只,健康的德国牧羊犬15只。
材料和方法
患犬在接受环孢素和酮康唑治疗的同时,每隔 30 天就诊 3 次,进行评估和拭子检测。 同时对健康的德国牧羊犬进行了采样。 部位包括直肠、肛周皮肤和腋窝。 对 16S 核糖体 RNA (rRNA) 基因的 V4 高变区进行测序后评估微生物组。
结果
健康犬和患犬在三个身体部位的 Alpha 多样性没有显着差异 (p > 0.5),但患犬的直肠和肛周 β 多样性在第 0 天与健康犬显着不同 (p = 0.004) 。 直肠和肛周普雷沃菌属的相对丰度。 肛周葡萄球菌增多。 随着时间的推移,患犬的相对丰度下降,与病变消退一致。
结论和临床意义
患有肛周瘘的德国牧羊犬的病变皮肤和直肠微生物群发生变化,并且在免疫调节治疗期间,随着时间的推移和病变消退而变化。 需要进一步研究皮肤和肠道微生物群在肛周瘘发病机制中的作用,以及微生物群的控制是否可以改善疾病。
Résumé
frContexte
Les fistules périanales constituent des ulcères ou des fistules douloureux, qui affectent de manière disproportionnée les bergers allemands et sont proposées comme modèle animal spontané de la maladie de Crohn fistulisante.
Objectifs
Caractériser le microbiote rectal et cutané chez les bergers allemands atteints de fistules périanales et en réaliser l’analyse longitudinale avec la résolution des lésions au cours d'une thérapie immunomodulatrice.
Animaux
Onze bergers allemands atteints de fistules périanales et 15 bergers allemands sains.
Matériels et méthodes
Les chiens atteints sont évalués cliniquement et subissent des prélèvements lors de trois visites, à 30 jours d'intervalle, durant un traitement à base de ciclosporine et de kétoconazole. Des bergers allemands sains sont échantillonnés en même temps. Les sites collectés sont le rectum, la peau périanale et axillaire. Le microbiome est évalué par séquençage de la région hypervariable V4 du gène de l'ARN ribosomal (ARNr) 16S.
Résultats
La diversité alpha n'est pas significativement différente entre les chiens sains et les chiens atteints sur chacun des trois sites corporels (p > 0,5), mais les diversités bêta rectale et périanale des chiens atteints différent significativement de celles des chiens sains au jour 0 (p = 0,004). L'abondance relative rectale et périanale de Prevotella spp. augmente et l'abondance relative périanale de Staphylococcus spp. diminue chez les chiens affectés au fil du temps, coïncidant avec la résolution de la lésion.
Conclusions et pertinence clinique
Des changements dans le microbiote cutané et rectal lésionnel se produisent chez les bergers allemands atteints de fistules périanales et évoluent dans le temps avec la résolution des lésions durantt la thérapie immunomodulatrice. Il est nécessaire de poursuivre les recherches sur le rôle du microbiote cutané et entérique dans la pathogénie des fistules périanales et de déterminer si la manipulation des populations microbiennes peut améliorer la maladie.
要約
ja背景
肛門周囲瘻は、ジャーマン・シェパード・ドッグに非対称性に発症する疼痛性潰瘍または瘻管であり、瘻孔を形成するクローン病の自然発症モデルとして提案されている。
目的
本研究の目的は、肛門周囲瘻を有するジャーマン・シェパード・ドッグの直腸および皮膚の微生物叢の特徴を明らかにし、免疫調節療法中の病変の消失に伴う縦断的な変化を調査することであった。
対象動物
肛門周囲瘻を有するジャーマン・シェパード・ドッグ11頭および健常ジャーマン・シェパード・ドッグ15頭。
材料と方法
シクロスポリンおよびケトコナゾールによる治療中、罹患犬に対し30日間隔で3回の来院時に評価およびスワブ採取を行った。健常ジャーマン・シェパード・ドッグも同時に採取した。採取部位は直腸、肛門周囲皮膚、腋窩であった。16SリボソームRNA(rRNA)遺伝子のV4超可変領域の塩基配列を決定し、マイクロバイオームを評価した。
結果
α多様性は3部位それぞれにおいて健常犬および罹患犬で有意差はなかったが(p > 0.5)、罹患犬の直腸および肛門周囲のβ多様性は0日目の健常犬と比較して有意差があった(p = 0.004)。罹患犬の直腸および肛門周囲のプレボテラ属菌の相対量は経時的に増加し、肛門周囲のブドウ球菌属菌の相対量は病変の消失とともに減少した。
結論と臨床的関連性
肛門周囲瘻を有するジャーマン・シェパード・ドッグでは、病変部の皮膚および直腸の微生物叢に変化が生じ、免疫調節療法中の病変の消失とともに経時的に変化した。肛門周囲瘻の病態における皮膚および腸内細菌叢の役割、ならびに微生物集団の操作が疾患を改善するかどうかについてのさらなる研究が必要である。
Resumo
ptContexto
As fístulas perianais são úlceras dolorosas ou tratos fistulosos que afetam desproporcionalmente os cães da raça pastor alemão e são propostas como um modelo animal espontâneo de doença de Crohn fistulosa.
Objetivos
Caracterizar a microbiota retal e cutânea em cães pastor alemão com fístulas perianais e investigar alterações longitudinais com resolução da lesão durante terapia imunomoduladora.
Animais
Onze cães pastor alemão com fístulas perianais e 15 cães pastor alemão saudáveis.
Materiais e Métodos
Os cães afetados foram avaliados e amostras foram coletadas em três visitas, com 30 dias de intervalo, durante o tratamento com ciclosporina e cetoconazol. Cães pastor alemão saudáveis foram amostrados simultaneamente. Os locais incluíram reto, pele perianal e axila. O microbioma foi avaliado após sequenciamento da região hipervariável V4 do gene 16S do RNA ribossômico (rRNA).
Resultados
A alphadiversidade não foi significativamente diferente entre cães saudáveis e afetados em nenhum dos três locais do corpo (p > 0,5), mas a betadiversidades retal e perianal de cães afetados diferiram significativamente daquelas de cães saudáveis no Dia 0 (p = 0,004). Houve aumento na abundância relativa retal e perianal de Prevotella spp. e redução na de Staphylococcus spp. perianal a abundância relativa diminuiu nos cães afetados ao longo do tempo, coincidente com a resolução da lesão.
Conclusões e Relevância Clínica
Alterações na microbiota lesional cutânea e retal ocorrem em cães da raça pastor alemão com fístulas perianais e mudam ao longo do tempo com a resolução da lesão durante a terapia imunomoduladora. São necessárias mais investigações sobre o papel da microbiota cutânea e intestinal na patogênese das fístulas perianais e se a manipulação de populações microbianas pode melhorar a doença.
RESUMEN
esIntroducción
las fístulas perianales son úlceras dolorosas o tractos sinusales que afectan desproporcionadamente a los perros pastores alemanes y se proponen como un modelo animal espontáneo de la enfermedad de Crohn fistulizante.
Objetivos
Caracterizar la microbiota rectal y cutánea en perros pastores alemanes con fístulas perianales e investigar los cambios longitudinales con resolución de la lesión durante la terapia inmunomoduladora.
Animales
Once perros pastores alemanes con fístulas perianales y 15 perros pastores alemanes sanos.
Materiales y métodos
Los perros afectados fueron evaluados y se obtuvieron muestras en tres visitas, con 30 días de diferencia, mientras recibían tratamiento con ciclosporina y ketoconazol. Al mismo tiempo se tomaron muestras de perros pastores alemanes sanos. Los sitios incluyeron el recto, la piel perianal y la axila. El microbioma se evaluó tras la secuenciación de la región hipervariable V4 del gen del ARN ribosomal (ARNr) 16S.
Resultados
La diversidad alfa no fue significativamente diferente entre perros afectadis y perros sanos en ninguna de las tres localizaciones (p= 0,5). Sin embargo la diversidad beta en las zonas rectal y perianal fue significativamente diferente entre perros afectados y sanos en el día 0 (p = 0,004). La abundancia relativa rectal y perianal de Prevotella spp. aumentó y la perianal de Staphylococcus spp. disminuyó en animales afectados con el transcurso del tiempo, coincidiendo con la resolución de la lesión.
Conclusiones y relevancia clínica
Se producen cambios en la microbiota cutánea y rectal de las lesiones en perros pastores alemanes con fístulas perianales y cambian con el tiempo con la resolución de la lesión durante la terapia inmunomoduladora. Se necesitan más investigaciones sobre el papel de la microbiota cutánea y entérica en la patogénesis de las fístulas perianales y si la manipulación de las poblaciones microbianas puede mejorar la enfermedad.
INTRODUCTION
Perianal fistulas are painful ulcers or sinus tracts that spontaneously develop in the skin and subcutis around the anus and predominantly affect German shepherd dogs.1-4 Accompanying dyschezia, tenesmus and histological evidence of colitis are common.5-7 A shared genetic basis for canine perianal fistulas, Crohn's disease and ulcerative colitis in humans has been suggested,8 and dogs have been proposed as a spontaneous model of human fistulising Crohn's disease.7, 9
Canine perianal fistulas are recognised to be immune-mediated and a dysregulated immune response to microbes in the anal or perianal region has been proposed.1, 10, 11 Faecal dysbiosis in dogs with perianal fistulas as compared with healthy dogs has been reported,7 yet the cutaneous and mucosal microbiota, and shifts of the microbiota associated with disease progression and remission, have not been investigated.
Oral ciclosporin is the current mainstay of medical management of canine perianal fistulas, with or without concurrent ketoconazole.12-19 Ketoconazole competitively inhibits metabolism of ciclosporin by hepatic cytochrome P450 microenzymes and inhibits P-glycoprotein-mediated efflux of ciclosporin into the intestinal lumen, increasing ciclosporin bioavailability and allowing dose reduction.16 Immunomodulatory therapy is often effective for management of canine perianal fistulas, yet long-term administration is typically required, and relapses are common with cessation.1 Prolonged immunomodulatory therapy may be prohibitively expensive for dog owners, and some dogs may be euthanised as a consequence of debilitating disease or adverse effects of therapy. Further investigations of the role of the cutaneous and enteric microbiota in this disease, and whether manipulation of microbial populations may alleviate disease, are needed.
The aims of this prospective study were to characterise the cutaneous and rectal microbiota in German shepherd dogs with perianal fistulas as compared with healthy German shepherd dogs; to investigate longitudinal shifts in the microbiota associated with lesion remission during therapy; and to evaluate the impact of immunomodulatory therapy (ciclosporin and ketoconazole) on the microbiota of a cutaneous site distant from the perianal region (the axilla).
MATERIALS AND METHODS
Ethics
The Institutional Animal Care and Use Committee of the Institutional Animal Care and Use Committee of the University of Pennsylvania (protocol 806,142) approved all procedures, and owner informed consent was obtained before study enrolment.
Study subject selection
German shepherd dogs were diagnosed with perianal fistulas based on the presence of characteristic perianal ulcers and/or sinus tracts. Owners provided information regarding clinical signs, diet, and any medications or supplements administered in the 30 days before each visit.
Dogs with perianal fistulas received modified ciclosporin (Atopica, Elanco; or generic) at 2–4 mg/kg/day and ketoconazole at 5–10 mg/kg/day. Other systemic or topical antimicrobial or immunomodulatory agents were not permitted during the study period, and affected dogs were not excluded if they were already receiving modified ciclosporin with or without ketoconazole if active lesions were present and dose adjustment was planned owing to ethical concerns associated with a withdrawal period. Dogs with perianal fistulas were evaluated and sampled at enrolment [Visit (V)1], at Day (D)30 (V2) and D60 (V3) of treatment (±2 days). Comorbidities were permitted in affected dogs, so long as no treatments were changed during the study period.
Healthy German shepherd dogs had no history of dermatological or gastrointestinal disease, normal physical and dermatological examinations, and received no antimicrobial or immunomodulatory therapies for 90 days before study enrolment. All healthy dogs were sampled at D0, and a subset of six healthy dogs were sampled at D30 to investigate stability of the cutaneous and rectal microbiota over time.
Clinical assessment
Affected dogs were evaluated by the same board-certified referral clinician at each visit. The number of perianal ulcers or sinus tracts was noted, the length and width of each lesion were measured (in mm) using callipers, and these measurements were used to calculate the total cross-sectional fistula area. Lesion depth was not measured owing to potential discomfort as sedation was not employed for most evaluations. Lesion improvement was assessed at V2 and V3 by changes in fistula number, area and severity, and each dog was assigned a score of >50% improvement, ≤50% improvement, no change or worsening lesions. The Wilcoxon signed-rank test was used to compare total fistula area between visits.
Microbiota sampling
The rectum, axilla and either the site of a perianal fistula or the perianal region at the junction of haired skin and the anus were swabbed using Catch-All Sample Collection Swabs (Epicentre Biotechnologies). Swabs were rubbed over the skin sites (3 cm2) for 10–15-s intervals and were inserted approximately 1 cm into the rectum. Swabs exposed to air at sampling and laboratory at extraction were used as negative control samples. Swabs were placed in 300 μL of Yeast Cell Lysis Solution (Epicentre Biotechnologies), and the tip of the swab was aseptically cut from the handle and stored at −80°C until extraction.
16S rRNA gene sequence analysis
Bacterial DNA extractions were performed as described previously.20 Identical DNA extractions also were performed on swab control samples, which never came into contact with skin, and water-only samples. These negative control samples, as well as a mock community pool containing purified genomic DNA from 12 known bacterial isolates, were amplified and sequenced in parallel to experimental samples.
A dual-index amplicon sequencing method was employed for PCR amplification of the V4 region of the 16S ribosomal RNA (rRNA) gene.21 The primer set 515F-806R was employed. Amplicons were quantified with Pico-green and sequenced on a MiSeq platform (Illumina) using 250-bp paired-end chemistry. Sequences were processed and analysed using QIIME2 (v2020.11.1). The QIIME2 plug-in DADA2 was used for sequence denoising and quality filtering, including size filtering to 224–250 bps. Taxonomic assignment was performed using a Naïve Bayes classifier trained on the Silva 138 99% database. Sequences were aligned using the QIIME2 MAFFT plug-in, and a phylogenetic tree was produced from this alignment using the FastTree plug-in.
Statistical methods
Statistical analysis and visualisation was performed using the (v1.38.0) and vegan (v2.6–2) packages in the R statistical computing environment.22, 23 Contaminants were identified based on prevalence in negative controls, and those labelled as chloroplasts and mitochondria were removed as described previously.21 During decontamination steps,24 sequences assigned to the Escherichia-Shigella genus were noted to be present in negative control samples and abundantly present in test samples (Figure S4) and were selectively included in the analysis (Figures S5–S9b).
Alpha and beta diversity indices were calculated using the phyloseq package. Beta diversity was calculated using the weighted UniFrac phylogenetic distances. Principal coordinate analysis (PCoA) was then performed on this distance matrix and visualised. Analysis of similarities (ANOSIM) and permutational multivariate analysis of variance (PERMANOVA) as implemented by the adonis function in the vegan package were used to compare differences between groups by disease status and body site. Redundancy analysis (RDA), plotting and permutation test for constrained correspondence analysis were performed with the vegan package and the anova.cca function. The R stats package (3.6.3) was used for all statistical computations. The Wilcoxon signed-rank test was used to compare differences in alpha diversity between groups.
RESULTS
Study subjects
Eleven German shepherd dogs with perianal fistulas and 15 healthy German shepherd dogs were enrolled. Signalment information for both groups is summarised in Table 1.
| Affected (n = 11) | Healthy (n = 15) | |
|---|---|---|
| Median age in months (range) | 72 (36–120) | 72 (24–132) |
| Sex M:F ratio (neutered) | 8:3 (7) | 12:3 (7) |
| Mean weight in kg (range) | 40.7 (31.7–47.0) | 33.7 (28.0–46.2) |
Clinical features of affected dogs are summarised in Table 2 (Table S1 includes more individual information about the affected dogs). Calculated total doses of ciclosporin were determined by doubling the daily dose of ciclosporin in combination with ketoconazole.25 Five dogs (45.5%) had acute disease of ≤3 months duration, while six dogs (54.5%) had chronic disease of >3 months duration. Three of the six dogs with chronic disease were receiving ciclosporin with or without ketoconazole at enrolment, and all underwent dose adjustment of ciclosporin and/or addition of ketoconazole. None of the dogs with acute disease had previously received ciclosporin. The mean dose of ciclosporin for affected dogs in this study was 3.0 mg/kg/day (range 2.1–4.4 mg/kg/day), and the mean dose of ketoconazole was 6.9 mg/kg/day (5.1–8.5 mg/kg/day).
| V1 | V2 | V3 | |
|---|---|---|---|
| Dogs with diarrhoea n (%) | 7 (63.6) | 6 (54.5) | 4 (36.4) |
| Dogs with tenesmus n (%) | 6 (54.5) | 7 (63.6) | 4 (36.4) |
| Dogs with haematochezia n (%) | 3 (27.3) | 1 (9.1) | 1 (9.1) |
| Dogs with ≥ 50% lesion resolutionn (%) | — | 5 (45.5) | 9 (81.8) |
| Median number of perianal fistulas (range) | 2 (1–4) | 2 (0–3) | 1 (0–2) |
| Mean fistula area in mm (range) | 169 (40–400) | 102 (0–450) | 116 (0–1000) |
Figure S1 shows Dog 20's lesions at V1 and following study conclusion. Dog 18 had full lesion resolution at V2, which was maintained at V3. Dog 24 had <50% lesion resolution at V3; this dog also was suspected to have a chronic enteropathy characterised by weight loss and hypocobalaminaemia. Dog 21 had worsened disease at V3; this dog had concurrent exocrine pancreatic insufficiency, and the owner also inadvertently discontinued ketoconazole following V2. The Wilcoxon signed-rank test indicated that fistula area at V3 was not statistically different than fistula area at V1 when all dogs were considered (Z = −1.96; p = 0.05). When Dog 21 was excluded from the analysis, fistula area at V3 was significantly decreased compared with fistula area at V1 (Z = −2.80; p < 0.005).
Microbial community structure
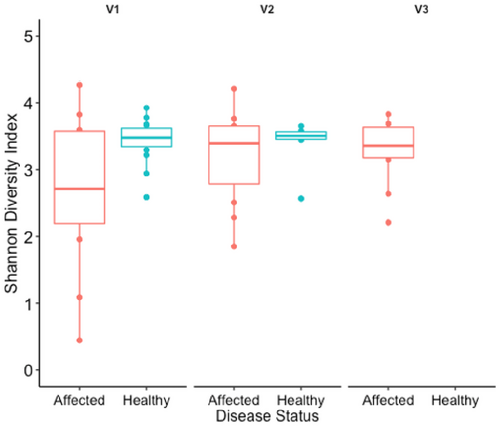
A total of 189 samples and 15 negative controls (204 samples total) were processed with a sum of 6,830,661 sequences (mean 31,918 reads/sample). Alpha diversity, measured by the Shannon diversity index, was not significantly different between affected and healthy dogs at each body site (rectal, axilla, and perianal or fistula site) at V1 (p > 0.5; Figure 1). Rectal alpha diversity of affected dogs was lower than in the healthy dogs and was lowest at V1 (Figure 2). Dogs with acute disease had lower rectal alpha diversity than dogs with chronic disease at the V1 although this difference was not statistically significant (p = 0.33; Figure S2). Alpha diversity at the axilla and fistula sites of affected dogs were stable, overall, at each visit (p > 0.5; Figure 1), with some variation over time at each site among individual dogs (Figure S3). Dog 18 was the only dog to achieve full lesion resolution at V2, which was maintained for V3 coincident with increased rectal alpha diversity from V1 to V2, maintained at V3 and decreased perianal alpha diversity from V1 to V3 (Figure S3).
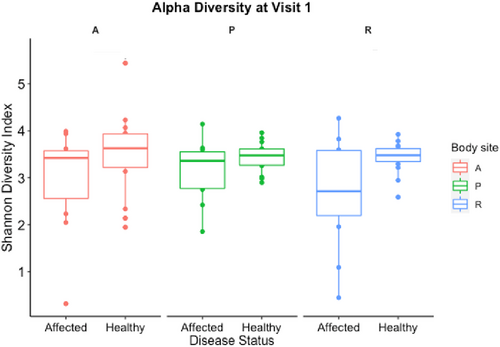
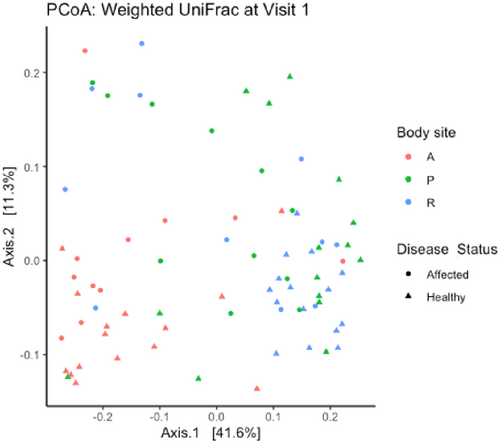
Beta diversity (differences between samples) from each body site of healthy and affected dogs at V1 is depicted by the principal coordinates analysis (weighted Unifrac metric) plot in Figure 3. Beta diversity differed significantly between body sites (independent of disease status; R = 0.24, p = 0.001; F = 8.18, p = 0.001) and between affected and healthy dogs (independent of body site; R = 0.14, p = 0.001; F = 6.12, p = 0.001). There was greater variation in beta diversity among rectal and fistula site samples of affected dogs than in healthy dogs (Figure 3). Beta diversity of rectal or fistula site samples from affected dogs differed significantly from that of healthy dogs at V1 using the weighted UniFrac metric (R = 0.38, p = 0.004). Further multivariate analysis (RDA) was performed to explore the relationship between disease status and site (predictor variables) with community composition (response variables) for the perianal and rectal sites at V1 (Figure S10). In this model, both disease status and site were significant factors to explain the variance in community composition (disease: variance = 21.25; F = 3.031; p = 0.001; Site: variance = 11.64; F = 1.65; p = 0.018) with disease status as a greater contributor.
Microbial community composition
Overall, similar bacterial taxa were identified from the perianal skin and rectum of healthy dogs; Bacteroides and Prevotella were the predominant bacterial genera identified, with lower abundance of Corynebacterium, Megamonas and Pseudomonas (Figure 4a). The fistula site of affected dogs had higher relative abundance of Staphylococcus and lower relative abundance of Prevotella than the perianal skin of healthy dogs at V1 and V2 (Figure 4b). At V3, Staphylococcus was less abundant from the fistula site with a corresponding increase in Prevotella relative abundance. Other predominant bacterial genera at the fistula site of affected dogs (with some individual variation over the three visits) were Bacteroides and Corynebacterium. Dog 18 had high abundance of Pseudomonas at the rectal and perianal sites, which was maintained over visits. The overall bacterial community composition of the fistula site of affected dogs approximated that of the perianal skin of healthy dogs at V3.
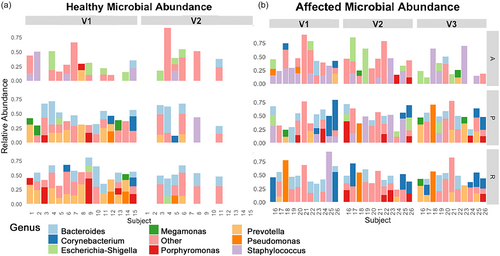
The relative abundance of Prevotella was lower from the rectum of affected dogs than from the rectum of healthy dogs at V1 and V2, and increased at V3. The overall rectal bacterial community composition of affected dogs approximated that of healthy dogs at V3. Other predominant bacterial genera from the rectum of affected dogs over the study visits were Bacteroides and Corynebacterium.
Staphylococcus was the predominant bacterial genus from the axilla of both affected and healthy dogs at V1, with higher relative abundance from the axilla of affected dogs as compared with healthy dogs. Overall, bacterial community composition from the axilla of affected dogs was stable between visits.
DISCUSSION
This study is the first to prospectively characterise the microbiota of German shepherd dogs with perianal fistulas with regard to lesion remission and the impact of ciclosporin therapy. The enteric microbiota heavily influences the perianal microbiota based on similarities between the rectal microbiota and perianal microbiota compared to more distant cutaneous sites. Findings are similar to those reported previously for the canine perianal region and nonmucosal haired skin with sequencing of the V4 hypervariable region of the 16S rRNA gene.26 Similarity of the rectal and fistula-associated microbiota of children with fistulising Crohn's disease was recently demonstrated, too.27
A combination of ciclosporin and ketoconazole was utilised in this study in an attempt to standardise therapy as much as possible while maximising cost-effectiveness for dog owners. Clinical trials have demonstrated efficacy of this combination for canine perianal fistulas,17-19 yet the increased risk of adverse effects (such as rare idiosyncratic hepatotoxicity with ketoconazole),28 regional prescribing regulations and potential for individual variation in ciclosporin serum levels29, 30 must be carefully weighed when using these medications together.
Faecal dysbiosis of German shepherd dogs with perianal fistulas, characterised by increased abundance of Bacteroides vulgatus and Escherichia coli, and decreased abundance of Megamonas spp. and Prevotella copri, was reported previously.7 Reduced relative abundance of Prevotella spp. from the rectum and perianal skin of dogs with perianal fistulas compared with healthy controls, which increased over time coincident with lesion resolution, was also found in this study. Shifts in the enteric microbiota are likely to be involved in the pathogenesis of canine perianal fistulas, as they are for Crohn's disease in humans.31 Tenesmus and diarrhoea were common in this study and persisted in some dogs beyond lesion improvement, suggesting that colitis was present. Changes in relative abundance of bacterial taxa and alpha diversity associated with histopathological evidence of inflammation or fibrosis from colonic biopsies of dogs with perianal fistulas have been reported.7 Similar alpha diversity trends were identified as in the previous study with affected German shepherd dogs having slightly lower, and not significantly different, Shannon diversity compared to healthy controls.7 Rectal alpha diversity of affected dogs in the present study increased over time to more closely approximate that of the healthy dogs, although this change was not statistically significant. Microbial diversity is also likely to be influenced by disease chronicity. Dogs with acute disease of <3 months duration also had lower, and not significantly different, rectal Shannon diversity than dogs with chronic disease at V1.
Bacteroidetes, which includes both Bacteroides and Prevotella, is commonly identified in the enteric microbiota across mammalian hosts. The balance between these genera can shift, potentially because they occupy similar intestinal microenvironmental niches.32, 33 These genera may also play a role in regulating inflammation, both within and outside the gastrointestinal tract. Some strains of Prevotella spp. may contribute to promotion of Th17-driven immune responses in human periodontal disease and rheumatoid arthritis.34, 35 Prevotella histicola, by contrast, may suppress disease activity in rheumatoid arthritis and multiple sclerosis.34, 36 These findings, together with the results of the present study, suggest that populations of Bacteroidetes (Prevotella spp. in particular) may play a critical role in immune homeostasis. Manipulation of these populations may represent a promising therapeutic option for inflammatory and immune-mediated disease.
Increased relative abundance of Staphylococcus from perianal fistula sites as compared with the perianal skin of healthy dogs was demonstrated in this study. Staphylococcus is a component of the resident microbiota in this region26 and can be isolated from rectal swabs.37 The increased abundance of Staphylococcus in affected dogs was likely to have been related to the presence of a persistent ulcer or sinus tract. Colonisation by Staphylococcus spp. is common in chronic cutaneous wounds.38 Differentiation of microbial colonisation from true infection may be difficult in the context of chronic wounds. Most dogs experienced marked lesion improvement and a concurrent decrease in perianal Staphylococcus abundance without targeted anti-staphylococcal therapy. Evidence of staphylococcal dermatitis such as papules, pustules, crusts or epidermal collarettes also was lacking in affected dogs. These findings support a nonpathogenic role for Staphylococcus spp. in canine perianal fistulas, although a potential role for microbial biofilms or virulence factors of colonising bacteria in delayed lesion healing warrants further evaluation.39
The increased relative abundance of Staphylococcus from the axilla of dogs with perianal fistulas as compared with healthy dogs was an unexpected finding. Although the reason for this is unknown, a dysfunctional innate immune response to microbes may play a role in the pathogenesis of perianal fistulas. This immune dysfunction may also influence barrier function.40 German shepherd dogs are predisposed to other immune-mediated or inflammatory disorders of the skin and mucosa including inflammatory bowel disease, cutaneous and mucocutaneous lupus erythematosus, deep pyoderma, atopic dermatitis (AD) and metatarsal fistulas.41 Dogs with AD also have a higher cutaneous burden of Staphylococcus,42 and Staphylococcus is a major contributor to differences in axillary microbial community composition between allergic and nonallergic German shepherd dogs.43 Only one dog (Dog 17) in this study had a documented clinical diagnosis of AD. Axillary microbial community composition of affected dogs was stable over time. This finding supports the minimal impact of ciclosporin therapy on the microbiota of nonlesional skin, as has been demonstrated previously in a small group of atopic dogs treated for 1 month with an immunomodulatory dose of ciclosporin.44
Functional defects of innate immune pattern recognition receptors, which recognise microbial pathogen-associated molecular patterns, are of particular interest in dogs with perianal fistulas. Single-nucleotide polymorphisms in the NOD2 gene and NOD2 dysfunction in monocytes/macrophages are associated with inflammatory bowel disease and perianal fistulas, respectively, in German shepherd dogs.10, 45 Likewise, mutations in the NOD2 gene, encoding for the intracellular nucleotide-binding oligomerisation domain 2 (NOD2) pattern recognition receptor, increase susceptibility to Crohn's disease.46 NOD2-deficient mice have an altered cutaneous microbiota and abnormal antimicrobial peptide expression associated with skin injury, contributing to delayed wound healing.47 Further studies are needed to elucidate the role of innate immune dysfunction on the cutaneous and enteric microbiota of perianal fistulas and other chronic inflammatory conditions in German shepherd dogs.
This study has some limitations. Perianal fistulas are distinct and are easily diagnosed in German shepherd dogs, and standard therapy is well-known. Three dogs (27%) were receiving ciclosporin at initial presentation. Although all dogs had active disease and underwent ciclosporin dose adjustment and/or addition of ketoconazole, prior immunomodulatory therapy may have affected the microbiota. Additionally, individual variation in microbial community structure and composition can occur over time or with different sampling methods.48 The V4 hypervariable region of the 16S rRNA gene was selected for analysis to allow comparison to prior studies assessing the canine cutaneous, mucocutaneous and enteric microbiota,20, 43, 49 yet is not without inherent limitations. For example, staphylococcal speciation may be better discerned with the V1–V3 hypervariable region.26, 42, 50 Study methodology should be closely scrutinised when making comparisons. Further studies sampling more diverse populations of German shepherd dogs are necessary to confirm these results.
CONCLUSIONS
Changes in the cutaneous and rectal microbiota occur in German shepherd dogs with perianal fistulas and shift over time during immunomodulatory therapy with lesion resolution. The role of the cutaneous and enteric microbiota in the pathogenesis of canine perianal fistulas, and whether manipulation of microbial populations, specifically Prevotella spp., may ameliorate disease, should be further investigated. Ciclosporin therapy appears to minimally impact the microbiota of nonlesional skin, yet dysbiosis may not be limited to lesional skin. German shepherd dogs with perianal fistulas may have a more widespread dysfunctional immune response to microbes.
AUTHOR CONTRIBUTIONS
Christine L. Cain: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; writing – review and editing; supervision; formal analysis. Ellen White: Methodology; investigation; writing – original draft; writing – review and editing; formal analysis; software; data curation; validation. Lindsey E. Citron: Investigation; funding acquisition; writing – review and editing. Qi Zheng: Methodology; writing – review and editing; software; formal analysis; resources; data curation. Daniel O. Morris: Conceptualization; writing – review and editing; methodology. Elizabeth A. Grice: Methodology; writing – review and editing; funding acquisition; formal analysis; software; supervision; data curation; resources. Charles W. Bradley II: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; writing – review and editing; formal analysis; supervision.
ACKNOWLEDGEMENTS
The authors acknowledge and thank Katherine Backel and Kathryn Rook for assistance with subject recruitment and enrolment, Max Grogan for analytical support and the PennVet Working Dog Center for assistance with subject recruitment and sampling.
FUNDING INFORMATION
Support for this work was provided by awards from the PennVet Center for Host-Microbial Interactions; the Penn Skin Biology and Diseases Resource-based Center, funded by NIH/NIAMS grant P30-AR069589; National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (T32-AR007465; to EW and EAG); and National Institutes of Health/Boehringer Ingelheim Summer Research Program (to LEC).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.




