Identification of 5α-reductase isoenzymes in canine skin
Abstract
enBackground
Alopecia X in dogs is a noninflammatory alopecia that may be caused by a hormonal dysfunction. It may be similar to androgenic alopecia in men that is caused by the effect of dihydrotestosterone (DHT). The 5α-reductase isoenzymes, 5αR1 and 5αR2, and a recently described 5αR3, are responsible for the conversion of testosterone into DHT. However, which 5α-reductases are present in canine skin has not yet been described.
Objectives
The main objective of this study was to determine the pattern of expression of 5α-reductase genes in canine skin.
Methods
Skin biopsies were obtained from healthy, intact young-mature beagles (three males, four females) at three anatomical sites normally affected by alopecia X (dorsal neck, back of thighs and base of tail) and two sites generally unaffected (dorsal head and ventral thorax). Prostate samples (n = 3) were collected as positive controls for 5α-reductase mRNA abundance measurement by real-time PCR.
Results
We detected mRNA encoding 5αR1 and 5αR3 but not 5αR2. There were no significant differences in 5αR1 and 5αR3 mRNA levels between the different anatomical sites, irrespective of gender (P > 0.05). Moreover, the mean mRNA abundance in each anatomical site did not differ between males and females (P > 0.05).
Conclusions and clinical importance
To the best of the authors' knowledge, this is the first study demonstrating the expression of 5α-reductases in canine skin and the expression of 5αR3 in this tissue. These results may help to elucidate the pathogenesis of alopecia X and to determine more appropriate treatments for this disorder.
Résumé
frContexte
L'alopécie X chez le chien est une alopécie non-inflammatoire qui peut être causée par une anomalie hormonale. Elle est semblable à l'alopécie androgénique de l'homme causée par l'effet de la dihydrotestostérone (DHT). Les iso enzymes 5α-réductases, 5αR1 et 5αR2, et une 5αR3 récemment décrite, sont responsables de la conversion de la testostérone en DHT. Cependant, les 5α-réductases présentes dans la peau du chien n'ont pas encore été décrites.
Objectifs
Le principal objectif de cette étude était de déterminer le patron d'expression des gènes de 5α-réductase dans la peau canine.
Méthodes
Des biopsies cutanées ont été obtenues de beagles jeunes à âgés, intactes (3 mâles et 4 femelles), sains sur trois sites anatomiques classiquement atteints par l'alopécie x (cou dorsal, arrière des cuisses, et base de la queue) et deux sites généralement épargnés (dessus de la tête et thorax ventral). Des échantillons prostatiques (n = 3) ont été prélevés comme contrôles positifs pour la mesure par PCR en temps réel de la quantité d'ARNm de 5α-réductase.
Résultats
Nous détectons l'ARNm codant pour 5αR1 et 5αR3 mais pas 5αR2. Il n'y avait pas de différence significative entre les taux d'ARNm de 5αR1 et de 5αR3 entre les différents sites anatomiques, quelque soit le genre (P > 0.05). En outre, la quantité moyenne d'ARNm dans chaque site anatomique ne variait pas entre les mâles et les femelles (P > 0.05).
Conclusions et importance clinique
A la connaissance des auteurs, ceci est la première étude démontrant l'expression de 5α-réductases dans la peau de chien et démontrant l'expression de 5αR3 dans ce tissu. Ces résultats peuvent aider à élucider la pathogénie de l'alopécie X et à déterminer des traitements plus appropriés pour cette atteinte.
Resumen
esIntroducción
la alopecia X en perros es una alopecia no inflamatoria que puede ser causada por una disfunción hormonal. Hay un alopecia similar androgénica en humanos que está causada por el efecto de la dihidrotestosterona (DHT). Las isoenzimas de la 5α-reductasa, 5αR1 y 5αR2, y una más recientemente descrita 5αR3, son responsables de la conversión de la testosterona en dihidrotestosterona. Sin embargo, no se ha descrito que reductasa 5α está presente en la piel canina.
Objetivos
el principal objetivo de este estudio fue determinar el patrón de expresión de los genes de 5α-reductasa en la piel canina.
Métodos
se obtuvieron biopsias de piel de perros sanos, enteros, jóvenes de raza Beagle (tres machos, cuatro hembras) de tres zonas anatómicas distintas normalmente afectadas por alopecia X (cuello dorsal, parte trasera de los muslos, y base de la cola) y de otras zonas normalmente no afectadas (cabeza dorsal y tórax ventral.). Se obtuvieron muestras de próstata (n = 3) como controles positivos para valorar la abundancia de mRNA de 5α-reductasa mediante PCR a tiempo real.
Resultados
detectamos mRNA codificando 5αR1 y 5αR3 pero no 5αR2. No hubo diferencias significativas en los niveles de mRNA 5αR1 y 5αR3 entre los diferentes lugares anatómicos en machos ni hembras (P > 0,05). Aún más, la abundancia media de mRNA en cada lugar anatómico no fue diferente entre machos y hembras (P > 0,05)
Conclusiones e importancia clínica
a nuestro entender es el primer estudio demostrando la expresión de las 5α-reductasas en la piel canina y demostrando la expresión de la 5αR3 en este tejido. Estos resultados pueden ayudar a determinar la patogénesis de la alopecia X y determinar tratamientos más apropiados para esta enfermedad.
Zusammenfassung
deHintergrund
Die Alopezia X des Hundes ist eine nicht entzündliche Alopezie, die durch eine hormonelle Störung verursacht sein könnte. Es ist ähnlich wie die androgene Alopezie des Menschen, die durch die Wirkung von Dihydrotestosteron (DHT) verursacht wird. Die 5α-Reduktase Isoenzyme, 5αR1 und 5αR2, und ein unlängst beschriebenes 5αR3, sind verantwortlich für die Umwandlung von Testosteron in DHT. Es wurde jedoch bisher nicht beschrieben, welche 5α-Reduktasen in der Haut des Hundes vorkommen.
Ziele
Das hauptsächliche Ziel dieser Studie war es, das Muster der Exprimierung des 5α-Reduktase Gens in der Hundehaut zu bestimmen.
Methoden
Es wurden Hautbiopsien von gesunden, intakten jung-adulten Beagles (3 männliche, 4 weibliche) an drei anatomischen Stellen, die normalerweise von Alopezia X betroffen sind (dorsal am Hals, kaudale Oberschenkel und Schwanzansatz) und von zwei Stellen, die normalerweise nicht betroffen sind (dorsal am Kopf und ventral am Thorax) genommen. Es wurden Prostataproben (n = 3) als positive Kontrollen für quantitative Messungen der 5α-Reduktase mRNA mittels real-time PCR genommen.
Ergebnisse
Wir fanden mRNA, welche 5αR1 und 5αR2, nicht aber 5αR3 codierte. Es bestanden keine signifikanten Unterschiede zwischen 5αR1 und 5αR3 mRNA Werten zwischen den verschiedenen anatomischen Lokalisationen, egal um welches Geschlecht es sich handelte (P > 0,05). Darüber hinaus unterschied sich die durchschnittliche mRNA Häufung an den diversen anatomischen Stelle nicht zwischen männlichen und weiblichen Tieren (P > 0,05).
Schlussfolgerungen und klinische Bedeutung
Nach bestem Wissen der Autoren handelt es sich hierbei um die erste Studie, die die Exprimierung der 5α-Reduktasen in der Hundehaut sowie die Exprimierung von 5αR3 im Gewebe demonstriert. Diese Ergebnisse könnten helfen, die Pathogenese der Alopezia X zu erhellen und dazu beitragen, sinnvollere Behandlungsmöglichkeiten für diese Erkrankung zu finden.
要約
ja背景
イヌにおけるアロペシアXはホルモン性機能不全により生じる可能性のある非炎症性脱毛症である。ジヒドロテストステロン(DHT)の影響により生じるという点でヒトにおける男性型脱毛と類似している。5αR1や5αR2の5αレダクターゼアイソエンザイムや最近述べられた5αR3はテストステロンからDHTへの変換に関係している。しかし、どの5α−レダクターゼがイヌの皮膚に存在しているかは示されていない。
目的
この研究の主な目的はイヌの皮膚における5α−レダクターゼ遺伝子の発現パターンを解明することである。
方法
健康で去勢や避妊されていない若い成犬のビーグル(3頭のオス、4頭のメス)の皮膚生検を、通常アロペシアXに罹患する(頚部背側、大腿部後面および尾根部)解剖学的な部位を3ヶ所および一般的に罹患していない部位(頭部背側および胸部腹側)から採取した。前立腺サンプル(n = 3)を陽性コントロールとして回収し、5α-レダクターゼmRNA量はリアルタイムPCRで測定した。
結果
筆者らは5αR1および5αR3をコードしているmRNAを特定したが、5αR2は特定できなかった。性別に関係なく、異なった解剖学的な部位の間に5αR1 ならびに5αR3mRNAレベルの有意差は見られなかった(P > 0.05)。さらに、それぞれの解剖学的な部位における平均mRNA量はオスとメスの間で変わらなかった(P > 0.05)。
結論および臨床的な重要性
筆者が知る限り、これはイヌの皮膚における5αレダクターゼの発現およびこの組織において5αR3の発現を示した最初の研究である。これらの結果はアロペシアXの病因の解明およびこの疾患に対するより適切な治療を究明することを助ける可能性がある。
摘要
zh背景
犬的X型脱毛是由激素失调引起的非炎症脱毛。与人的雄激素性脱毛相似,其由二氢睪酮(DHT)作用引起。5α-还原酶如5αR1、5αR2、以及最近发现的5αR3,可将睾酮转换为DHT。然而,5α-还原酶在犬皮肤中是否存在还未被研究过。
目的
本次试验的主要目的为判断5α-还原酶基因在犬皮肤中的表达模式。
方法
从健康、未绝育成年比格犬(3只公犬、4只母犬)上取皮肤活检样本,取3个X型脱毛常发的解剖部位(颈背、大腿后侧和尾根)以及2个通常不发病的区域(头背侧和胸腹侧)。采集前列腺样本(n = 3)作为阳性对照,通过实时PCR进行5α-还原酶mRNA丰度测定。
结果
我们检测到mRNA编码5αR1和5αR3而不编码5αR2。5αR1和5αR3的mRNA等级在不同的解剖位点没有显著差别,与性别无关(P > 0.05)。此外,各个解剖位点的平均mRNA丰度没有性别差异(P > 0.05)。
总结与临床意义
据作者所知,本研究是第一次演示5α-还原酶在犬皮肤中的表达,以及5αR3在该组织中的表达。结果有助于阐述X型脱毛的发病机理,并确定此病更适宜的治疗方法。
Introduction
Alopecia X (hair cycle arrest) has been described in dogs as a noninflammatory alopecia that affects predominantly ‘plush-coated’ breeds (chow chow, keeshound, Siberian husky, Alaskan malamute and Pomeranian) and miniature poodles.1 This condition occurs in both sexes, and generally in adult dogs. The initial clinical sign is the loss of hair in frictional areas (around the neck, tail, caudal thighs and perineum). Gradually, the alopecia establishes in the truncal region and eventually the exposed skin may become hyperpigmented. The head and distal legs usually remain unaffected. The reason why some areas are more affected by alopecia X than others remains unknown.1-3
The pathogenesis of this condition is unclear and several hypotheses have been proposed. Genetic predisposition to a nonidentified hormonal imbalance,4 primary disorder of the hair growth cycle,5 and imbalance of gonadal and/or adrenal hormones may be involved3, 6 However, there is strong evidence of androgen influence on alopecia X. Treatments shown to induce hair regrowth include deslorelin,7 a gonadotropin releasing hormone superagonist, anti-androgenic drugs such as osaterone acetate,8 and surgical castration.9, 10 These results suggest that this disorder is influenced by sex hormone modulation and could resemble androgenic alopecia (AGA) in humans, which is characterized by a genetic predisposition to progressive decline in the duration of the anagen phase of hair follicle growth caused by dihydrotestosterone (DHT).11 DHT causes continuous miniaturization of androgen-responsive hair follicles which is accompanied by perifollicular fibrosis of follicular units.12 However, the exact pathomechanism by which DHT induces AGA is not well understood.
DHT is an androgen hormone converted from testosterone by the 5α-reductase (5αR) isoenzymes. In humans, type 1 (5αR1) and type 2 (5αR2) isoenzymes, and a recently described type 3 5αR (5αR3)13 are associated with DHT production. Studies of patients with AGA indicate a correlation between 5α-reductase levels and hair loss in the affected areas.14 However, it is not known which 5α-reductases are present in the skin in dogs.
The main objective of this study was to determine the pattern of expression of 5α-reductase genes in normal canine skin. This is a crucial preliminary step for future studies that aim to clarify a potential correlation between 5α-reductase activity and alopecia X in dogs.
Material and methods
Study population
All procedures related to animal care and experimentation were approved by the institutional animal care and use committee and followed the guidelines established by the Canadian Council on Animal Care. Seven healthy, unrelated, intact beagle dogs (three males and four females) were used for this study. The animals had no history of drug treatments or skin disease and their ages varied from 9 months to 3 years (mean age 1.5 years). All dogs came from the same colony (CiToxLAB, Laval, QC, Canada) and were housed in the same manner in a controlled environment (21°C; artificial photoperiod of 12 h light and 12 h of darkness).
Biopsy collection
Skin samples were obtained with an 8-mm biopsy punch immediately after euthanasia, from three anatomical sites typically affected by alopecia X (dorsal neck, back of thighs and base of tail) and two sites typically unaffected (dorsal head and ventral thoracic region). Prostate samples were collected as positive controls for 5α-reductase gene expression; three samples were taken from random parts of the prostate from three dogs and pooled for each animal. The samples were stored in RNAlater (Qiagen, Mississauga, ON, Canada) at −80°C until RNA extraction.
Nucleic acid extraction and RT-PCR
Total RNA was extracted from the skin biopsies using TRIzol (Invitrogen Life Technologies, Burlington, ON, Canada) according to the manufacturer's instructions and quantified by absorbance at 260 nm. Total RNA (1 μg) was first treated with 1 U of DNase (Promega, Madison, WI, USA) at 37°C for 5 min to digest any contaminating DNA, followed by incubation at 65°C for 5 min for DNase inactivation. The RNA was reverse-transcribed in the presence of 1 mmol/L oligo(dT) primer and 4 U of Omniscript RTase (Omniscript RT Kit, Qiagen; Mississauga, ON, Canada), 0.25 mM dideoxy-nucleotide triphosphate (dNTP) mix and 19.33 U of RNase Inhibitor (GE Healthcare, Baie-d'Urfé, QC, Canada), in a volume of 20 μL at 37°C for 1 h. The reaction was terminated by incubation at 93°C for 5 min.
Real-time PCR was performed in a real-time thermal cycler (CFX96; BioRad, Mississauga, ON, Canada) with SsoAdvanced Universal SYBR Green (BioRad). Canine-specific primer sequences were taken from the literature for ribosomal protein L13a (RLP13A),15 whereas those for 5αR1, 5αR2 and 5αR3 were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA). Amplicons of 5αR1 and 5αR3 spanned exon–exon boundaries, whereas the amplicon of 5αR2 was located within exon 2; all primers had optimal annealing temperatures of 60°C (sequences given in Table 1). PCR was performed with dilutions of 1:32, 1:4, 1:2 and 1:2 cDNA for gene RLP13A, 5αR1, 5αR2 and 5αR3, respectively. Common thermal cycling parameters (3 min at 95°C, 40 cycles of 45 s at 95°C, 30 s at 60°C and 30 s at 72°C) were used to amplify each transcript. Amplicon identity was established by sequencing products of new primers and by melting-curve analyses conducted after every reaction. Samples were run in duplicate, with a coefficient of variation of 0.2, 0.4 and 0.6% (Ct values) for RLP13A, 5αR1 and 5αR3, respectively. Data were expressed relative to RLP13A, a stably expressed housekeeping gene in canine whole skin, and were normalized to a calibrator sample using the Pfaffl ΔCt-based relative quantification method with correction for amplification efficiency.16
| Gene | Sense (5′ – 3′) | Antisense (3′ – 5′) | Accession number |
|---|---|---|---|
| 5αR1 | CGCAACGGTCCTTGATTT | GGGCATCGGCTTTCCT | NM_001287100.1 |
| 5αR2 | ACTCATTGCTCACTAGAGG | CTCAGCGCAGTAAATCAGA | NM_001287064.1 |
| 5αR3 | GCTGTGATTCACGTCGTCCA | ACATAGACGTTCCTGCCATCC | XM_539274.4 |
Statistical analysis
Data did not follow a normal distribution (Shapiro–Wilk test) and were transformed to logarithms. Anatomic region and/or animal gender were used as main effects in the F-test. Differences between means were tested with the Tukey–Kramer HSD test. All analyses of data were performed with JMP software (SAS Institute, Cary, NC, USA). Data are presented as means ± SEM.
Results
mRNA encoding 5αR1, 5αR2 and 5αR3 was detected in canine prostate, with mean Ct values of 32, 31 and 32. In canine skin we detected 5αR1 and 5αR3 with mean Ct values of 34 and 31, respectively; 5αR2 mRNA was not detected after 40 cycles of amplification. There were no significant differences in 5αR1 and 5αR3 mRNA levels in skin samples between the different anatomical sites generally affected by alopecia X and sites typically unaffected by alopecia X (P > 0.05). Moreover, the mean mRNA abundance for both 5αR1 and 5αR3 in each anatomical site did not differ between males and females (P > 0.05; Figure 1a). The average of mean mRNA values combined for all five anatomical sites was not significantly different between the sexes for 5αR1 and 5αR3 (P > 0.05; Figure 1b).
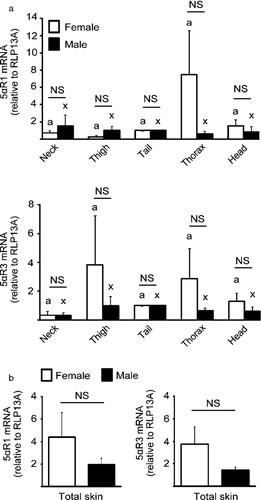
Discussion
Androgenic alopecia in humans involves DHT production from local activity of 5α-reductase isoenzymes in the skin of affected areas.17, 18 We have shown for the first time, to the best of the authors' knowledge, that 5αR1 and 5αR3 – but not 5αR2 – are expressed in the skin of healthy dogs. Previous studies have identified the isoforms 5αR1 and 5αR2 in the prostate, adrenal gland, cerebellum, cerebrum, liver, testis, cardiac muscle, bladder wall, lung and pectoral muscle of dogs.19-21 Our finding of mRNA encoding 5αR1 and 5αR3 in canine skin is consistent with studies in humans, in which 5αR3 is the predominant isoenzyme detected and 5αR2 levels are low in comparison to the other isoenzymes.22, 23 Our inability to detect 5αR2 mRNA in canine skin may represent a species-specific difference, or it could be that the mRNA levels in the canine skin samples were too low to be detected by qPCR.
In terms of the pattern of gene expression, there is a correlation between 5α-reductase levels and AGA in people. Both young men and women had higher levels of 5αR1 and 5αR2 in areas affected by AGA as compared to normal areas.14 In the present report, we failed to find a significant difference for 5αR1 and 5αR3 mRNA levels between the different anatomic regions studied, irrespective of gender. These results were not unexpected because healthy animals with no history of skin disorder were used. It therefore remains to be determined whether mRNA encoding 5α-reductases may be more abundant in affected areas than in unaffected areas in dogs with alopecia X.
Our results also indicate that 5αR1 and 5αR3 mRNA levels do not vary between males and females. Considering that alopecia X does not occur exclusively in intact males, this may suggest that the local conversion of testosterone into DHT in the skin may be a crucial step for the pathogenesis of this disorder in dogs. In humans, cutaneous 5α-reductases convert testosterone entering the skin from the circulation into DHT, and hair follicles affected with AGA demonstrate an increase in 5α-reductase activity and consequently increased local levels of DHT.23-25 Although we have evaluated 5α-reductases at the mRNA level and not their activity, our results suggest the existence of a DHT conversion system in the skin of dogs.
The 5αR3 gene was first identified in human prostate cancer cells and knock-down of the enzyme significantly reduced DHT secretion,13 demonstrating the relevance of this isoform for DHT production. The presence of this enzyme in skin and prostate may have implications for the therapeutic use of 5α reductase inhibitors, as inhibitors may not be equally effective against all three isoforms. The two predominant inhibitors used in the treatment of AGA in humans are finasteride and dutasteride.26, 27 Their use in dogs for treatment of alopecia has not been reported, although they are used in dogs to treat prostate cancer.19, 28-30 Both compounds inhibit 5αR1 and 5αR2 activities; finasteride is selective for 5αR2 and dutasteride is selective for 5αR1 and 5αR2.The ability of these drugs to inhibit 5αR3 is not clear, as one study showed potent inhibition of 5αR3 activity by dutasteride in HEK293 cells stably expressing this gene,23 whereas another study demonstrated that dutasteride did not effectively inhibit exogenous 5αR3 activity in a hamster ovary cell line.31 Finasteride appears to be 50-fold less potent than dutasteride in the inhibition of 5αR3,23 and as canine skin had very low levels of 5αR2, it seems likely that dutasteride would be the inhibitor of choice for use in dogs.
In summary, the results of the present study demonstrate for the first time the pattern of mRNA expression for 5αR1 and 5αR3 in the skin of healthy dogs. Further studies are needed to investigate the distribution, characteristics and activity of 5αR isoenzymes in canine skin. We intend to expand our investigations by studying the potential implications of 5αR activity to the pathogenesis of alopecia X, the results of which may help to determine more appropriate treatments for this disorder.
Acknowledgements
The authors wish to thank CiToxLAB North America for their technical support and tissue collection.




