Hematocrit estimates comparing centrifugation to a point-of-care method in beef cattle living at high altitude
Abstract
Background
The hematocrit (Hct) or packed cell volume (PCV) reflects the blood volume occupied by red blood cells. The development of point-of-care (PoC) instruments can accelerate the ease of measuring Hct/PCV compared with traditional capillary centrifugation (TCC) methods. However, no studies have compared Hct/PCV levels in cattle at high elevation with other measurement methods.
Objectives
In this study, we aimed to compare methods to estimate Hcts/PCVs of rangeland cattle at high elevation. We specifically wanted to determine if Hct/PCV levels measured with a commercial PoC instrument (i-Stat with CHEM8+ cartridges [PoCi]) were comparable to Hct/PCV levels measured with traditional laboratory methods.
Methods
We assessed the Hct/PCV of 94 mature beef cattle (black Angus; Bos taurus) at ~2195 m above sea level using paired analyses of the PoCi and TCC methods from each animal. We used paired samples t-tests to compare mean Hct/PCVs. Correlation analyses relative to the line of identity and Passing-Bablok regression were used to assess systematic and proportional differences, respectively, and Bland-Altman plots were used to assess agreement between the two methods.
Results
The PoCi estimated a Hct of 28.2% ± 0.7% (SE), which was lower than the TCC estimated PCV of 39.2% ± 0.5%. The Bland-Altman plot revealed poor agreement between the two methods in addition to a −11% bias for the PoCi. The Passing-Bablok regression revealed both systematic and proportional bias between the two methods.
Conclusions
Point-of-care blood instruments were not comparable to TCC methods for quantifying Hct/PCVs of cattle living at high elevations.
1 INTRODUCTION
Since the advent of automated hematology analyzers, the terms hematocrit (Hct) and packed cell volume (PCV) have been used interchangeably. Importantly, however, Hct is a value calculated by multiplying the mean corpuscular volume (MCV) by the red blood cell (RBC) count, whereas the PCV is directly measured after blood sample centrifugation. Regardless, both measurements reflect the blood volume occupied by red blood cells, expressed as a percentage.1 From a clinical pathology perspective, Hct/PCV is an insightful hematologic measurement because it reflects the ability of oxygen transport in the circulatory system and can indicate an animal's physiologic condition.2 For hematologic and oncologic purposes, the Hct/PCV is a component of the complete blood count, along with hemoglobin concentrations and white blood cell counts, which can help diagnose anemia, erythrocytosis, inflammation, leukemia, bone marrow failure, and adverse drug reactions.3 Specifically for bovine veterinary medicine and production agriculture, the Hct/PCV can be influenced by reproductive status (gestation and lactation), can vary by season and animal age, and can also be affected by diet.4, 5 Sex can also influence the Hct/PCV, as reported for female cattle that were shown to have significantly higher levels than their male counterparts.6 Finally, the environment that animals interact with also influences Hct/PCV levels.7 Hct/PCVs increase as elevation increases; however, the association between Hct/PCV levels and elevation at altitudes above 1470 m is less understood.8
Early methods for measuring PCV were developed in the 1920s, and for decades, laboratory procedures consisted of centrifuging blood and quantifying the proportion of red blood cells relative to the plasma volume.3, 9 Over time, the need for hematologic analyses that were more rapid and logistically efficient led to technological innovation in the development of point-of-care (PoC) hand-held blood analyzers.10 PoC instruments increasingly offer clinicians and researchers rapid chemistry/electrolyte, hematology, blood gas, coagulation, endocrinology, and cardiac marker results.
Early assessments of PoC instruments compared with commercial high-throughput dry chemistry analyzers for Hct provided a coefficient of variation (CV) of less than 3.5%, which is lower than the 5% generally suggested for large automated instruments.11 However, more recent assessments comparing laboratory methods with PoC instruments for Hct, hemoglobin (Hb), Na, and K, found that the PoC instrument consistently underestimated all hematologic parameters.12 The reported low bias was within US CLIA standards for all hematologic parameters with the exception of Hcts.12 Researchers have specifically found similar falsely low Hct levels when measured with other PoC instruments.13 In an experiment comparing three PoC instruments, researchers concluded that important clinical differences and limitations of the measurements should caution clinicians from relying on PoC data as the only information for blood transfusion decisions.14
Thus, given (1) the importance in understanding Hct/PCV values for veterinary clinical pathology, diagnoses, and treatments, (2) the implications of variable animal-environment interactions particularly related to Hct elevations, and (3) questions about the accuracy and precision of PoC instruments for measuring Hct, we developed a comparative experimental design.15 In this study, PoC instrument-measured Hcts were compared with PCVs measured using traditional laboratory methods established specifically for veterinary clinical pathologists in cattle living at high elevations.15
2 MATERIALS AND METHODS
2.1 Animal use and hematological procedures
Following the University of Wyoming (UW) Institutional Animal Care and Use Committee protocol (UW-IACUC) 20190516DS00364-01 approved on May 16, 2019, we collected blood samples from 94 beef cattle on May 20, 2019. With a range ratio of 2.6 and 2.1 for the PoC and traditional capillary and centrifugation (TCC) methods, respectively, this sample size was determined to be sufficient in detecting two deviations from the slope and intercept.16 The cattle of this study reside on native rangeland and improved hay meadows near Laramie, WY, USA (~2200 m above sea level) and were provided by the UW Agriculture Experiment Station's Beef Unit. All animals were in the early-lactation stage with calves averaging 46 days old. Blood samples were acquired by first physically immobilizing cattle in a hydraulic squeeze chute and then collecting blood. Blood was collected using a 3-mL vacutainer with a 20-gauge needle to draw <3 mL of blood from the coccygeal vein with access via a palpation cage at the rear of the animal. After the first few animals were sampled, vacutainers were replaced with 3mL syringes to further decrease the time between the blood draw and analysis. Paired blood samples from each animal were simultaneously analyzed to determine Hct/PCV levels, as indicated by Hct/PCV, using the following two methods17: (1) PoC instrument, specifically an i-Stat with CHEM8+ (Abbott Laboratories, Abbott Park, IL, USA) cartridges (referred to as PoCi, indicating a specific combination of the manufacturer and cartridge type) and (2) TCC using a laboratory bench top microhematocrit capillary tube (Medline Industries, Northfield, IL, USA) method with centrifugation and visual assessment against a microhematocrit card. All venipuncture and hematologic procedures were conducted by Veterinarians and Animal Care Specialists of the US Army's 438th Medical Detachment—Veterinary Service Support.
2.2 Statistical analyses
We first calculated the mean, standard deviation, minimum, and maximum Hct/PCV values for the PoCi and TCC methods, and then calculated the absolute difference (Δ; delta) between the values. Prior to all analyses, the Hct/PCV was converted to proportions (0 to 1) and analyzed for normality using a Shapiro-Wilks test, which indicated a deviation from normality (W statistic = 0.897; P < .001). We then applied the arcsine transformation for all analyses. To compare mean Hct/PCV values derived from the two methods, we first ran a paired samples t-test at the 95% confidence level. Then, to determine method correlations, we plotted the PoCi method on the y-axis and TCC method on the x-axis and plotted a line of identity where x = y (ie, the 1:1 line). Then, we applied linear least squares regression to assess the coefficient of determination (r2) as an indicator of correlation strength. We also performed a Pearson correlation test and calculated the Pearson correlation coefficient (r) of the sample.15 We then performed Passing-Bablok regression to compare the two methods and calculated between-cattle variation using the coefficient of variation (CVG) for each method.15 Coefficient of variations were calculated for the 94 cattle, and one sample per cow was analyzed using the TCC and POCi methods. Finally, we calculated the combined inherent imprecision of the two methods and plotted the interval lines where differences between the methods should fall if they are identical and where the inherent imprecision of both methods should fall.15
3 RESULTS
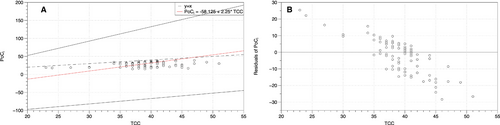
The mean PoCi Hct was 28.2% (± 6.6% standard deviation [SD], CV 23.4%) and the mean TCC PCV was 39.2% (±4.4% SD, CV 11.2%) (Table 1), and the differences were statistically significant (t = −15.17, df = 93, P <.001; Figure 1A). The mean absolute difference (Δ) between the PoCi and TCC values was −11% (±6.8%, CV −61.8%) (Table 1). PoCi values were lower than TCC values 99% of the time (93 out of 94 cattle). The minimum-maximum range for PoCi values was 15%-39%, and that for TCC values was 23%-51%, and the Δ range between PoCi and TCC values was 0%-29%. Moreover, the PCV values derived from the two methods were poorly correlated (r2 = 0.08); Pearson correlation (r) = 0.288. The Bland-Altman plot revealed poor agreement between the two methods as well as a −11% bias (Figure 1B). The Passing-Bablok regression and residuals revealed both systematic and proportional bias between the two methods as 0 was not included in the intercept CI (−127.0 to −27.5) and 1 was not included within the slope CI (1.5-4.0) (Figure 2A,B). Finally, PoCi had more than 2x greater between-cow variation than TCC as indicated by CVG (23.4% and 11.2% respectively; Table 1).
| Mean | StDev | 1CVG |
Range [Minimum - Maximum] |
95% Credible Interval [Lower, Upper] |
Range Ratio | |
|---|---|---|---|---|---|---|
| PoC Hct | 28.2% | ± 6.6 | 23.4% | 15%-39% | 26.84%-29.54% | 2.60 |
| TCC PCV | 39.2% | ± 4.4 | 11.2% | 23%-51% | 38.25%-40.04% | 2.22 |
| Δ PCV | −11.0% | ± 6.8 | −29%-0% |
Note
- 1CVG indicates between-cow variation following Jensen and Kjelgaard-Hansen (2006). Δ PCV = PoC Hct-TCC PCV.
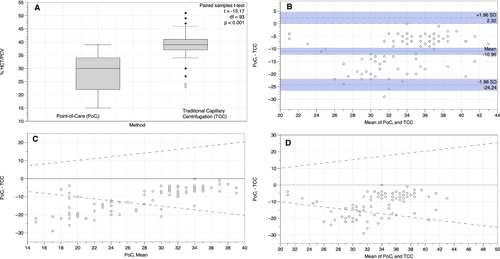
Examining the inherent combined imprecision (Figure 1C,D) of the two methods clearly shows that Hct/PCV derived from PoCi and TCC methods are not identical and cannot be used interchangeably, given that less than 95% of the differences fall within the dotted lines (1.96 * combined inherent CV of the two methods [1.96*0.2589]) and the differences are not symmetrically distributed around zero.
4 DISCUSSION
In this comparison of methods to estimate Hct/PCV, PoCi underestimated Hct/PCV values by a margin of 11%, suggesting that the two methods might not be interchangeable. Evaluating the inherent imprecision of both methods visually and with multiple quantitative methods, we found that the two methods are indeed not identical and cannot be used interchangeably. The bias seen with the PoC method in underestimating Hct/PCV has also been shown in human medicine.12, 13 While the technological features of the PoCi could be due to confounding factors in our study, we also surmise that the expected elevations in the Hct/PCV values seen in this study are likely caused by the high-elevation habitats of these animals.18, 19 The causes of these Hct/PCV differences must be understood, particularly if they are due to the methodology, because diagnoses could vary depending on the method used.
Falsely low values have important implications for diagnostic interpretations in veterinary clinical pathology and medicine. Falsely high Hct/PCV values could lead to a suspicion of secondary polycythemia as triggered by increased erythropoietin production that occurs under hypoxic conditions; the primary causes being high altitude (physiologic) or pulmonary/cardiac disorders (pathologic).17 In addition, renal tumors, cysts, and/or hereditary polycythemia could also be possible in certain breeds of cattle or poultry.17, 20, 21 Clinicians unaware of falsely low Hct/PCV values, coupled with lower reported hemoglobin concentrations, might try to sort out the many complex, and at times comorbid, causes of anemia in the affected animals.
5 CONCLUSIONS
In our methods comparison study, 94 beef cattle living in high-elevation rangeland habitats yielded a significantly different Hct/PCV between the PoCi and TCC methods. These results also agree with a prior study indicating that PoC technologies falsely under report Hct levels compared with traditional centrifugation techniques in human medicine.12 Although we documented significant differences between Hct/PCV estimation methods, PoC technologies continue to play a vital and emerging role in veterinary medicine. The number of diagnostic tests that can be performed on PoC devices has grown exponentially and is anticipated to continue to grow as the technology advances. More empirical investigations are needed to further analyze the causes of these differences that are attributed to technology and/or environmental factors. Ultimately, the Hct values obtained with PoC technology must be carefully considered for use in the clinical laboratory, as we found that this technology greatly underestimated the Hct/PCV values in cattle living at high elevations, where elevated Hct/PCV values are usually seen.15, 19, 21
ACKNOWLEDGMENTS
The authors acknowledge the support provided by University of Wyoming (UW) Beef Unit of the Laramie Research and Extension Center (UW), the National Institute of Food and Agriculture (NIFA) Crop Protection and Pest Management (CPPM) and Extension Implementation Program (EIP) (Award# 2017-70006-27281), the US Army Medical Department - Veterinary Corps (VC) Long Term Health Education and Training (LTHET) program and the 438th Medical Detachment (Veterinary Service Support). The authors have no conflict of interest to declare.
DISCLOSURE
The authors have indicated that they have no affiliations or financial involvement with any organization or entity with a financial interest in, or in financial competition with, the subject matter or materials discussed in this article.




