Optical coherence tomography for surgical margin evaluation of excised canine cutaneous and subcutaneous tumours
Abstract
Currently, intraoperative tumour margin imaging is not routinely utilized in veterinary medicine. Optical coherence tomography (OCT) allows for real-time assessment of tissue morphology of 1–2 mm depth. The aims of this study were (1) to compare the histologic and OCT features of excised canine skin and subcutaneous specimens, and (2) to determine the diagnostic accuracy of OCT for surgical margin evaluation. The authors hypothesized that OCT imaging would correlate well with histopathology and that OCT would be sensitive for detection of incomplete margins. Eighty dogs were prospectively enrolled. Tumours were excised, and the surgical margins were imaged using a spectral domain OCT system. The tumour type and completeness of excision were determined by histopathology. Nine blinded observers received training in OCT image interpretation and were then given a set of OCT images and videos. The observers assigned each image/video a grade from 1 (no tumour) to 4 (tumour) and the results were compared to histopathology. The overall median sensitivity and specificity of OCT imaging for detection of incomplete margins were 86.7% and 84.6%, respectively. A potential limitation is that observers had varied experience with OCT image interpretation, ranging from no prior experience to participating in a previous OCT project. OCT is sensitive for detection of incomplete margins and could be a promising real-time surgical margin imaging modality. Further study is needed to evaluate intraoperative applications of OCT and its impact on tumour recurrence and long-term outcome.
1 INTRODUCTION
Tumours of the skin and subcutaneous tissues represent 32%–43% of all canine tumours, with 21%–37% of skin tumours being malignant.1-6 For these tumours, surgical resection is the mainstay of treatment; however, a critical challenge in surgical oncology is tumour margin assessment. Excision of canine mast cell tumours (MCT) and soft tissue sarcomas (STS) with complete margins has been associated with decreased tumour recurrence rates, longer disease-free intervals and longer survival times.6-13 Histopathology is currently the gold standard for surgical margin assessment; however, current cross-sectional or radial tissue trimming methods only evaluate less than 1% of the total surgical margins.14-16 This could yield inaccurate results, which could explain local tumour recurrence despite having reportedly complete margins.8, 10, 13, 17-20 Furthermore, histopathology can take many days for results to finalize.
Traditional intraoperative tumour margin evaluation includes visual inspection and palpation, but these methods are often imprecise and inadequate.21, 22 Optical coherence tomography (OCT) was first described in 1991 and has become the standard of care for human retinal imaging to quantify vascular plaques and intravascular imaging for coronary disease.23 This technology uses near infrared (NIR) light waves directed at tissues, then measures the delay and intensity of the reflected light, which is similar to an optical analog of ultrasound. It can detect tissue architecture and morphology up to a depth of 1–2 mm, analogous to the detail seen in low-power histopathology with micron-scale resolution.24, 25 Furthermore, it produces real-time high-resolution images, is label-free (no tissue preparation or staining required) and can be integrated into surgical handheld instruments.24, 26-28 OCT has been used for surgical margin imaging in human breast cancer patients, where intraoperative use following lumpectomy resulted in a sensitivity of 100% and specificity of 82% for completeness of surgical excision.25 Additionally, OCT has been used to image tumours of human skin, gastrointestinal, respiratory and urogenital tracts.29-32
In veterinary medicine, intraoperative OCT has been evaluated in canine STS to compare histopathologic and OCT features of normal and abnormal (neoplastic) tissues, and to assess textural imaging characteristics associated with these different tissue types.32, 33 Recent clinical trials evaluating the use of OCT on surgical margin assessment in canine tumours show promising results. In a recent study of canine MCT, the overall sensitivity and specificity for OCT to accurately identify completeness of excision were 90% and 56.2%, respectively.34 In another recent study of canine STS, the overall sensitivity and specificity of ex-vivo OCT imaging were 82.5% and 93.3%, respectively, with no difference detected between observers of varying experience and specialties.35 Given the promising results in these prior studies, the authors would like to evaluate the diagnostic accuracy of OCT in a larger, more heterogeneous population of dogs.
The aims of this study were (1) to compare normal and abnormal histological features with corresponding OCT images for surgical margins from excised canine cutaneous and subcutaneous tumour specimens; these OCT images and their correlations with histopathology would later form a training set for observers; (2a) to determine the diagnostic accuracy of OCT for assessment of surgical margins for excised canine cutaneous and subcutaneous tumours, and (2b) to determine the frequency of incomplete margin detection for the aforementioned tumours. The authors hypothesized that OCT evaluation of surgical margins in canine cutaneous and subcutaneous tumours will be sensitive for detection of incomplete margins.
2 METHODS
2.1 Enrollment and inclusion criteria
Client-owned dogs that were undergoing surgical resection of a skin or subcutaneous tumour were eligible for enrollment in this prospective clinical study. The study protocol was approved by the Institutional Animal Care and Use Committee at the Ohio State University. For inclusion, dogs needed cytologic or histopathologic tumour assessment with suspicion of a malignant or benign skin or subcutaneous tumour; de novo and recurrent tumours were both accepted. Dogs were excluded if the final histopathology revealed lipomata or non-neoplastic lesions.
2.2 Sample size calculation
In previous clinical studies performed by the authors, five cases were enrolled to develop an imaging training set; for aim 1 in this study to account for variability in tumour diagnoses, 10 cases were enrolled. For aim 2, the sample size was estimated based on methods described by Flahault et al. and Zhou et al.38, 39 A confidence interval half-width of 0.3, a power of 80% and an alpha of 0.05 were chosen. The sensitivity was estimated at 90% based on a human breast cancer clinical trial.26 The estimated number of cases needed with incomplete margins was eight. Seventeen cases with complete margins were required using an estimated incomplete margins prevalence of 33% based on a recent study following surgical resection of malignant canine and feline cutaneous tumours.9 This resulted in a total of 25 cases needed for this study. To account for a dropout rate of 20% due to cases diagnosed with lipomata or non-neoplastic lesions, we estimated requiring a minimum of 30 cases for aim 2.
2.3 Patient data collection
Patient information was collected preoperatively including age, sex and neuter status, breed, anatomic location of the tumour, and tumour dimension based on calliper measurement. The tumours were excised by an American College of Veterinary Surgeons (ACVS) board-certified surgeon either primarily or supervising a trainee. The ACVS surgeon determined the surgical dose for tumour excision, which was not influenced by study enrollment. Information was collected at the time of surgery including the surgery date and the surgical dose. The surgical dose used was adapted from the Enneking system.47 Marginal excision was defined by incision and dissection around the tumour pseudocapsule or through the tumour's reactive zone (grossly <1 cm from the tumour). Otherwise, the planned lateral surgical margins (≥1 cm from the tumour) were determined at the time of surgery, based on the tumour's preoperative cytologic or histopathologic diagnosis, anatomic location and dimensions. Tumours undergoing radical excision were not enrolled. The excised tumour specimens were wrapped in gauze soaked in sterile saline to prevent specimen drying prior to OCT imaging (performed within 1–6 h after excision).
2.4 OCT imaging & specimen inking
Optical coherence tomography imaging was performed using a spectral-domain OCT system (TELESTO, 1300 nm, 76 kHz, Thorlabs, Lubeck, Germany) with a handheld probe in B-mode (2D image display), which allowed continuous scanning of the tissue comparable to the use of ultrasound. All specimens were evaluated grossly and through OCT imaging to identify areas of interest (1 × 1 cm), defined as either suspicious areas where the tumour may be extending to surgical margins or normal tissue types (if no suspicious areas were found). One to four areas of interest were identified for each specimen, and static OCT images were acquired. The boundary of the imaged area was marked in a three-sided box shape with surgical ink (Davidson Marking System, Bradley Products, Bloomington, MN). Digital photographs of all specimens were obtained with smartphones (iPhone 12, Cupertino, California, and Samsung Galaxy S8, Seoul, South Korea) before and after inking to create a record of the sample and inking. For Aim 1, these steps were performed to allow comparison of the OCT and the histologic images for the training set. For Aim 2, all the surgical margins were additionally imaged with OCT in a systematic way working around the entire lateral margins, then the entire deep margin. The OCT images were evaluated in real-time by one or more investigators (EC and/or LES) trained in OCT surgical margin assessment to ensure that the images were of sufficient quality for evaluation.
2.5 Histopathologic assessment
For all specimens, the surgical ink was allowed to dry for 5 min or was sprayed with 5% acetic acid; then, the specimen was placed in 10% neutral buffered formalin at a 1:10 ratio in a plastic container. After 24–48 h, the specimens were removed from formalin and trimmed by an American College of Veterinary Pathology board-certified pathologist (RNJ). Specimens underwent radial trimming, which involved sectioning the specimens along the short axis of the ellipse through the tumour, followed by sectioning each half of the ellipse in half through the long axis of the ellipse (Figure 1A). Aim 1 specimens had a cross-section (perpendicular to the specimen surface) taken from the centre of each of the inked areas of interest for comparison with OCT images. Aim 2 specimens were then trimmed additionally using a tangential trimming technique (Figure 1B), resulting in 2–3 mm thickness sections covering the entire surface area of the specimen including tangential sections of the inked areas. All sections were paraffin-embedded, slides were created, and stained with haematoxylin and eosin (H&E). For only Aim 1 specimens, the slides for inked areas were digitized in a pathology software (Aperio ImageScope, Leica Biosystems Imaging, Vista, California), viewed at 4× magnification and digitally orientated based on inked areas for comparison to the OCT images. The pathologist assessed standard tumour sections, tangential sections and inked areas while being blinded to the results of the OCT imaging.

For all specimens, the pathologist determined the tumour type and grade (if applicable) according to the established grading scheme and produced a diagnostic histopathology report.8, 47-49 For Aim 1, the pathologist provided histopathologic interpretation of the tissue types and findings in the inked areas. For Aim 2, the pathologist also evaluated the slides generated from the tangential tissue trimming to determine what kind of tissues are present (normal vs. abnormal). Using the radial sections of each specimen, it was determined if the margins were complete or incomplete, and if complete, the distance between tumour cells and the surgical margin. Complete margins were defined as tumour cells ≥2 mm away from the surgical margin or the presence of an intact fascial barrier for the deep margin. Incomplete margins were defined as tumour cells <2 mm away from the lateral surgical margin or tumour cells <2 mm away from the deep surgical margin without an intact fascial barrier. This 2-mm histologic margin was chosen so it corresponds with the depth of tissue imaged by OCT. For Aim 2, the entire specimen was considered to have complete margins if this was observed in all radial and tangential sections for the specimen (including inked sections). If at least one slide showed incomplete margins, the entire specimen was determined to have incomplete margins. The tangential sections of the OCT inked area(s) were considered as complete or incomplete using the same criteria. The results of standard histopathologic assessment were considered complete if no incomplete margins were detected in only the radial sections. The results from the complete histopathologic margin assessment (radial and tangential sections) were utilized as the gold standard for comparison to OCT in Aim 2 to allow calculation of diagnostic accuracy and determination of whether each specimen had incomplete margins.
2.6 OCT image assessment
Following completion of Aim 1, a training set of OCT images and videos was established and evaluated by two investigators (EC and LES) through a comparison of OCT and digitized histopathology. These investigators identified OCT image and video features that characterized normal and abnormal (tumour) tissues at surgical margins for observer training in Aim 2.
For Aim 2, an investigator (EC) created a library of OCT images and videos of inked areas grouped by dog specimen (with identification concealed) for review by nine observers. Within the library, 10 specimens were randomly repeated (as a mirror image of the original OCT image) to allow assessment of intraobserver variability. The observers received a virtual training session, which involved an hour-long presentation to introduce them to OCT imaging and explain the OCT image features for identification of different normal and abnormal (tumour) tissues at surgical margins. Then, the blinded observers were provided a practice set of five static OCT images and five OCT videos from Aim 1 and Aim 2. For each image or video, the observer independently determined whether it was normal or abnormal (contains tumour) and assigned a grade according to a numeric grading scale (1: tumour is not present; 2: tumour is most likely not present; 3: tumour is most likely present; 4: tumour is present). The investigators (EC and LES) graded the observers' practice sets and provided feedback if needed. Then, the nine blinded observers independently evaluated OCT images and videos (the data set) using the same criteria for Aim 2 cases within 14 days of the training session. Practice set images and videos were not repeated in the data set. Images and videos with abnormal features were classified as incomplete because the OCT imaging depth was within the 2 mm range used to define incomplete margins. The results of all the OCT images and videos for each specimen were collated and if at least one image or video was determined incomplete, then the specimen margins were determined incomplete on the OCT imaging result.
2.7 Statistical analysis
For Aim 2, data analysis consisted of calculation of sensitivity, specificity and incorrect classification rate for OCT assessment of surgical margins. For each specimen, the result for all OCT images and videos was compared to its complete histopathologic margin assessment (combined results of radial and tangential sections). Tangential tissue trimming technique was thought to represent the most complete evaluation of surgical margins possible and as such represented the gold standard for OCT assessment comparison.14 In addition for Aim 2, we calculated the proportion of specimens with incomplete margins detected by OCT compared to complete histopathologic margin assessment. These were compared using contingency table analysis and Fisher's exact test. To determine inter- and intraobserver variability in OCT image and video assessment, the observers' incorrect classification rates were calculated.
Cell Line Validation Statement: Cell lines were not used in this study.
3 RESULTS
3.1 Aim 1: Comparisons of OCT images with histopathology
Ten client-owned dogs were enrolled. The median age of the dogs at the time of surgery was 11.3 years (range: 4.5–13.3 years). Four dogs were mixed breed dogs, and there was one dog of each of the following pure breeds: German Shorthaired Pointer, Boxer, English Bulldog, Golden Retriever, English Springer Spaniel and West Highland White Terrier. These dogs were affected by MCT (5), STS (2), poorly differentiated carcinoma (1), fibroepithelial polyp (1) and fibroma (1).
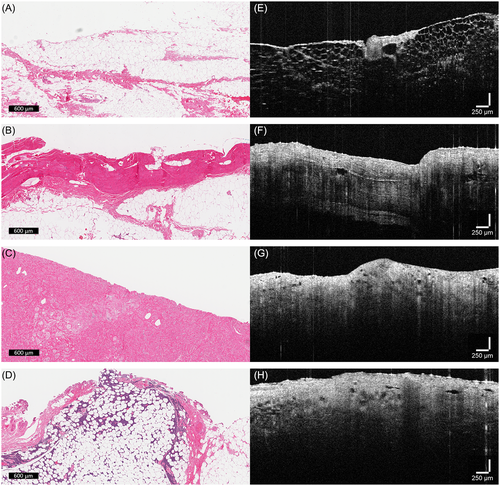
The histopathologic and OCT features for Aim 1 cases are shown in Table 1. The observed tissue types included fat, skeletal muscle, fascia and tumour. Fat appeared as organized honeycomb and vacuolated appearance of adipocytes, with a low-scattering (dark) OCT signal (Figure 2A,E). Skeletal muscle demonstrated intermediate-scattering which appeared brighter on OCT. White lines representing fascia surrounding muscle bundles could be observed with OCT and compared similarly to H&E stained histology of skeletal muscle (Figure 2B,F). Skeletal muscle had different OCT appearances when scanning longitudinally vs. cross-sectionally (Figure 3). Fascia had the highest-scattering OCT signal (white and brightest), which reflected its normal dense microstructural architecture composed of collagen-rich fibres. However, it was difficult to capture fascia on histopathology, likely an artefact of tissue processing and sectioning. Tumours were seen as disorganized and high-scattering on OCT (Figure 2G,H). Different tumours showed varying degrees of high light scatter, but they were consistently irregular and disorganized with no discernible tissue architecture. On histopathology, this was characterized by tumour cells rapidly multiplying with no pattern or invading through normal tissues (Figure 2C,D).
| Case | Histopathology | OCT | ||
|---|---|---|---|---|
| Diagnosis | Tissue Types | Margin | Tissue Characteristics | |
| 1 | STS - perivascular wall tumour (grade I) | Skeletal muscle Fascia |
Incomplete (1 mm) | Skeletal muscle appeared as intermediate-scattering with organized bundles. Fascia appeared as highest-scattering dense tissue. |
| 2 | Cutaneous MCT (low grade/grade II) | Fat | Complete | High-scattering and dense fascia with fat underneath which appeared as low-scattering honeycomb and vacuolated tissue. |
| 3 | Cutaneous MCT (low grade/grade I) | Skeletal muscle Fat |
Complete | Skeletal muscle appeared as intermediate-scattering with organized bundles. Fat appeared as low-scattering honeycomb and vacuolated tissue. |
| 4 | Poorly differentiated carcinoma | Skeletal muscle | Complete | In cross sections of intermediate-scattering skeletal muscle, there was no distinct stroma separating the muscle bundles. |
| 5 | Fibroepithelial polyp | Blood vessel with surrounding connective tissue | Complete | Cystic structure with pinpoint stippling consistent with blood content surrounded by intermediate-scattering stroma. |
| 6 | STS - perivascular wall tumour (grade I) | Skeletal muscle Fat |
Complete | Skeletal muscle appeared as intermediate-scattering with organized bundles. High-scattering and dense fascia with fat underneath which appeared as low-scattering honeycomb and vacuolated tissue. |
| 7 | Cutaneous MCT (high grade/grade III) | Skeletal muscle Tumour |
Incomplete (1.5 mm) | Skeletal muscle appeared as intermediate-scattering with organized bundles. In this case, tumour appeared as intermediate-scattering as well but with no discernible organization. |
| 8 | Subcutaneous fibroma | Tumour | Incomplete (1.5 mm) | Tumour appeared as high-scattering, irregular, and disorganized tissue. |
| 9 | Subcutaneous MCT | Tumour invading through adipocytes | Incomplete (1 mm) | Tumour appeared as high-scattering and indistinct tissue intertwining with vacuolated fat tissue |
| 10 | Cutaneous MCT (low grade/grade II) | Fat | Complete | High-scattering and dense fascia with fat underneath which appeared as low-scattering honeycomb and vacuolated tissue. |

3.2 Aim 2: Assessment of diagnostic accuracy
Seventy client-owned dogs were enrolled. One dog was excluded because postoperative histopathology revealed a diagnosis of fibroadipose tissue despite preoperative cytology being highly suspicious for a mesenchymal malignant neoplasm. The mean (± standard deviation) age of the dogs was 10.0 ± 2.7 years at the time of surgery, and dogs of the following breeds were represented: mixed breed dog (23), Labrador Retriever (9), American Pit Bull Terrier (5), Golden Retriever (3), Bernese Mountain Dog (2), Australian Cattle Dog (2), and one each of Bichon Frise, Jack Russell Terrier, Weimaraner, Chesapeake Bay Retriever, Cocker Spaniel, Coonhound, Shih Tzu, Standard Schnauzer, Siberian Husky, American Bulldog, Miniature Pinscher, Boxer, Beagle, Yorkshire Terrier, Miniature Dachshund, Vizsla, Miniature Schnauzer and Pug. Some dogs were enrolled more than once for different tumours. These dogs were affected by MCT (34), STS (24) and others (see below). One tumour contained both a MCT and a STS.
The median age of the dogs with MCT was 10.0 years (range: 3.9–13.5 years) at the time of surgery. The median tumour size at its largest dimension was 1.7 cm (range: 0.4–8.0 cm). The median surgical margin was 2 cm (range: 1–3 cm), excluding the tumours that underwent marginal excision (2 of 34 cases). Incomplete margins were present in 41% (14/34) of the MCT cases. 59% (20/34) of the MCT cases had complete margins, with a median of 9 mm (range: 2–20 mm) at the narrowest margin. Table S1 summarizes the enrolled MCT cases.
The median age of the dogs with STS was 11.0 years (range: 3.8–14.3 years) at the time of surgery. The median tumour size at its largest dimension was 3.4 cm (range: 1.3–11.0 cm). The median surgical margin was 3 cm (range: 1–3 cm), excluding the tumours that underwent marginal excision (13 of 24 cases). This resulted in incomplete margins in 75% (18/24) of the STS cases. 25% (6/24) of the STS cases had complete margins, with a median of 6.5 mm (range: 2–15 mm) at the narrowest margin. Table S2 summarizes the enrolled STS cases.
Diagnoses in the remainder of enrolled cases included sebaceous adenoma (2), fibroadnexal hamartoma (2), trichoepithelioma (2), hemangioma (2), hemangiosarcoma (1), perianal adenoma (1) and melanocytoma (1). The median age of the dogs with these tumours was 10.6 years (range: 7.2–14.1 years) at the time of surgery. The median tumour size at its largest dimension was 2.5 cm (range: 1.0–7.5 cm). The median surgical margin was 1 cm (range: 1–2 cm), excluding the tumours that underwent marginal excision (6 of 11 cases). This resulted in incomplete margins in 73% (8/11) of these cases. 27% (3/11) of these cases had complete margins, but the narrowest margins were not specified in the histopathology reports. Table S3 summarizes these cases.
The median sensitivity of OCT imaging for canine cutaneous and subcutaneous tumours was 86.7% (range: 73.3%–93.3%) (Table 2). The median specificity of OCT imaging was 84.6% (range: 76.9%–92.3%). The observers correctly graded the OCT images and videos as tumour or no tumour 85.4% (315/369) of the time. There was interobserver variability with an incorrect classification of OCT images and videos occurring 14.6% (54/369) of the time. There was intraobserver variability with an incorrect classification of mirrored OCT images occurring 3.3% (3/90) of the time.
| Observer | Sensitivity | Specificity |
|---|---|---|
| 1 | 86.7% | 76.9% |
| 2 | 93.3% | 88.5% |
| 3 | 73.3% | 92.3% |
| 4 | 93.3% | 88.5% |
| 5 | 80.0% | 80.8% |
| 6 | 80.0% | 92.3% |
| 7 | 80.0% | 84.6% |
| 8 | 86.7% | 80.8% |
| 9 | 93.3% | 84.6% |
| Median | 86.7% | 84.6% |
| Range | 73.3%–93.3% | 76.9%–92.3% |
After completion of the training session, observers took a median of 3 days (range: 0–14 days) to complete the practice set and a median of 14 days (range: 2–15 days) to complete the data set. The observers who attended the training session in real-time had a higher median specificity (92.3%) compared to those who did not (84.6%). The observers who completed the data set in 0–7 days had a higher median sensitivity and specificity (93.3% and 88.5%, respectively) compared to those who completed the data set in 8–14 days (80.0% and 84.6%, respectively).
4 DISCUSSION
The OCT image features for surgical margin tissues from resected skin and subcutaneous tumours were consistent with those previously described.37-39 Different tumours had high-scattering tissue with a lack of organization, and normal tissues had consistent structured appearances.37-39 In addition, we found that OCT was sensitive and specific in the detection of incomplete margins with a median sensitivity of 86.7% and a median specificity of 84.6%.
The most common tumours in this study were MCT which represented 49.4% (39/79) of cases and STS which represented 32.9% (26/79) of cases. In other large canine populations, MCT has been reported to represent 14–21%, and STS represent 8–15% of all neoplasms.3-5 In the current population of canine tumours, a higher distribution of MCT and STS was present, likely because these cases were preferentially referred to our institution due to tumour size, location and complexity. These same factors could also explain the high incomplete resection rates for the enrolled tumours. 71.4% (10/14) of incompletely excised MCT and 77.8% (14/18) of incompletely excised STS occurred on the extremities where typically smaller surgical doses was used to allow for primary wound closure. For the remainder of the tumours, many of these had benign etiologies based on preoperative diagnostics. Thus, marginal excision (in 6/11 cases) or excision using 1-cm lateral margins (in 3/11 cases) were electively performed which undoubtedly contributed to its high incomplete resection rate (73%) as well. Furthermore, some references utilized the classification ‘complete but close’ as tumour cells extending to within 2 mm of the surgical margin.9, 12 Consequently, some tumours in the current study were classified as incomplete when other studies may classify the same tumours as complete but close, leading to a high incomplete resection rate for the enrolled tumours.
Optical coherence tomography images/videos were acquired from tissues after excision but before formalin fixation, and these were compared to the histopathologic assessment after formalin fixation. MCT and STS have been shown to undergo significant reductions in circumferential lengths of surgical margins post-excision.49 Compared to in vivo grossly normal surgical margins, MCT and STS measured a median of 3.0 and 2.5 mm shorter, respectively, immediately after excision, and a median of 8.8 and 5.0 mm shorter, respectively, during histopathologic assessment after formalin fixation. However, the same study found that tumour depth remained unchanged or even increased after formalin fixation.49 Due to tissue shrinkage in the fixation process, compared to OCT, histopathology can overestimate the number of incompletely excised tumours depending on which surgical margin is evaluated (lateral vs. deep). Further studies are needed to elucidate the difference between the length of surgical margins using OCT vs. with histopathology.
Seven of the Aim 2 cases were recurrent tumours. Only one of these 7 had a disagreement in the interpretation of tissue types between OCT and the histopathologic evaluation. This tumour was suspected to be a recurrent angiosarcoma preoperatively, and the histopathology revealed a final diagnosis of cutaneous hemangiosarcoma. In one area of interest, tumour was noted by the OCT, but on histopathologic assessment, there was a focal granuloma with multifocal myofiber degeneration and necrosis. OCT may not be able to discern between tumours and scar tissue with abundant collagen which also has high light scatter. Different tumour types had varying degrees of OCT light scattering signal, but these were consistently unorganized with no discernible tissue pattern, which may potentially mimic the early stages of scar tissue formation when the collagen is immature and unorganized. This was not the focus of the current study, and future work is needed to evaluate the difference between OCT and histopathology of de novo vs. recurrent tumours.
One previous study had found that OCT had a specificity of 93.3% for the detection of incomplete margins after STS excision.44 In the current study, a lower specificity (84.6%) was found. There were two potential reasons for this difference. Firstly, in two cases, all observers misinterpreted skeletal muscle as tumour. One case was represented by a static image, and the other case was represented by a video. This is likely because muscle had a different OCT appearance if scanning in the longitudinal vs. the cross-sectional fashion (Figure 3). In cross-section, skeletal muscle could be incorrectly identified as a tumour since the muscle bundles are less well-defined. However, skeletal muscle still had an intermediate-scattering OCT signal compared to tumours which were generally high-scattering and disorganized. In a recent study by Lages and Selmic, 93.5% of skeletal muscle had lines of fascia surrounding muscle bundles, as opposed to 0% of sarcomas.45 Differentiating skeletal muscle based on the method of OCT scanning should be incorporated into the standardized training session for observers, which may result in the elimination of these systematic errors and improve accuracy. Another possible reason for the lower specificity could be that six of the enrolled tumours for Aim 2 arose from digits that were excised by digital and partial foot amputations. These samples had particularly irregular surgical margins which may have resulted in some distortion of OCT images and videos. Furthermore, OCT images and videos of less commonly encountered tissue types such as ligaments and tendons were not presented to the observers during the training session (as none of the Aim 1 cases were digital tumours). However, observers were requested to evaluate these tissue types in the data set as several digital tumours were enrolled for Aim 2. Even though these collagen-rich tissue types appeared organized and linear on OCT, which were starkly different from OCT features of tumours, the observers were unfamiliar with them which may have resulted in their inaccurate classification by 5 out of 9 observers in one case (#40 - digital fibroadnexal hamartoma). If this contributed to decreased accuracy, then observer performance may be improved through a more comprehensive OCT library and training session.
Another previous study had found that OCT had a sensitivity of 90.0% for the detection of incomplete margins after MCT excision.41 In the current study, a lower sensitivity (86.7%) was found. There were two possible reasons for this difference. Firstly, in some cases, tumours can be captured by OCT beneath a layer of normal tissues such as fat (e.g. case #61 - melanocytoma). These cases were misconstrued by some observers as solely normal tissues as they might not have evaluated the whole OCT image/video. OCT light penetration is greatest for adipose tissue, and it can readily detect tissues deeper to fat such as tumours.49 Adding more examples of these types of cases to the training session may have increased the sensitivity of OCT in the evaluation of surgical margins of resected canine tumours in this study. Additionally, some of the cases contained adipose tissue that had been infiltrated with various gradations of neoplastic cells. Some specimens contained mostly high-scattering and disorganized tumour that completely effaced fat tissue with no discernible honeycomb structure, while others showed less neoplastic cell infiltration between the adipocytes. During OCT imaging of milder cases of infiltrated fat, borders of fat vacuoles were less distinct and blurrier. This could appear similar to tissue artefact and may confuse observers, leading to the inaccurate classification of infiltrated fat as normal fat by 5 out of 9 observers in one case (#77 - sebaceous adenoma). Providing more examples of normal fat with tissue artefact in comparison to neoplastic infiltration of fat in the training session may help to improve accuracy. With the aforementioned examples, it is possible that these challenges with benign tumours could have led to a decrease in OCT's sensitivity to detect incomplete surgical margins, but there were only 10 benign tumours in Aim 2, so the accuracy for tumour types was not ascertained. Further studies are necessary to assess the difference between benign and malignant tumours using OCT.
The standardized training session for this study was delivered virtually (Zoom, San Jose, California) due to public health safety mandates as a result of the COVID-19 pandemic. Only 33.3% (3/9) of the observers attended this virtual session in real-time, while the rest were required to listen to its recording first before receiving the practice set. The observers who attended the training session in real-time had a higher median specificity (92.3%) than those who did not (84.6%), so the findings of this study may have been improved had this training session been held in-person or as a real-time webinar to improve observer engagement and to allow better instruction of OCT tissue features. Another potential way to improve the findings of this study is to shorten the amount of time provided to complete the data set. Observers were allotted 14 days from the training session to complete the data set. They were also instructed to refer to the guidelines, recording and feedback from both the standardized training session and the practice set as they worked through the data set. The observers who completed the data set in the first week after the training session had a higher median sensitivity and specificity (93.3% and 88.5%, respectively) than those who completed it in the second week (80.0% and 84.6%, respectively). Thus, a shorter amount of time between the training session and data interpretation may result in better recall of the important OCT characteristics taught in the training session, leading to more accurate classification of the OCT images and videos.
Study observer #6 completed the data set 1 day beyond the deadline. Nonetheless, with this individual's data omitted, the median sensitivity and specificity of OCT imaging for the enrolled canine tumours remained unchanged. For this reason, we elected to include this individual's data as part of the study. However, this individual was one of two observers who had the highest specificity (92.3%) in this study. Both individuals had participated in previous OCT studies by the authors, which may have influenced these findings.38, 39, 47, 48 Interestingly, the other individual (observer #3) had the lowest sensitivity (73.3%) of all observers. These two individuals may have relied on their prior knowledge and experience with OCT when completing the data set and less on applying the OCT interpretation instructions provided during this study's training session. When grouping the observers based on previous experience with OCT interpretation, those with no prior experience had a median sensitivity of 90.0% (range: 80.0%–93.3%), while those with limited prior experience had a median sensitivity of 80.0% (range: 73.3%–93.3%). The two groups had similar median specificities (no prior experience: 86.5% vs. limited prior experience: 84.6%). This indicated that those with no prior experience were more likely to label an OCT image/video as having tumour or suspicious for tumour (leading to fewer false negative results), likely due to a fear of mislabeling.
In summary, study investigators demonstrated that OCT is sensitive and specific for the detection of incomplete surgical margins from excised canine cutaneous and subcutaneous tumours. By providing thorough and standardized training with representative examples of common tissue types, study investigators were able to train observers with limited to no prior experience to accurately assess OCT images and videos to differentiate normal versus abnormal tissues. Thus, OCT could be a promising imaging modality for real-time intraoperative tumour margin assessment, but its clinical utility needs to be evaluated to investigate the impact on patient outcomes.
ACKNOWLEDGEMENTS
This project was supported by a canine intramural grant at The Ohio State University and the AKC Canine Health Foundation (Grant No. 02758). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the view of the foundation. The authors would like to acknowledge Jenny Bolon for digitization of histopathologic specimens.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this report.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.