Lifetime prevalence of malignant and benign tumours in companion dogs: Cross-sectional analysis of Dog Aging Project baseline survey
A full list of the Dog Aging Project Consortium members is included in the Appendix.
Funding information: National Cancer Institute of the National Institutes of Health, Grant/Award Number: F32CA247088; National Institute on Aging of the National Institutes of Health, Grant/Award Number: U19AG057377
Abstract
Although cancer is widely regarded as a major contributor to canine morbidity and mortality, its frequency in companion dogs has only infrequently been characterised. We analysed cross-sectional data from the baseline survey of owners of 27 541 living companion dogs enrolled in the Dog Aging Project as of 31 December 2020 to estimate the lifetime prevalence of malignant and benign tumours and several potentially-associated characteristics. Survey questions elicited information on history of ‘cancer or tumors’ including organ site and histologic type. Owners reported 819 malignant tumours (56% sited in the skin, muscle or other soft tissue) and 404 benign tumours (69% sited in the skin, muscle or other soft tissue). The lifetime prevalence of malignant tumours (29.7/1000 dogs) was approximately double the lifetime prevalence of benign tumours (14.7/1000 dogs). Lifetime prevalence of both malignant and benign tumours increased with dog age at survey completion. There were no statistically discernable differences in age-adjusted lifetime prevalence of malignant (prevalence ratio (PR) = 0.93 [95% confidence interval (CI) 0.82, 1.07] or benign tumours (PR = 1.10, 95% CI 0.91, 1.34) in mixed vs. purebred dogs. The lifetime prevalence of malignant tumours increased with increasing dog size class; compared to toy and small dogs, the age-adjusted PRs (95% CIs) for medium, standard, large, and giant dogs were 1.65 (1.28, 2.11), 2.92 (2.35, 3.64), 3.67 (2.92, 4.62) and 2.99 (1.23, 4.02), respectively. Similar though less pronounced patterns in relation to dog size class were observed for benign tumours. Ongoing prospective data collection will permit future studies on risk factors for canine tumour incidence.
1 INTRODUCTION
Cancer is a leading cause of canine morbidity and mortality.1, 2 Although population-based frequencies comparable to those available for humans have only been infrequently reported, attempts at such an assessment suggest that the incidence of malignant tumours is higher among female than male dogs; and varies at least 3–4 fold among breeds.3-9 Notably, reports from the US registries3, 4 are 40–50 years old, and more recent reports from European settings6-9 likely include much larger proportions of intact dogs than are owned in the United States.
Despite the paucity of data on the precise frequency of canine cancer, considerable interest exists in determining the causes of different cancer types in companion dogs.10 In addition to the potential benefit to dog owners and veterinary medicine that can be gained from this knowledge, there is considerable potential benefit to human health. Pet dogs are a powerful comparative model for human cancers because they develop some of the same cancer types that humans do, are often treated with the same medications as human patients, and—as opposed to the commonly used laboratory animal models of cancer—they share their living environments (and thus associated risk factors) with humans.11-14 In addition, the shorter lifespan of canines means that research on cancer causes or outcomes can be conducted more rapidly than is possible in humans. Some rare human cancers (e.g., osteosarcoma and hemangiosarcoma) are relatively common in dogs, enabling larger studies than would be feasible in a human population.
The Dog Aging Project (DAP, dogagingproject.org) is a large-scale, longitudinal cohort of companion dogs living in the United States.15 Broadly, the DAP's goal is to provide a multi-disciplinary platform to address unanswered questions about the factors that influence healthy ageing in dogs. As an initial step towards better characterising the occurrence of cancer in dogs and its causes, and thus how cancer impacts dog healthspan (the period of life spent in good health, free from the chronic diseases and disabilities of ageing),16 we analysed baseline survey data from the initial recruitment wave of dogs to assess the lifetime prevalence of both malignant and benign tumours, as well as their relationships to age, sex, purebred status (purebred vs. mixed breed) and dog size class.
2 METHODS
2.1 Study setting
An overview of the DAP rationale and methods has been published.15 The DAP was launched in Fall 2019 when a call for U.S. dog owners to enrol their companion dogs was announced through a wide variety of media outlets. By the end of December 2020 27 541 living dogs had been enrolled in the DAP by their owners.17 This group of dogs is officially known as the ‘DAP Pack’. Enrollment consisted of completing a baseline web-based Health and Life Experiences Survey (HLES) that elicited from the dog owner information on a wide array of topics including physical activity, environmental features, behaviour, diet components, medications, health status and history of the dog and demographic characteristics of the dog and owner. We used these baseline HLES data to conduct a cross-sectional study.
2.2 Data sources
2.2.1 Lifetime malignant and benign tumour prevalence
Information on malignant and benign tumour diagnoses came from owner responses to the HLES question about whether the dog had ever been diagnosed with ‘Cancer or Tumors’; it was accompanied by a list of 34 potential body locations. A positive response to that question directed the owner to provide information on the date of diagnosis, whether surgery or hospitalisation was required, and whether or not follow-up care was ongoing. Next, the owner was asked to choose the dog's body locations that were affected from among the 34 specific body locations listed (Figure S1A), as well as an option to write in a body location that was not listed. The owner was next prompted to indicate which type(s) of cancer or tumour was diagnosed from among 36 specific types (e.g., ‘adenocarcinoma’), to write in a type that was not listed or to indicate a lack of knowledge of the type (Figure S1B). We used the information on reported tumour types to categorise the dogs with a history of cancer or tumour into those with a history of malignant tumours and those with a history of benign tumours (Table S1). We also reviewed the owner reported free text response for ‘Other type of cancer’ so as to classify them as malignant, benign, not tumour or unknown (the latter including situations where the owner selected ‘other type of cancer’ but did not provide a free text response).
In addition to information on cancer locations and types, the HLES elicited information on (1) dog age; (2) dog sex and spay/neuter status; (3) whether or not the dog was purebred (and if purebred, which breed); and 4) dog weight. We classified each dog as to its size class (Toy and Small, Medium, Standard, Large or Giant) differently for adult dogs and puppies. For adult dogs, we used owner-reported information on dog weight to define dogs as Toy & Small (<10 kg), Medium (10 to <20 kg), Standard (20 to <30 kg), Large (30 to <40 kg) or Giant (≥40 kg). For puppies we used the above weight categories but relied on the owner's responses to the HLES question ‘What is your dog's expected adult weight?’
2.3 Statistical analysis
Lifetime prevalence is the proportion of a population that, at some point in life has ever had the characteristic.18 We calculated lifetime cancer prevalence as the number of dogs reported to have a history of tumours (either malignant or benign) per 1000 DAP participants. We estimated crude lifetime prevalence in relation to current dog age, sex, purebred status and dog size class. As the vast majority (92%) of enrolled dogs had been spayed or neutered we did not attempt to analyse lifetime cancer prevalence in relation to spay/neuter status. Associations between purebred status (purebred vs. mixed), dog size and lifetime cancer prevalence were estimated using prevalence ratios (PRs). Adjusted PRs were estimated using multivariable Poisson regression models, and 95% confidence intervals (CI) on the PRs were constructed from robust standard errors.19 PRs were adjusted for age (in integer years) at baseline survey completion because attained age is strongly associated with cancer prevalence and thus could be a confounder of associations between purebred status and size class and cancer prevalence. To test the null hypothesis that the pattern of PRs did not exhibit a monotonic trend we used Wald tests.
3 RESULTS
Selected baseline characteristics of the enrolled dogs are shown in Table 1. The age distribution of the DAP Pack is wide (<1 to 26 years) with a median of 7 years. Male (50.2%) and female (49.8%) dogs, and purebred (49.5%) and mixed breed (50.6%) dogs, were equally represented. The top five breeds enrolled in the DAP Pack were Labrador Retrievers (12.3%), Golden Retrievers (10.8%), German Shepherds (4.5%), Poodles (4.0%) and Australian Shepherds (3.2%) (data not shown). Approximately one-quarter of the cohort dogs were Large or Giant. The enrolled dogs lived in all regions of the United States, with the largest proportion (>one-third) coming from the Western Census regions (Mountain and Pacific). Approximately 60% of the enrolled dogs lived in suburban neighbourhoods.
| Malignant tumour diagnoses (n = 819) | Benign tumour diagnoses (n = 404) | Entire cohort(n = 27 541) | |
|---|---|---|---|
| Characteristic | Number of dogs (%) | ||
| Age (years) | |||
| 0–2 | 1 (0.1) | 4 (1.0) | 4178 (15.2) |
| 3–5 | 39 (4.8) | 27 (6.7) | 6655 (24.2) |
| 6–7 | 80 (9.8) | 40 (9.9) | 3893 (14.1) |
| 8–9 | 120 (14.7) | 82 (20.3) | 3799 (13.8) |
| 10–11 | 219 (26.7) | 90 (22.3) | 3807 (13.8) |
| 12–26 | 360 (44.0) | 161 (39.9) | 5210 (19.0) |
| Sex | |||
| Male | 384 (46.8) | 211 (52.2) | 13 814 (50.2) |
| Female | 435 (53.1) | 193 (47.8) | 13 727 (49.8) |
| Purebred status | |||
| Purebred | 415 (50.3) | 188 (46.5) | 13 618 (49.5) |
| Mixed breed | 404 (49.7) | 216 (53.5) | 13 923 (50.5) |
| Dog Size Class | |||
| Toy and Small | 108 (13.2) | 56 (13.9) | 6177 (22.4) |
| Medium | 133 (16.2) | 74 (18.3) | 5596 (20.3) |
| Standard | 291 (35.5) | 152 (37.6) | 8230 (29.9) |
| Large | 220 (26.9) | 88 (21.8) | 5188 (18.8) |
| Giant | 67 (8.2) | 34 (8.4) | 2352 (8.5) |
| Residence locationa | |||
| New England | 60 (7.3) | 25 (6.2) | 1756 (6.4) |
| Mid-Atlantic | 73 (8.9) | 36 (8.9) | 2505 (9.1) |
| North Central | 154 (18.8) | 53 (13.1) | 5756 (20.9) |
| South Atlantic | 166 (20.3) | 87 (21.6) | 4928 (17.9) |
| South Central | 79 (9.6) | 37 (9.1) | 2994 (10.8) |
| Mountain | 81 (9.9) | 50 (12.4) | 2850 (10.4) |
| Pacific | 206 (25.2) | 115 (28.5) | 6742 (24.5) |
| Residence neighbourhood type | |||
| Urban | 142 (17.3) | 76 (18.8) | 4785 (17.4) |
| Suburban | 527 (64.4) | 244 (60.4) | 17 081 (62.0) |
| Rural | 150 (18.3) | 84 (20.8) | 5676 (20.6) |
- a Primary residence according to US Census designation.
Owners reported that 1751 dogs (6.4%) had a history of cancer or tumours. Following classification as to malignant vs. benign behaviour, and review of owner-reported tumour types (including exclusion of types written in by owners that were, in fact, not tumours), 819 dogs had a history of malignant tumours (74% had one malignancy, 20% had 2 malignancies and 6% had ≥3 malignancies); 404 dogs had a history of benign tumour (72% had one benign tumour, 21% had 2 benign tumours and 7% had ≥3 benign tumours). Fifty-four (54) dogs had a history of both malignant and benign tumours. The corresponding lifetime prevalences of malignant and benign tumours were 29.7 per 1000 dogs and 14.7 per 1000 dogs, respectively. Lifetime prevalence for both malignant and benign tumours rose with increasing age at enrollment (Figure 1). The 10 most commonly reported body locations of malignant and benign tumours are shown in Figure 2. The predominant locations, whether malignant or benign, were skin and muscle or other soft tissue.
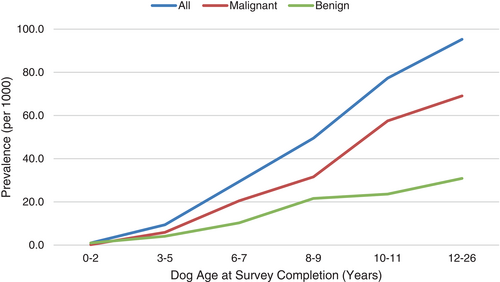
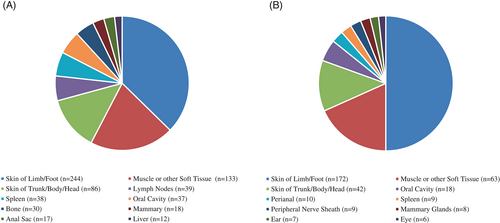
Table 2 shows the age-adjusted prevalence ratios for the association between purebred status, dog size class and lifetime prevalence of malignant and benign tumours. The prevalences of both malignant and benign tumours in purebred dogs were essentially identical to mixed breed dogs; this finding was unchanged when we also adjusted for dog size class. Compared to small dogs, dogs in medium, standard and large size classes had increasingly higher lifetime prevalence of malignant tumours; giant size dogs did not have a higher age-adjusted lifetime prevalence of malignant tumours compared to standard or large size dogs. In contrast, the corresponding PRs for benign tumours increased from medium to standard, large and giant dogs. For both malignant and benign tumours the PRs the null hypothesis of no monotonic trend was rejected.
| Malignant tumours | Benign tumours | |||
|---|---|---|---|---|
| Characteristic | Crude Prevalencea | PR(95% CI)b | Crude Prevalencea | PR(95% CI)c |
| Purebred status | ||||
| Purebred | 30.5 | 1.00d- | 13.8 | 1.00d- |
| Mixed breed | 29.0 | 0.93 (0.82, 1.07) | 15.5 | 1.10 (0.91, 1.34) |
| Dog size class | ||||
| Toy and small | 17.5 | 1.00d | 9.1 | 1.00d |
| Medium | 23.8 | 1.65 (1.28, 2.11) | 13.2 | 1.70 (1.21, 2.41) |
| Standard | 35.4 | 2.92 (2.35, 3.64) | 18.5 | 2.74 (2.02, 3.73) |
| Large | 42.4 | 3.67 (2.92, 4.62) | 17.0 | 2.62 (1.87, 3.66) |
| Giant | 28.5 | 2.99 (1.23, 4.02) | 14.5 | 2.61 (1.70, 4.01) |
| pe | 4.03 x 10−39 | 3.30 x 10−12 | ||
- a Per 1000 dogs.
- b Prevalence Ratio, adjusted for age at survey completion (continuous years).
- c 95% confidence interval.
- d Referent group for PR calculations.
- e p-Value from Wald test of the null hypothesis that the PRs are not consistent with a monotonic trend.
4 DISCUSSION
Among the companion dogs participating in the DAP Pack 3.0% had a history of a malignant tumour and 1.5% had a history of a benign tumour. These figures correspond to lifetime prevalences of 29.7 per 1000 dogs and 14.7 per 1000 dogs, respectively. Although no other report has comparably estimated lifetime prevalence of malignant or benign tumours, several reports of cancer incidence from population-based veterinary registries have been published.3, 4, 6-9 Although the lifetime prevalence of cancer in the dog populations served by those registries is not known, assuming that a non-trivial proportion of animals survived following their diagnoses and treatment, we can broadly expect that the lifetime prevalence would necessarily be higher than the annual incidence because prevalence is a function of incidence and disease duration (i.e., survival).20 In that respect, we note that the cancer incidence estimates from the population-based registry studies are all an order of magnitude lower than our lifetime prevalence estimate for malignant tumours.3, 4, 6-9 Importantly, both cancer incidence and prevalence measurements in dogs are likely underestimated due to the tendency for internal organ malignancies to be under-ascertained (as noted below).
4.1 Characteristics associated with lifetime cancer prevalence
Purebred Status Mixed breed dogs have been shown to live longer than purebred dogs of the same size.21 There is a long-standing belief that, due to their strongly human-engineered evolution, purebreds have a higher incidence of health conditions due both to inbreeding22 and limited effective population sizes as well as linkage of disease-susceptibility loci with loci that contribute to certain phenotypic breed characteristics. The extent to which these factors contribute to malignant or benign tumour susceptibility has rarely been addressed. Our results showing no difference in lifetime prevalence of malignant or benign tumours between purebred and mixed breed dogs are consistent with one prior study that relied on cancer prevalence23 and the majority of prior studies that relied on incidence3, 9, 24, 25 (Two studies measuring cancer incidence reported higher rates in purebred compared to mixed breed dogs4, 7).
Dog Size Class In theory, larger mammalian species should be at increased risk of malignant tumours compared to smaller mammalian species because the more cells that comprise an animal, the greater the chances of cancer developing.26 That this relationship is not observed across mammalian species (aka ‘Peto's Paradox’) has stimulated extensive research to identify the reasons for departures from expectation. Companion dogs provide an intriguing model for addressing this issue due to the wide range of animal sizes within the same species. The extent to which Peto's Paradox holds among dogs, however, has only infrequently been assessed. Risks of appendicular osteosarcoma and hemangiosarcoma have been reported to be increased in several large dog breeds and reduced in small dog breeds.24, 27 Further, whether cancer risk differs according to dog size class may vary according to cancer site.9
We found that the age-adjusted lifetime prevalence of malignant and benign tumours increased with increasing size class. That the PRs for the larger size classes were not uniformly larger than for smaller size classes could well be due to imprecision in the PR estimates (as evidenced by wide confidence intervals). For example, for malignant tumours the PRs for giant and standard dogs were numerically very similar yet the confidence interval for the former was substantially wider than for the latter. While our results suggest that increasing size class is associated with increasing lifetime prevalence of canine malignancy, the challenges with making etiologic inference in the absence of prospective incidence data substantially limit the strength of our conclusions.
4.2 Limitations
Our results have multiple key limitations. First and most important, is that the data for each dog come from a single point in time and thus are cross-sectional. Second, the enrolled study population did not come from a defined sampling frame; rather, the dogs and their owners all volunteered in response to multiple requests for participants. We therefore do not know the extent to which the enrolled participants are generalisable to (i.e., reflect) the theoretical study base (essentially, all dogs living in US households in late 2019 through 2020). In limited comparisons, we were able to make with nationwide survey data from 2016 published in the American Veterinary Medicine Association (AVMA) Pet Ownership & Demographics Sourcebook,28 we determined that the DAP Pack and AVMA survey have the same proportion of dogs that are mixed breed (51%) vs. purebred (49%), but the DAP Pack has a much lower proportion of dogs that come from households with <$20 000 annual income (2% vs. 13%). Third, because lifetime prevalence of tumours is a function of incidence and survival, we cannot make strong inferences about the factors that only influence incidence (i.e., potential risk factors). Nonetheless, certain characteristics that might influence risk, such as purebred status, are determined at birth and thus years before malignant or benign tumours typically develop. Fourth, our estimate of lifetime prevalence of malignant tumours in particular may be underestimated for at least three reasons: (1) some owners whose dogs had developed advanced cancer and were in poor health may have chosen not to enrol their pets in the DAP Pack; (2) owners whose dogs had previously developed cancer and subsequently died due to the disease could not have enrolled their dogs; and (3) owners of older dogs might be less motivated to subject their pets to extensive diagnostic measures when symptoms are present. We do not know the extent of this underestimation, but it most likely varies by malignancy type, location and dog age. Regarding the latter, the lifetime prevalence of malignancies that would have typically been diagnosed in (and resulted in the death of) juvenile dogs might be most severely underestimated in DAP Pack dogs that were older at baseline. Fifth, owner recall of tumour diagnoses, particularly the histologic type of tumour, may be incorrect, leading us to over- or under-estimate the different tumour types that are prevalent in companion dogs. Sixth, it is possible that owners focused on malignant tumour types when answering HLES questions, and placed less importance on (or had less recollection of) benign tumours. Malignant and benign tumours may also be undiagnosed if they never caused significant symptoms for the dog or went undetected because a dog received infrequent veterinary care. Even if a malignancy caused symptoms an owner may have chosen not to have an internal tumour biopsied or resected. The impact of these types of errors generally would be that our absolute estimates of lifetime prevalence are lower than the truth, and particularly so for ‘internal’ tumours. Unfortunately, the amount of the downward bias is impossible to know without studies designed to estimate the extent of under-ascertainment. Work is ongoing to better understand these factors by examining the extent of concordance of owner-reported information and the dog's veterinary medical records. Seventh, some types of tumours reported by owners (e.g., mast cell tumours and soft tissue sarcomas) exhibit heterogeneity in grade and/or location that ultimately reflect their likelihood of negatively impacting the dog's lifespan.29, 30 Unfortunately, we lacked such information and thus could not classify the reported tumours more precisely. Specifically, some tumours we have classified as ‘malignant’ could truly exhibit relatively ‘benign’ behaviour whereas others could have truly exhibited aggressively. Our results, however, would only be impacted to the extent that this misclassification was associated with the characteristics we investigated. Eighth, since the incidence of both malignant and benign canine tumours vary substantially across age and breed,14 our estimates of the lifetime prevalence of these conditions similarly depend on the distribution of those characteristics among the dogs currently enrolled in the DAP Pack.
Finally, despite the very large size of the DAP cohort enrolled to date we were unable to assess the lifetime prevalence of specific malignant or benign tumours due to the small numbers of each type. Similarly, we could not study breed-specific lifetime tumour prevalence due to the small numbers of dogs in all but a few breeds.
5 CONCLUSIONS
A central aim of the DAP is to identify the characteristics that influence a dog's ‘healthspan’ (the portion of a dog's lifespan which is spent free from illness or infirmity).16 Given the frequency of new cancer diagnoses in companion dogs as reported by others,3, 4, 6-9 it is likely that malignancies contribute substantially to shortening a dog's healthspan. Improving the rigour of our ascertainment and documentation of malignancies is necessary if we are to determine accurately the extent to which these diseases influence the healthspan of dogs and ascertain risk factors. To this end, we are currently collecting veterinary medical records for enrolled DAP Pack members, and piloting data mining strategies to allow for validation of diagnoses. We are also seeking additional funding through which we will be able to establish a more robust protocol for ascertaining and documenting malignant tumour diagnoses among the DAP Pack.
The DAP Pack will eventually consist of approximately 100 000 companion dogs, each of which will be followed annually for updated HLES information, including new diagnoses of malignant and benign tumours. At the time of submission of this manuscript, the DAP Pack includes 36 130 dogs. We therefore anticipate that within a few years we will be able to begin to address outstanding questions about known, suspected and heretofore unexamined risk factors for canine tumour development using data on incidence of these conditions. This powerful emerging dataset will inform ongoing comparative and veterinary studies of cancer in dogs. Precise prevalence and incidence data will aid substantially in the assessment of lifestyle, environmental and molecular risk factors.
ACKNOWLEDGEMENTS
The authors are grateful for the enthusiastic participation of the dogs (and their owners) in the Dog Aging Project. The authors also thank Heather L. Gardner, DVM, PhD, DACVIM (Oncology), Tufts University Cummings School of Veterinary Medicine for her very helpful remarks on the penultimate version of the manuscript. This research is based on publicly available data collected by the Dog Aging Project, which is supported by grant U19AG057377 (PI: Daniel E. L. Promislow) from the National Institute on Aging, a part of the National Institutes of Health, and by additional grants and private donations. Kate Megquier is supported by the National Cancer Institute of the National Institutes of Health under Award Number F32CA247088. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Data used in the analyses described are stored on the Google Cloud Platform, and can be accessed through the Terra workspace at the Broad Institute of MIT and Harvard.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
APPENDIX: DOG AGING PROJECT CONSORTIUM MEMBERS
Joshua M. Akey, Lewis-Sigler Institute for Integrative Genomics, Princeton University; Brooke Benton, Department of Laboratory Medicine and Pathology, School of Medicine, University of Washington; Elhanan Borenstein, Department of Clinical Microbiology and Immunology, and Blavatnik School of Computer Science, Tel Aviv University, Tel Aviv, Israel; Marta G Castelhano, Cornell Veterinary Biobank, College of Veterinary Medicine, Cornell University; Amanda E. Coleman, Department of Small Animal Medicine and Surgery, College of Veterinary Medicine, University of Georgia; Kate E. Creevy, Department of Small Animal Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University; Kyle Crowder, Department of Sociology, University of Washington; Benjamin S. Wilfond, Treuman Katz Center for Pediatric Bioethics, Seattle Children's Research Institute; Matthew D. Dunbar, Center for Studies in Demography and Ecology, University of Washington; Virginia R. Fajt, Department of Veterinary Physiology and Pharmacology, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University; Annette L. Fitzpatrick, Department of Family Medicine, School of Medicine, University of Washington; Departments of Epidemiology and Global Health, School of Public Health, University of Washington; Unity Jeffery, Department of Small Animal Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University; Erica C Jonlin, Department of Laboratory Medicine and Pathology, School of Medicine, University of Washington; Matt Kaeberlein, Department of Laboratory Medicine and Pathology, School of Medicine, University of Washington; Elinor K. Karlsson, University of Massachusetts Medical School / Bioinformatics and Integrative Biology; Kathleen F. Kerr, Department of Biostatistics, School of Public Health, University of Washington; Jonathan M. Levine, Department of Small Animal Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University; Jing Ma, Biostatistics Program, Public Health Sciences Division, Fred Hutchinson Cancer Center; Robyn L McClelland, Collaborative Health Studies Coordinating Center, Department of Biostatistics, School of Public Health, University of Washington; Daniel E.L. Promislow, Department of Laboratory Medicine and Pathology, School of Medicine, University of Washington, Department of Biology, University of Washington; Audrey Ruple, Department of Population Health Sciences, Virginia-Maryland College of Veterinary Medicine, Virginia Polytechnic Institute and State University; Stephen M. Schwartz, Epidemiology Program, Public Health Sciences Division, Fred Hutchinson Cancer Center, and Department of Epidemiology, School of Public Health, University of Washington; Sandi Shrager, Collaborative Health Studies Coordinating Center, Department of Biostatistics, School of Public Health, University of Washington; Noah Snyder-Mackler, School of Life Sciences and Center for Evolution and Medicine, Arizona State University; M. Katherine Tolbert, Department of Small Animal Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University; Silvan R. Urfer, Department of Laboratory Medicine and Pathology, School of Medicine, University of Washington.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request and establishment of data use agreements authorised by the Dog Aging Project's institutions (University of Washington and Texas A&M University).