Expression of the hedgehog signalling pathway and the effect of inhibition at the level of smoothened in canine osteosarcoma cell lines
Hwa-Young Youn and Kyoung-Won Seo contributed equally to this work.
Funding information: Research Institute for Veterinary Science and BK21 Plus Program for Creative Veterinary Science Research, Seoul National University; Seoul National University, Grant/Award Number: BK21
Abstract
Osteosarcoma (OSA) is the most common malignant bone cancer in dogs. Canine and human OSA share several features, including tumour environments, response to traditional treatment, and several molecular pathways. Hedgehog (Hh) signalling is known to contribute to tumorigenesis and progression of various cancers, including human OSA. This study aimed to identify the role of the Hh signalling pathway in canine OSA cell lines, including Abrams, D17, and Moresco, focusing on the signal transducer Smoothened (SMO). mRNA and protein levels of Hh pathway components, including SHH, IHH, SMO, and PTCH1, were aberrant in all examined OSA cell lines compared with canine osteoblast cells. The SMO inhibitor cyclopamine significantly decreased cell viability and colony-forming ability in the canine OSA cell lines in a dose-dependent manner. Moresco cells, which expressed the highest level of SMO protein, were the most sensitive to the anticancer effect of cyclopamine among the three canine OSA cell lines tested. Hh downstream target gene and protein expression in canine OSA cell lines were downregulated after cyclopamine treatment. In addition, cyclopamine significantly increased apoptotic cell death in Abrams and Moresco cells. The findings that Hh/SMO is activated in canine OSA cell lines and cyclopamine suppresses OSA cell survival via inhibition of SMO suggest that the Hh/SMO signalling pathway might be a novel therapeutic target for canine OSA.
1 INTRODUCTION
Osteosarcoma (OSA) is a predominant type of bone cancer in both humans and dogs, but it is more prevalent (approximately 10 times) in dogs than in humans.1 In dogs, the cancer occurs mainly at middle age, and in large or giant breeds, such as Rottweiler, German Shepherd, Boxer, Doberman Pinscher, and Irish Setter.2-4 Although surgical resection and chemotherapy can be effective, OSA easily spreads to other organs, including the lungs; approximately 80% of dogs with OSA die from metastasis.2, 3, 5
The aetiology and pathophysiology of OSA are not well known and have been the focus of various studies. Some studies revealed that the hedgehog (Hh) signalling pathway is activated in OSA and is associated with OSA onset and progression.6, 7 Hh signalling, which involves Hh ligands, including Sonic Hh (SHH), Indian Hh (IHH), and Desert Hh (DHH), Patched (PTCH) receptor, and Smoothened (SMO), and the glioma-associated oncogene (GLI) family of transcription factors, plays an important role in embryonic development and cell differentiation in normal physiological conditions.8, 9 PTCH normally inhibits SMO activation in the absence of Hh ligand in adult tissues. However, if one of the Hh ligands is present and activated, it binds to PTCH. In that case, SMO is no longer inhibited, and activated SMO enters the cytoplasm and activates GLI1 and GLI2 proteins. After GLI family members enter the nucleus, they regulate Hh target genes, including GLI itself and PTCH.9, 10
Among the Hh components, mainly SMO and GLI have been investigated as therapeutic targets for cancer. Especially SMO has been regarded as a primary target for Hh pathway inhibition, because SMO inhibition enables the blockage of GLI transcription by preventing its downstream activation.11 The representative SMO inhibitor cyclopamine strongly binds to SMO and suppresses tumour growth in vitro and in vivo.12-16 While studies revealed that Hh signalling is reactivated in several types of human cancers, including OSA, and contributes to carcinogenesis and metastasis in these cancers, a few studies on aberrant Hh or GLI signalling in canine cancer have been reported.17-19 One of these studies reported that GLI signalling was present in canine OSA and inhibition of GLI with GANT61 prevented cancer cell proliferation.17 Another study revealed variable expression levels of Hh components in canine OSA tumour tissues.19 This study aimed to evaluate whether Hh signalling, including the key transducer SMO, is activated in canine OSA cells using the well-studied SMO inhibitor cyclopamine.
2 MATERIALS AND METHODS
2.1 Cell line validation statement and cell culture
The Abrams and Moresco canine OSA cell lines were kindly provided by Dr. Douglas Thamm, Department of Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University. The canine osteoblast cells (Cn406-05) and D17 OSA cell line (CCL-183) were purchased from Cell Application Inc. and American Type Culture Collection (ATCC), respectively. The cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM; PAN-Biotech, Aidenbach, Germany) supplemented with 10% heat-inactivated fetal bovine serum (FBS; PAN-Biotech) and 1% penicillin–streptomycin (PAN-Biotech) in a humidified atmosphere of 5% CO2. All cell lines have been validated and used in previous studies.17, 20, 21 The cells were evaluated for morphology and maintenance of growth characteristics and tested free of mycoplasma.
2.2 Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells, including canine osteoblast cells and the three OSA cell lines, using an Easy-BLUE Total RNA Extraction kit (Intron Biotechnology, Seongnam, Gyeonggi, Korea) according to the manufacturer's instructions. cDNA was synthesized from 1 μg of total RNA using SuPrimeScript RT Premix (GeNet Bio, Daejeon, Korea). The mRNA expression levels of IHH, SHH, SMO, and PTCH1 were quantified by RT-qPCR using AMPIGENE qPCR Green Mix Lo-ROX with SYBR Green dye (Enzo Life Sciences, Farmingdale, NY, USA). Sequence information for the primers used for RT-qPCR is taken from previous studies18, 22-24 and is presented in Table 1. PCR conditions consist of 2 min at 95°C, followed by 40 cycles of 5 s at 95°C and 25 s at 60°C. Reactions included no template control and were performed in triplicate. Transcript expression levels were normalized to the level of hypoxanthine phosphoribosyltransferase 1 (HPRT1) and quantified using 2−ΔΔCT method.18, 24 To investigate effect of the SMO inhibitor cyclopamine on expression of genes downstream of Hh signalling in OSA cells, RT-qPCR was performed using cyclopamine or vehicle-treated cells. The cyclopamine was purchased from Santa Cruz (Dallas, TX, USA) and was dissolved in methanol.
| Target gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Product size (bp) |
|---|---|---|---|
| IHH | CTGGAAGGGATCTGAGTTGG | GGTCAAGTTGCAATGGTGTG | 75 |
| SHH | GCTTCGACTGGGTGTACTACG | GTGAGGAAGTCGCTGTAGAGC | 213 |
| SMO | TGGTGCTCATTGTGGGAGGTTACT | ACTCAGCCTGGTTGAAGAAGTCGT | 210 |
| GLI1 | CAGCAGCTGAACCTTATGGA | GGGTGGTTCAGGATAGGAGA | 116 |
| GLI2 | GCCTCAAGAAAGTGGGAAGA | TGGAGAAACAGGATTGGGTAAA | 103 |
| PTCH1 | TGTCTGTAATCCTTCATGGGC | AAAGAGATGCCTTGGACCTG | 139 |
| HPRT1 | TGCTCGAGATGTGATGAAGG | TCCCCTGTTGACTGGTCATT | 191 |
- Note: Reverse transcription polymerase chain reaction.
2.3 Western blot analysis
Cell pellets were resuspended in PRO-PREP Protein Extraction Solution (Intron Biotechnology, Seongnam, Korea), incubated on ice for 20 min, and centrifuged at 13 000 rpm for 5 min at 4°C. The protein in the supernatant was quantified using the Bio-Rad DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions. Equal amounts of proteins were separated on an 8%–13% SDS polyacrylamide gel and then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline containing Tween-20 (TBS-T) at room temperature for 1 h. Then, the membranes were incubated with following primary antibodies at 4°C overnight: anti-SHH (1:500, Santa Cruz, CA, USA, Cat# sc-365 112), anti-SMO (1:500, Aviva Systems Biology, San Diego, CA, USA, Cat# OAEB01776), anti-PTCH1 (1:500, Aviva Systems Biology, Cat# ARP44249_P050), and anti-β-Actin (1:1000, Santa Cruz, Cat# sc-47 778). After three washes with TBS-T, appropriate HRP-conjugated secondary antibody (1:4000) was hybridized to the membrane at room temperature for 2 h. The bands were detected by enhanced chemiluminescence (Advansta, Menlo Park, CA, USA) and imaged using an ImageQuant LAS 4000 Mini imaging system (GE Healthcare Biosciences, Uppsala, Sweden). Three independent experiments were performed. Additional antibodies anti-GLI1 (1:500, Aviva Systems Biology, Cat# ARP32368_T100) and anti-GLI2 (1:200, Santa Cruz, Cat# sc-271 786) were used to examine whether cyclopamine treatment affect GLI proteins in canine OSA cell lines. Densitometric analyses were performed using the ImageJ software (NIH, Bethesda, Maryland, USA) and protein expression levels were normalized to the corresponding β-Actin loading control.
2.4 Cell viability assay
Canine Abrams, D17, and Moresco cells were seeded into 96-well cell culture plates at 3000 cells/well, in triplicate. After overnight incubation, the medium was replaced with 2% FBS medium containing 2.5, 5, 10, 15, 20, 25, or 30 μM cyclopamine. The cells were incubated at 37°C and 5% CO2 for 24, 48, 72, or 96 h, and then, 10 μl CCK-8 reagent (D-Plus™ CCK cell viability assay kit, Dongin Biotech, Seoul, Korea) was added to each well. Following a 2-h incubation, absorbances at 450 nm were measured using a Model 680 Microplate Reader (Bio-Rad, Hercules, CA, USA). Four replicates of each treatment were conducted. Cells treated with the same volume of vehicle were used as a control.
2.5 Clonogenic assay
Cells of all three canine OSA cell lines were seeded at 400 cells/well in 6-well cell culture plates. After attachment of the cells to the plates after 6 h, the cells were treated with 0, 2.5, 5, 10, 15, or 20 μM cyclopamine for 96 h, and then cultured in fresh, drug-free medium for an additional 9 days. To stain colonies, the plates were rinsed with phosphate-buffered saline (PBS), and 2 ml of a mixture of 6.0% glutaraldehyde and 0.5% crystal violet was added. The plates were left to stand for 30 min and were then carefully washed with tap water. Digital images of stained colonies were captured, and the colonies were counted.
2.6 Drug treatment for qRT-PCR and western blot analysis
Cells of the three canine OSA cell lines were seeded into 6-well cell culture plates. After overnight incubation, the cells were treated with 10 μM or 20 μM of cyclopamine, or with a volume of the vehicle (methanol). Cells harvested by scraping at 48 and 96 h of incubation were used for qRT-PCR and western blot analysis. Two independent experiments were performed in triplicate.
2.7 Flow cytometry
Cells were seeded into 6-well cell culture plates and were treated with cyclopamine or an equal volume of vehicle for 96 h. The dose of cyclopamine for each cell line, including Abrams, D17, and Moresco, was 10, 15, and 8 μM, respectively, which was determined by IC50 values calculated from data of the cell viability assay. The cells were harvested and stained using the FITC Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer's instructions. Briefly, cells were washed with cold PBS and resuspended in 1× binding buffer. Then, 5 μl of FITC Annexin V and 5 μl of propidium iodide (PI) were added to 100 μl of the cells. After incubation at room temperature in the dark for 15 min, 400 μl of 1× binding buffer was added. Each sample was analysed on a FACSAria II instrument (BD Biosciences).
2.8 Statistical analysis
Data are presented as the mean ± standard deviation (SD). IC50 values, the concentrations of cyclopamine at which cell survival was reduced by 50%, were determined from cell viability data fitted to a non-linear regression model. Means were compared by repeated measures ANOVA after the Shapiro–Wilk normality test using GraphPad Prism v.6.01 software (GraphPad Inc., La Jolla, CA, USA). p Values <.05 were considered statistically significant.
3 RESULTS
3.1 Hh expression in canine OSA cell lines
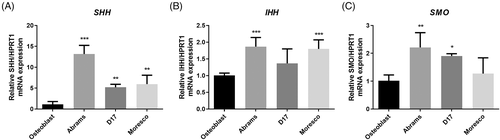
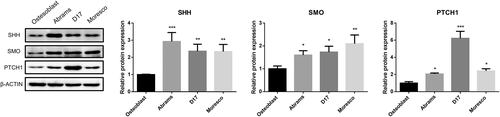
The mRNA and protein expression levels of Hh components in three canine OSA cell lines were compared with those in normal canine osteoblast cells. The SHH mRNA level was higher in all examined canine OSA cell lines than in canine osteoblast cells (p < .01). Especially, SHH mRNA expression in Abrams cells was approximately 13 times higher than that in canine osteoblast cells (Figure 1A). Abrams and Moresco cells showed significantly higher IHH expression compared with osteoblast cells by 1.87-fold and 1.8-fold, respectively (p < .001) (Figure 1B). The SMO transcript was significantly higher in Abrams and D17 cells than in the canine osteoblast cells with differences of 2.2-fold and 1.9-fold, respectively (p < .05) (Figure 1C). Protein expression of SHH, SMO, and PTCH1 was higher in all three canine OSA cell lines than in osteoblast cells (p < .05) (Figure 2). SHH protein level for Abrams cells was higher 2.9 times, and PTCH1 for D17 cells was higher 6.2 times compared with those for osteoblast (p < .001).
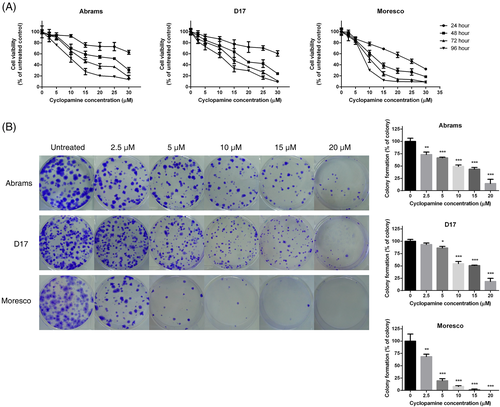
3.2 Cyclopamine reduces cell viability and colony formation in canine OSA cell lines
A CCK-8 assay was used to determine the effect of cyclopamine on cell viability. Cyclopamine treatment reduced cell viability in a dose- and time- dependent manner in all three canine OSA cell lines (Figure 3A). Cell viability was the most strongly declined in Moresco cells. The IC50 values of each cell line at 72 and 96 h, derived from data fitted to a non-linear regression model, are shown in Table 2. Cell viability was not affected in vehicle-treated cells (data not shown).
| Cell line | 72 h | 96 h | ||
|---|---|---|---|---|
| IC50 (95% CI) | R2 | IC50 (95% CI) | R2 | |
| Abrams | 14.67 (9.485–22.69) | 0.9575 | 9.898 (8.796–11.14) | 0.9782 |
| D17 | 25.34 (10.98–58.48) | 0.9727 | 16.20 (10.23–25.66) | 0.9724 |
| Moresco | 10.08 (9.289–10.93) | 0.9815 | 7.940 (7.767–8.116) | 0.9989 |
- Note: The IC50 value for each cell line was determined by nonlinear regression using the CCK-8 cell proliferation data, and the 95% confidence interval is shown in parentheses. Correlation coefficients (R2) were evaluated to measure the strength of the relationship among the dose–response values.
The colony-forming ability was inhibited by cyclopamine in all three canine OSA cell lines (Figure 3B). The number of colonies dose-dependently decreased in the three canine OSA cell lines after cyclopamine treatment, except for D17 cells treated with 2.5 μM cyclopamine (p < .05). Moresco cells were the most sensitive to the effect of cyclopamine on colony formation.
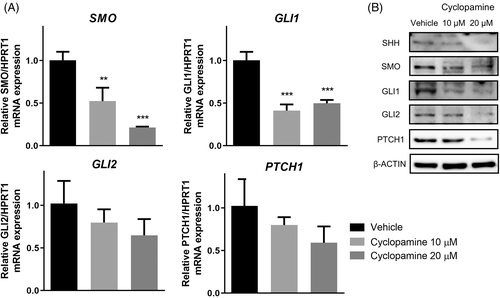
3.3 Cyclopamine downregulates expression of Hh components in Moresco cells
The Moresco cell line was selected to examine the effect of cyclopamine on the expression of Hh pathway components because it had the highest SMO protein level and its cell viability and colony-forming ability were the most strongly affected by cyclopamine among the three OSA cell lines. After treatment with 10 or 20 μM cyclopamine for 48 h, which was close to IC50 value at 48 h (10.98) and the double dose, mRNA levels of SMO and GLI1 were significantly downregulated as determined by qRT-PCR (p < .01); SMO represents a fall of nearly one fifth by 20 μM of cyclopamine (Figure 4A). At 20 μM, cyclopamine suppressed the expression of all proteins examined, including SHH, SMO, GLI1, GLI2, and PTCH1, whereas at 10 μM, it affected only GLI1 in Moresco cells, as indicated by western blotting (Figure 4B).
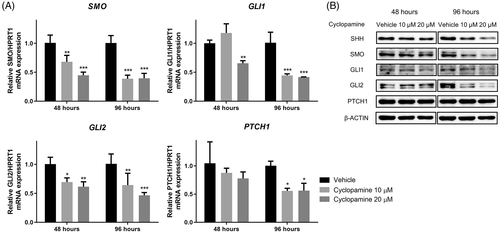
3.4 Dose- and time-dependent effect of cyclopamine on Hh pathway expression in Abrams cells
The Abrams cell line, which showed relatively low sensitivity for cyclopamine, was used to verify the effect of cyclopamine dose (10 μM or 20 μM) and treatment time (48 or 96 h) on Hh gene and protein expression. At 48 h, 10 μM or 20 μM cyclopamine significantly downregulated mRNA expression of SMO and GLI2, whereas GLI1 mRNA level was decreased by 20 μM cyclopamine (p < .05) (Figure 5A). All examined doses cyclopamine suppressed mRNA levels of SMO, GLI1, GLI2, and PTCH1 after 96-h, especially with means of 0.39 for SMO compared to vehicle-treated cells. SHH, SMO, GLI1, and GLI2 protein levels were dose-dependently decreased after treatment with of cyclopamine for 96 h (Figure 5B). PTCH1 protein expression was not affected by cyclopamine at any dose or treatment time tested.
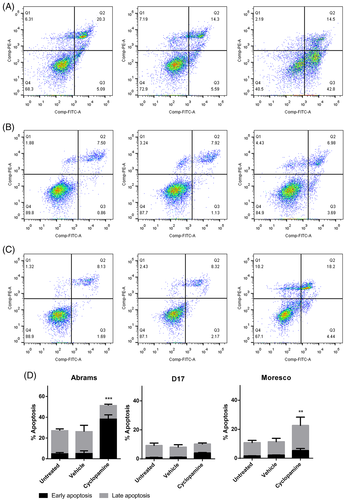
3.5 Cyclopamine increases apoptosis in Abrams and Moresco cells
Abrams, D17, and Moresco cells were treated with 8, 10, or 15 μM cyclopamine for 96 h and then stained with FITC Annexin V and PI to differentiate live, apoptotic, and necrotic cells. Figure 6A–C shows the populations of viable, early-apoptotic, late-apoptotic, and necrotic cells. Compared with non- or vehicle-treated cells, the early-apoptotic fraction was significantly increased in Abrams and Moresco cells, and the late-apoptotic fraction was also increased in Moresco cells after cyclopamine treatment (p < .01) (Figure 6D). D17 cells were not affected by the treatment.
4 DISCUSSION
Previous studies have revealed that overexpression or dysregulation of Hh signalling contributes to tumour formation, proliferation, and invasion in many types of human cancers, including basal cell, medulloblastoma, pancreatic, breast, colon, ovarian, and small cell lung carcinomas.25, 26 GLI signalling is expressed in canine OSA cell lines, variable expression levels of Hh family mRNA detected in canine OSA tissues, and IHH and the Hh mediators GLI and PTCH1 are activated in canine transitional cell carcinoma (TCC).17-19 In the present study, expression levels of SHH, IHH, SMO, and PTCH1 in three canine OSA cell lines were compared with those in canine osteoblast cells. All examined OSA cell lines had high mRNA levels of SHH, IHH, and SMO, with SHH mRNA expression in Abrams cells being the highest. A previous study reported that IHH was strongly expressed in four out of five TCC cell lines, whereas the SHH gene was not amplified from any of the TCC cell lines, suggesting that IHH rather than SHH plays a role in canine TCC.18 This is in contrast to human urothelial carcinoma, which is closely related with SHH.27 Several studies have shown overexpression of SHH, IHH, and DHH in human OSA in vivo and in vitro, and their correlation with tumour size, survival, and progression. However, which of these Hh ligands has the largest contribution has not been revealed.7, 28 Canine OSA seems more obviously influenced by SHH, based on the current findings.
Protein expression of the above Hh signalling components was also higher in the three canine OSA cell lines than in osteoblast cells; however, the degree of differences did not correspond with those on the mRNA level. For example, SHH protein expression in Abrams cells was only three times higher than that osteoblast cells, which was a smaller difference than that observed in mRNA expression. The SMO protein level appeared in reverse order of that of mRNA. PTCH1 mRNA expression was lower in Moresco cells than in osteoblast cells in a previous study,17 whereas PTCH1 protein expression in Moresco cells was two times higher than that in osteoblast cells in our study. The Hh mRNA expression levels did not necessarily represent the protein expression in the OSA cells treated with cyclopamine. Treatment with vismodegib significantly decreased GLI1 mRNA but did not change in the protein expression in HMPOS OSA cells.19 The generally poor correlation between mRNA and protein expression has been reported in numerous studies, although transcript levels are still often used as an estimate for the corresponding protein levels.29-31 There are various possible reasons for the discrepancy, including varied and complex post-translational modification, different half-life of proteins, and experimental error, which is not negligible.32, 33 Nevertheless, transcript and protein abundances of all signalling components evaluated were higher in the three canine OSA cell lines than in canine osteoblast cells, indicating that Hh/SMO signalling was activated in the canine OSA cell lines.
The inhibitory effect of cyclopamine on cell viability in canine OSA was the most obvious in Moresco cells, which exhibited the highest SMO protein expression. In a previous study, D17 cells, with the highest expression of GLI, were the most strongly affected by GANT61.17 Similar results have been obtained for canine TCC cell lines; K9TCC cells were more sensitive to the inhibition of cyclopamine than K9TCC-sh, as the former cells express higher levels of IHH.18 However, some studies have reported opposite effects; for example, SHEP1 human neuroblastoma cells are highly sensitive to cyclopamine, despite lower SHH signalling.34 The differential drug susceptibilities in different cell lines might result from variable expression of various Hh components. Colony formation was also suppressed by cyclopamine and showed trends similar to those observed in cell viability. These findings suggest that Hh/SMO signalling plays an important role in the proliferative potential of the canine OSA cell lines. Additional migration assay or in vivo experiments are necessary to identify whether cyclopamine affect the malignancy of canine OSA.
Regulation of cell survival by Hh signalling is associated with B-cell lymphoma 2 (BCL-2), an anti-apoptotic protein,35 and cyclopamine induces apoptosis by suppressing transcription of BCL-2 in some malignancies.36, 37 Cyclopamine significantly increased early apoptosis in Abrams cells, and both early and late apoptosis in Moresco cells, but it did not induce apoptosis in D17 cells. The differential effects on cell proliferation and apoptosis have been also found in several other cancer cell lines, including human biliary tract cancer and canine TCC cells.18, 38 Interestingly, more than 20% of non-and vehicle-treated Abrams cells were in the state of apoptosis. The high rate of spontaneous apoptosis of tumour cells reflects a high rate of cell turnover, which is an indicator of high proliferation.39 It was suspected that the high background level of apoptosis in canine TCC cell lines resulted from a high sensitivity to anoikis, a form of apoptosis or programmed cell death that occurs when anchorage-dependent cells are dislodged from the extracellular matrix.18, 40 The cell viability of Abrams appeared most sensitive by vismodegib treatment among three canine OSA cell lines including D17 and HMPOS but colony formation did not significantly decreased.19 The variable effects of SMO antagonist on cell viability, colony formation, and apoptosis in the different cell lines indicate the need for more specific and personalized cancer treatment.
Cyclopamine was the first molecule discovered to act as an SMO antagonist. The discovery of cyclopamine enabled Hh studies to expand from basic to preclinical translational research.41 However, because of chemical instability, limited potency, and side effects, its clinical application potential is limited.42, 43 Other compounds, including vismodegib and sonidegib, have been approved by the FDA for the treatment of locally advanced basal cell carcinoma. The anticancer effects of five SMO antagonists derived from acylguanidine and acylthiourea were determined in the MNNG human OSA cell line.44 In addition, a recent study have revealed that the vismodegib reduced canine OSA cell growth and proliferation.19 The results of the current study using cyclopamine indicate not only a role of SMO in canine OSA, but also the therapeutic potential of SMO inhibitors.
In conclusion, this study revealed that aberrant expression of Hh signalling in canine OSA cell lines contributes to OSA cell survival, which is inhibited by SMO blockade with cyclopamine. While further studies using canine OSA tumour samples and xenografts need to be done, the current findings are expected to be helpful for investigating the etiopathogenesis of canine and human OSA and developing new treatments for canine OSA.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Douglas Thamm who gifted us the Abrams and Moresco canine osteosarcoma cell lines. This manuscript is written based on the PhD thesis of author Aryung Nam. This study was supported by the Research Institute for Veterinary Science and BK21 Plus Program for Creative Veterinary Science Research, Seoul National University.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.