Mutation and methylation status of KIT and TP53 in canine cutaneous and subcutaneous mast cell tumours
Abstract
Cutaneous and subcutaneous mast cell tumours (MCTs) are counted among the most frequent cancers in dogs. However, the genetic aetiology of their development is still mostly unknown, with the exception of KIT and tumor protein p53 (TP53) mutations reported in less than a half of cutaneous MCTs. In subcutaneous MCTs, no gene alterations were previously detected. We analysed KIT and TP53 mutations in cutaneous and subcutaneous MCTs, and identified methylated CpG sites in KIT and TP53 promoters and adjacent exon 1 regions. The mutation analysis focused on KIT exons 8, 9 and 11, and TP53 exons 5-8, and revealed mutations in 26% and 7% cutaneous MCT cases, respectively. Moreover, we report a first case of KIT mutation ever detected in subcutaneous MCTs. KIT exon 11 mutations and high Kiupel and Patnaik grades were associated with reduced survival in this study. Both KIT and TP53 gene were generally unmethylated in canine cutaneous MCTs. A sporadic methylation of the CpG positions in KIT promoter and adjacent exon 1 was detected in 70.4% of cutaneous and 82% of subcutaneous MCTs. A sporadic methylation of the CpG positions in the TP53 promoter and exon 1 was observed in 36.8% of the analysed cutaneous MCT samples. Only in two subcutaneous MCTs, we observed more than 30% of clones showing KIT methylation at the CpG positions 13 or 14. The CpG position 14 is involved in a predicted binding site for Sp1 transcription factor. However, the significance of KIT promoter methylation at this specific position needs further evaluation.
Canine mast cell tumours (MCT) are among the most frequent malignancies in dogs, accounting for approximately 20% of canine skin tumours.1 Their clinical behaviour is highly variable, ranging from benign to highly aggressive with metastases and a high morbidity.2 Prognostic assessments are mostly based on clinical staging and histopathological grading.1-4 A negative prognosis was previously also found associated with a deregulated expression of the KIT (KIT proto-oncogene encoding the KIT tyrosine kinase receptor).5-7 Activating mutations in exons 8, 9 and 11 of the KIT gene representing a negative prognostic factor were described in 8%-40% of cutaneous MCTs.6, 8-10 Recently, also mutations in the tumor protein p53 (TP53) gene were detected in canine cutaneous MCTs.11 TP53 is a well-known tumour suppressor gene, and mutations in its DNA binding domain encoded by exons 5-8 were well documented in a variety of human cancers.12, 13 No gene mutations were revealed in the less studied canine subcutaneous MCTs so far, and MCTs located in subcutis usually show more favourable outcomes regarding recurrence rates, metastasis and survival times than the cutaneous MCT forms.14, 15
The available data indicate that KIT and TP53 mutations are not the only factors involved in MCT pathogenesis. In human cancers, a reduced TP53 expression was found associated with CpG methylation of the TP53 promoter.16 On the other hand, hypermethylation of the KIT gene was previously reported not only in association with the expected epigenetic silencing17 but, paradoxically, also with KIT overexpression in human tumours.18, 19 Both canine TP53 and KIT promoter sequences contain CpG sites and share a high homology with humans.20, 21
In this study, we tested a hypothesis that not only KIT and TP53 mutations but also their promoter methylation can play a role in canine MCT pathogenesis. We searched for mutations in KIT (exons 8, 9 and 11) and TP53 (exons 5-8) in cutaneous and subcutaneous MCTs, and identified MetCpG positions in the KIT and TP53 promoter regions. We used the technique of sequencing and cloning, which reveals molecule-specific DNA methylation patterns.22
A total of 27 cutaneous and 11 subcutaneous MCT samples were obtained from client owned dogs at several veterinary clinics in the Czech Republic at the time of surgery. The central part of each tumour (approx. 0.5 cm3) without any signs of necrosis was separated for the molecular genetic analysis. All tumours were histopathologically confirmed as MCTs and graded using previously published grading systems.3, 4 Only tumours located solely in the subcutis were classified as subcutaneous, not those extending into the subcutis from the cutaneous location. The dogs' survival was followed for at least 12 months after surgery. The characteristics of the dogs and their MCTs are summarized in Table 1.
| Histological grade | Mutation | |||||||
|---|---|---|---|---|---|---|---|---|
| ID | Breed | Age at surgery | Localization | Survival (months) | Patnaik | Kiupel | KIT | TP53 |
| C1 | Bull Terrier | 10 | Trunk | 12 | I | Low | wt | wt |
| C2 | Amer. Bulldog | 11 | Leg | 13 | I | Low | wt | wt |
| C3 | Boston Terrier | 8 | Head | 14 | I | Low | wt | wt |
| C4 | Bernese Mount. dog | 9 | Leg | 13a | II | Low | wt | wt |
| C5 | Golden Retriever | 6 | Head | 21 | II | Low | wt | wt |
| C6 | Weimaraner | 9 | Trunk | 13 | II | Low | wt | wt |
| C7 | Miniature Schnauzer | 10 | Trunk | 13 | II | Low | wt | wt |
| C8 | Weimaraner | 6 | Trunk | 17 | II | Low | wt | wt |
| C9 | Dachshund | 12 | Scrotum | 13 | II | Low | wt | wt |
| C10 | Rhodesian Ridgeback | 8 | Trunk | 21 | II | Low | wt | wt |
| C11 | Rhodesian Ridgeback | 8 | Leg | 17 | II | Low | wt | wt |
| C12 | Yorkshire Terrier | 9 | Scrotum | 13 | II | Low | wt | wt |
| C13 | Labrador Retriever | 9 | Trunk | 20 | II | Low | wt | wt |
| C14 | Labrador Retriever | 7 | Trunk | 30 | II | Low | ex8 ITD417-421b | wt |
| C15 | Boxer | 7 | Leg | 14 | II | Low | wt | wt |
| C16 | Crossbreed | 8 | Trunk | 10a | II | Low | ex11 ITD571-595c | wt |
| C17 | Rhodesian Ridgeback | 7 | Head | 13 | II | Low | wt | wt |
| C18 | Border Collie | 9 | Leg | 14 | II | Low | wt | wt |
| C19 | Amer. Pitbull Terrier | 8 | Leg | 12 | II | Low | wt | wt |
| C20 | Pug | 13 | Scrotum | 9a | II | High | wt | wt |
| C21 | Labrador Retriever | 8 | Trunk | 8a | II | High | ex11 ITD576-590d | wt |
| C22 | French Bulldog | 11 | Leg | 10a | II | High | ex11 ITD575-588e | wt |
| C23 | Chinese Shar-Pei | 13 | Head | 6a | III | High | wt | ex6 S203R |
| C24 | German Shepherd | 9 | Leg | 7a | III | High | wt | wt |
| C25 | Crossbreed | 12 | Trunk | 8a | III | High | ex11 ITD580-595f | wt |
| C26 | Flat Coated Retriever | 6 | Head | 6a | III | High | ex11 ITD574-594g | wt |
| C27 | Boxer | 6 | Leg | 1a | III | High | ex11 P554L | ex7 S229F |
| S1 | Bernese Mount. dog | 4 | Leg | 24 | II | Low | ex8 ITD417-421b | wt |
| S2 | Am. Stafford. Terrier | 11 | Trunk | 14 | II | Low | wt | wt |
| S3 | Pug | 5 | Leg | 3a | II | Low | wt | wt |
| S4 | Labrador Retriever | 12 | Trunk | 15 | II | Low | wt | wt |
| S5 | Rhodesian Ridgeback | 13 | Trunk | 4a | II | Low | wt | wt |
| S6 | Bernese Mount. dog | 8 | Leg | 12 | II | Low | wt | wt |
| S7 | Jack-Russell Terrier | 7 | Leg | 12 | II | Low | wt | wt |
| S8 | Cross | 8 | Trunk | 12 | II | Low | wt | wt |
| S9 | Cross | 10 | Leg | 12 | II | Low | wt | wt |
| S10 | Weimaraner | 9 | Trunk | 13 | II | Low | wt | wt |
| S11 | Golden Retriever | 10 | Leg | 12a | II | High | wt | wt |
- Abbreviations: C, cutaneous; ex, exon; ITD, internal tandem duplication; MCT, mast cell tumour; S, subcutaneous; wt, wild type.
- a Dead.
- b ITD417-421 AATCCTGACTCA.
- c ITD571-595 TGTTTACATAGACCCAACACAGCTTCCTTACGATCACAAATGGGAGTTTCCCAGAAACAGGCTGAGCTTTGGTCA.
- d ITD576-590 CCAACACAGCTTCCTTACGATCACAAATGGGAGTTTCCCAGAAAC.
- e ITD575-588 CCCAACACAGCTTCCTTAYGATCACAAATGGGAGTTTCC.
- f ITD580-595 CTTACGATCACAAATGGGAGTTTCCCAGAAACAGGCTGAGCTTTGGTC.
- g ITD574-594 AGACCCAACACAGCTTCCTTACGATCACAAATGGGAGTTTCCCAGAAACAGGCTGAGCTT.
Tumour genomic DNA was isolated from the fresh MCT samples using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and purified by MinElute PCR Purification Kit (Qiagen). The mutation analysis of the KIT (exons 8, 9 and 11) and TP53 (exons 5-8) gene was performed as described previously.11 The primer sequences and polymerase chain reaction (PCR) conditions are displayed in Table 2. The isolated tumour genomic DNA was further subjected to bisulphite modification using EpiJET Bisulfite Conversion Kit (Thermo Fisher Scientific, Waltham, Massachusetts) and the promoter and exon 1 regions of KIT and TP53 were amplified using specific primers designed to anneal to both methylated and unmethylated DNA (Table 2). The canine sequence KF471023 (KIT) and a part of canine sequence NC_006587 homologous to human X54156 (TP53) were converted in silico and used for the selection of primers. The PCR products were run on a 2% agarose gel; the specific bands were extracted from the gel, and cloned into pDrive Cloning Vector (Qiagen). Screening of the recombinant clones was performed by PCR using the VEC primers derived from pDrive Vector sequence (Table 2). Methylation status of at least six clones per each MCT sample was analysed by sequencing.
| Analysis | Target | Primer | Primer sequence | Amplicon size | Annealing temp. | Cycles |
|---|---|---|---|---|---|---|
| KIT mutation | Exon 8 | cKIT8 | GCTCCCCTTTGAATRTGTTCC | 549 bp | 55°C | 30 |
| TTACTCTGTCCTGGAAAATTGC | ||||||
| Exon 9 | cKIT9 | TTTCCTAGAGTAAATCCAGG | 278 bp | 55°C | 30 | |
| CTAAACATCCCCTTAAATTGG | ||||||
| Exons 10-12 | cKIT11 | TAGAACAAATCCATCCCCACACCC | 713 bp | 61°C | 30 | |
| CATCTTAACGGCAACAGTCATGGC | ||||||
| TP53 mutation | Exon 5 | TP-5 | CTCTGGGGAGCAGCCTTGTCC | 267 bp | 63°C | 30 |
| ATGCCCTGACCTGTCCATCTGTCC | ||||||
| Exon 6, 7 | TP-6,7 | AGGGTGATGATAGTGAGGATGGG | 377 bp | 57°C | 30 | |
| CTGGACGACAGAAACACTTTTCG | ||||||
| Exon 8 | TP-8 | CTCCTCTTGTCTTGCTTGCTTACC | 260 bp | 57°C | 30 | |
| TAGTCTGTAGGCTTTGGCTCTACG | ||||||
| Methylation | KIT promoter | MET-KIT | GAGAGTYGGTGATATGTAGG | 254 bp | 49°C | 40 |
| ATCRCRATAACTACGCTAAAACC | ||||||
| TP53 promoter | MET-TP | TTCATGTCTGACACAGGAGCAGGT | 446 bp | 50°C | 43 | |
| GGGTAAGCTCCTGACTGAGCCA | ||||||
| Vector | VEC | CAGCTATGACCATGATTACGCC | 56°C | 30 | ||
| TCACGACGTTGTAAAACGACG |
- Note: Y—C or T; R—A or G.
We revealed heterozygous KIT mutations in 26% (7/27) of cutaneous and 9% (1/11) of subcutaneous MCT cases (Table 1). In canine cutaneous MCTs, mutations in the KIT gene, namely exons 8, 9 and 11, were previously reported in 9%-40% of the cases.6, 7, 9, 10 The internal tandem duplications (ITD) in exon 11 detected here were localized to a genomic region known to be frequently altered in canine cutaneous MCTs.7-10, 23 Also, the ITD417-421 in exon 8 encoding the immunoglobulin-like domain of the KIT receptor was repeatedly described in canine cutaneous MCTs.7, 9-11 Subcutaneous MCTs were previously genetically analysed only in two studies which did not reveal any mutations.15, 24 The 12 bp ITD417-421 in KIT exon 8 detected in this study thus represents a first case of KIT mutation found in subcutaneous MCTs to date. It was previously shown that the ITD417-421 in exon 8 and the ITD572-586 in exon 11 located in the same region as the five ITDs in this study cause growth factor-independent proliferation by constitutively activating the KIT tyrosine kinase.9 KIT activating mutations in exon 11 were previously shown as negative prognostic factors in canine cutaneous MCTs.5, 6, 8-10 Similarly in this study, KIT mutations in exon 11 were significantly associated with high tumour grades (P = .001, rs = 0.602) and reduced survival time (P = .001, rs = −0.611) (Spearman's correlation, Statistical Package for the Social Sciences - SPSS software ver. 24, SPSS, Chicago, Illinois). However, any association of the ITD417-421 in exon 8 with high grade and negative prognosis was not proved in this study. This might have been caused by the small number of KIT exon 8 mutation cases but a similar lack of a significant association between KIT mutations and tumour related death was previously observed in other studies.7, 11 The logrank test and Kaplan-Meier survival curves showed a significant association (P < .001) of KIT mutations, and high Patnaik and Kiupel grades with reduced survival times (Figure 1).
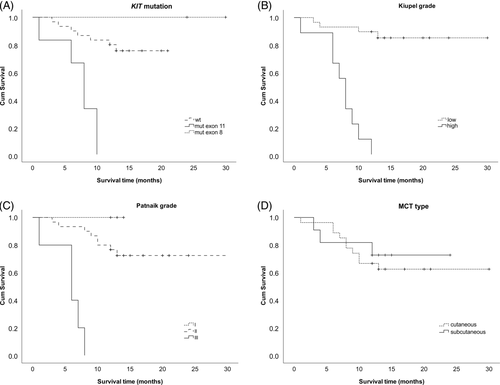
The TP53 gene is a major tumour suppressor altered in a wide range of human and canine cancers.12, 13, 25, 26 Mutations in TP53 exons 5-8 were previously found in 15% of canine cutaneous MCTs.11 In this study, we detected heterozygous missense mutations in TP53 exon 6 and 7 in 7.4% of cutaneous MCT samples (2/27). These alterations (S203R and S229F) were analogous to the known human loss of function substitutions (S215R and S241F) (International Agency for Research on Cancer - IARC TP53 Database, ver. R19, November 2018).13 Although the low number of TP53 mutations did not allow proper statistical evaluation, both TP53 mutations in this study were associated with a high histopathological grade and short survival time. In this first attempt to assess the TP53 mutation status also in subcutaneous MCTs, we did not detect any TP53 alterations in this type of MCTs.
Presuming that DNA methylation can possibly be an alternative cause of canine MCT pathogenesis,27 we analysed the methylation status of KIT and TP53 regulatory regions.
In order to identify CpG sites susceptible to methylation, we assessed the methylation status of 38 CpGs in the KIT promoter and adjacent exon 1 in 27 cutaneous and 11 subcutaneous MCTs. In 19 cutaneous and 9 subcutaneous MCT samples, clones showing methylation in at least one CpG position were observed (Figure 2). However, the MetCpGs were rare, their positions varied in the individual clones, and the gene was generally unmethylated. DNA methylation was most frequently detected at the CpG position 38 in cutaneous and 14 in subcutaneous MCTs. Only in two subcutaneous cases, we found 33% clones showing methylation in the CpG positions 13 and 14, respectively. Using TFBIND software (http://tfbind.hgc.jp/, accessed March 6, 2019), we revealed that the methylated CpG at position 14 of the KIT promoter is located in a predicted binding site (sequence GGGGCGTGGC) for Sp1 transcription factor which is known to be involved in the KIT transcription activation.28 Given the expected transcription inactivation caused by the inhibition of Sp1 binding by hypermethylation of its binding site, we hypothesise that in some subcutaneous MCTs the KIT CpG methylation might be associated with their favourable prognosis. However, the KIT immunohistochemical staining patterns were similar in all MCT samples analysed in this study (pattern 2: perimembrane and stippled cytoplasmic KIT localisation) not depending on their methylation or mutation status. An analysis including quantitative expression data in a higher number of MCT cases is needed to verify this hypothesis.
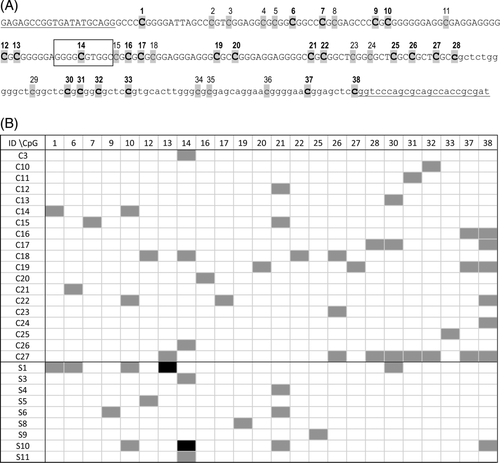
The previously reported A/G single nucleotide polymorphism (NC_006595 position 47 108 373) of the KIT promoter21 was also observed in this study. Briefly, the nucleotide A was detected in 52.6% (20/38) of the cases; its frequency did not differ between cutaneous and subcutaneous MCTs, and it did not correlate with MCT grade or survival time.
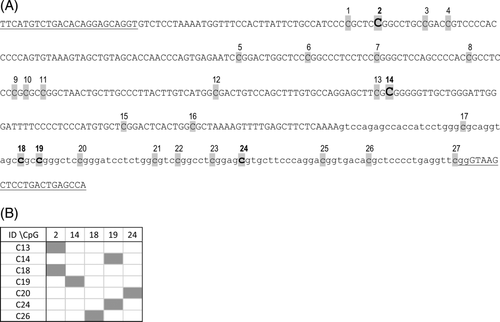
DNA methylation was reported as an alternative mechanism of inactivation of tumour suppressor genes including TP53 in various human cancers.16, 29 We analysed methylation status of 27 CpG sites in the TP53 promoter and adjacent exon 1 region in 19 cutaneous MCTs. Clones with sporadic MetCpGs were detected in seven samples (Figure 3). Five different CpG positions were methylated in the individual clones, but the methylation rates were low and the gene was generally unmethylated.
In this pilot study, we identified several MetCpG positions in the KIT regulatory regions, which can be exploited in future studies using methylation sensitive PCR. Our hypothesis that an inhibition of Sp1 binding to the KIT promoter through KIT hypermethylation might lie behind the previously reported favourable prognosis in subcutaneous MCT cases needs further testing. Future research taking into account both methylation status and quantitative expression data is needed to better understand the role of KIT methylation in pathogenesis of canine MCTs, and evaluate its potential prognostic and therapeutic value. No difference in survival times between cutaneous and subcutaneous MCTs was observed in this study. The first case of KIT mutation in subcutaneous MCTs detected in this study (ITD417-421 in exon 8) suggests that KIT mutations are not limited to cutaneous MCT forms. Despite the fact that KIT mutations are considered as negative prognostic factors in canine MCT, the ITD417-421 in exon 8 was not associated with a high histopathological grade or decreased survival time in this study.
The study complies with the current laws of the Czech Republic. All applicable international, national and institutional guidelines for the care and use of animals were followed.
ACKNOWLEDGEMENTS
This study was supported by the grant 16-26655S from the Czech Science Foundation (GA CR), by the Ministry of Agriculture (RO 0518) and by the Ministry of Education, Youth and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601). The authors are grateful to the dog owners and to the veterinarians who collected the tumour samples for analysis.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.