Ultra-frequent HRAS p.Q61R somatic mutation in canine acanthomatous ameloblastoma reveals pathogenic similarities with human ameloblastoma
Funding information: College of Veterinary Medicine; Cornell University
Abstract
Ameloblastoma is a locally aggressive odontogenic tumour that occurs in humans and dogs. Most ameloblastomas (AM) in humans harbour mutually-exclusive driving mutations in BRAF, HRAS, KRAS, NRAS or FGFR2 that activate MAPK signalling, and in SMO that activates Hedgehog signalling. The remarkable clinical and histological similarities between canine acanthomatous ameloblastoma (CAA) and AM suggest they may harbour similar driving mutations. In this study, aimed at characterizing the mutational status of SMO, BRAF, HRAS, KRAS, NRAS and FGFR2 in CAA, we used RNA sequencing, Sanger sequencing and restriction fragment length polymorphism assays to demonstrate that 94% of CAA (n = 16) harbour a somatic HRAS p.Q61R mutation. The similarities in MAPK-activating mutational profiles between CAA and AM implicate conserved molecular mechanisms of tumorigenesis, thus, qualifying the dog as a potentially useful model of disease. Given the relevance of RAS mutations in the pathogenesis of odontogenic tumours and other types of cancer, the results of this study are of comparative, translational, and veterinary value.
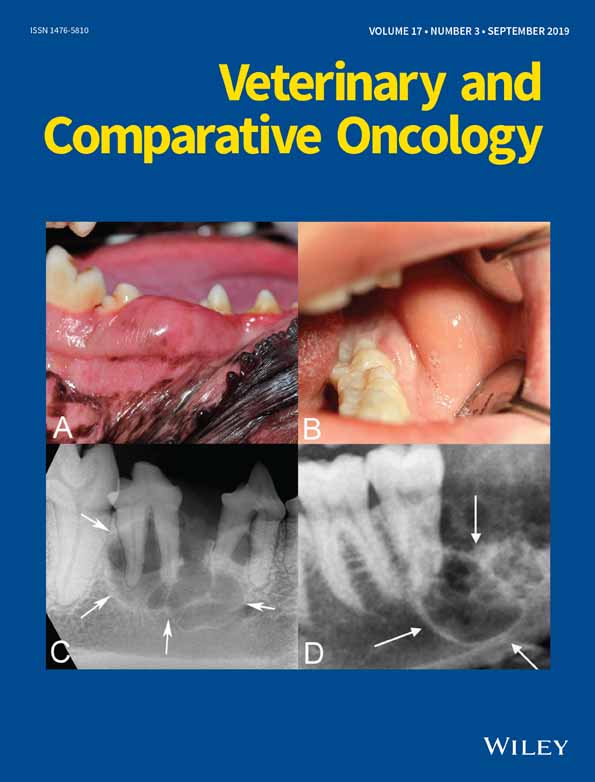
Ameloblastoma is an odontogenic tumour that is rare in humans but is relatively common in dogs, particularly in golden retrievers, cocker spaniels, Akitas, Shetland sheepdogs and mixed breed dogs.1-4 As in humans, ameloblastomas in dogs are more common in the mandible than in the maxilla, and are typically locally aggressive (Figure 1).3, 5, 6 The standard of care for ameloblastomas has traditionally been wide surgical excision of the affected area of the mandible or maxilla.7, 8 However, surgery is highly invasive and may result in significant disfigurement and dysfunction.4, 9 Novel therapeutic approaches based on molecular oncogenic mechanisms are expected to replace or complement current surgical solutions and thus help minimize patient morbidity while improving possible outcomes.10
Recent discoveries have shown that the majority of human ameloblastomas (AM) are driven by mutually-exclusive BRAF, HRAS, NRAS, KRAS or FGFR2 mutations that activate the MAPK pathway regulating cell survival.9, 11-15 Co-occurring SMO mutations that alter the Hedgehog signalling pathway to regulate cell fate have also been detected, especially in maxillary tumours.12, 13 Both pathways are mutated in various cancers and have been targeted for therapy, making them of particular interest in personalized oncology.9
Tooth development is similar in humans and dogs, and canine acanthomatous ameloblastomas (CAA) and AM show remarkable clinical and histological similarities.3, 14, 16 Therefore, we hypothesize that both tumours may harbour oncogenic mutations in the same genes, making the canine disease directly relevant as a model of human odontogenic tumours.
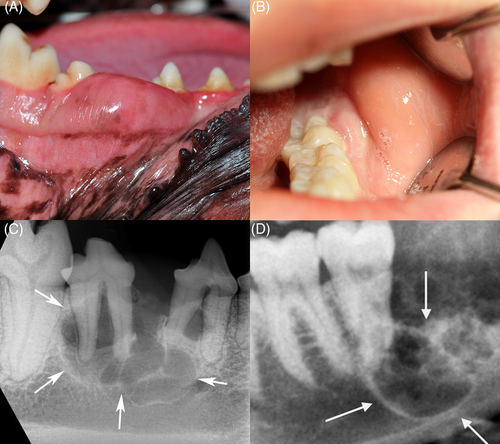
To determine whether mutational similarities underlie the clinical and histological features shared by CAA and AM, our study focused on the molecular characterization of candidate oncogenic mutations in SMO, BRAF, HRAS, KRAS, NRAS and FGFR2 in 16 CAA cases from client-owned dogs (Table 1). Eight gingival oral squamous cell carcinoma (OSCC) tumour samples, as well as four healthy gingival samples, all from different dogs, were used as a comparator set. Dog owners signed an informed consent for use of tissues and blood for research purposes prior to collection. Sample collection and experimental procedures were approved by Cornell University's Institutional Animal Care and Use Committee. All tumours were independently confirmed by histological assessment of routine haematoxylin and eosin (H&E) stained formalin-fixed and paraffin-embedded tumour sample by a board-certified veterinary pathologist (GED), in accordance with previously described diagnostic criteria4 (Figure 2). The estimated tumour content based on histological assessment was >90%.
| Case No. | Dog breed | Sex | Age (years) | Diagnosis | Tumour/tissue location | HRAS status in tumour tissues | HRAS status in genomic DNA |
|---|---|---|---|---|---|---|---|
| 1 | Great Dane | Male | 7 | CAA | Rostral maxilla | Q61R | Wild-type |
| 2 | Samoyed | Male | 12 | CAA | Rostral maxilla | Q61R | Wild-type |
| 3 | Shiba Inu | Male | 11 | CAA | Rostral maxilla | Q61R | Not tested |
| 4 | Golden retriever | Male | 10 | CAA | Rostral maxilla | Q61R | Wild-type |
| 5 | Neapolitan mastiff | Male | 10 | CAA | Rostral mandible | Q61R | Wild-type |
| 6 | Mixed | Male | Unknown (adult) | CAA | Rostral mandible | Q61R | Not tested |
| 7 | Mixed | Male | 6 | CAA | Rostral mandible | Q61R | Not tested |
| 8 | Mixed | Male | 12 | CAA | Rostral mandible | Q61R | Not tested |
| 9 | Labrador retriever | Female | 12 | CAA | Rostral mandible | Q61R | Wild-type |
| 10 | Labrador retriever | Male | 9 | CAA | Rostral mandible | Q61R | Not tested |
| 11 | Airedale terrier | Female | 10 | CAA | Caudal mandible | Q61R | Wild-type |
| 12 | Labrador retriever | Male | 9 | CAA | Caudal mandible | Q61R | Not tested |
| 13 | Labrador retriever | Female | 11 | CAA | Caudal mandible | Q61R | Not tested |
| 14 | Labrador retriever | Female | 8 | CAA | Caudal mandible | Q61R | Wild-type |
| 15 | Staffordshire bull terrier | Female | 9 | CAA | Caudal mandible | Q61R | Not tested |
| 16 | Labrador retriever | Male | 5 | CAA | Caudal mandible | Wild-type | Not tested |
| 17 | German shorthair pointer | Female | 12 | OSCC | Caudal mandible | Wild-type | Not tested |
| 18 | Great Dane | Male | 5 | OSCC | Rostral mandible | Wild-type | Not tested |
| 19 | Boxer | Male | 9 | OSCC | Caudal maxilla | Wild-type | Not tested |
| 20 | Mixed | Male | 12 | OSCC | Rostral mandible | Wild-type | Not tested |
| 21 | Mixed | Male | 4 | OSCC | Rostral mandible | Wild-type | Not tested |
| 22 | Boxer | Male | 12 | OSCC | Rostral mandible | Q61L | Not tested |
| 23 | Mixed | Female | 10 | OSCC | Caudal maxilla | Wild-type | Not tested |
| 24 | Mixed | Female | 10 | OSCC | Rostral maxilla | Wild-type | Not tested |
| 25 | Beagle | Male | 3 | Healthy gingiva | Rostral mandible | Wild-type | Not tested |
| 26 | Staffordshire bull terrier | Male | 10 | Healthy gingiva | Caudal mandible | Wild-type | Not tested |
| 27 | Pit-bull mix | Male | 0.3 | Healthy gingiva | Rostral maxilla | Wild-type | Not tested |
| 28 | Mixed | Female | Unknown (adult) | Healthy gingiva | Caudal maxilla | Wild-type | Not tested |
- Abbreviations: CAA, canine acanthomatous ameloblastoma; : RNAseq, RNA sequencing; OSCC, oral squamous cell carcinoma.
Frozen study tissues (flash frozen in liquid nitrogen or preserved in RNAlater [Thermo Fisher Scientific, Waltham, Massachusetts] before freezing at −80°C) were homogenized in Trizol (Thermo Fisher) using a bead mill without being allowed to thaw. RNA was isolated following the manufacturer's protocol with the following modifications: an extra chloroform extraction was added prior to precipitation, 1 μL glycoblue (Thermo Fisher) was added immediately prior to precipitation, and the RNA pellet was washed twice with 75% ethanol. RNA concentration was measured with a Nanodrop (Thermo Fisher) and integrity determined with a Fragment Analyser (Advanced Analytical).
PolyA+-enriched RNAseq libraries were generated with the NEBNext Ultra II Directional library prep kit (New England Biolabs, Ipswich, Massachusetts) using 250 ng input total RNA. Single-end 85 nt reads were generated on a NextSeq500 instrument (Illumina). Reads were trimmed with cutadapt17 and mapped to the CanFam3 reference genome with Tophat18 v2.1.1 to investigate polymorphisms in candidate oncogenic driver genes. Candidate sequence polymorphisms were discovered in SMO, BRAF, HRAS, KRAS, NRAS and FGFR2 guided by GATK best practices for calling variants in RNAseq, using picard tools MarkDuplicates (http://broadinstitute.github.io/picard), GATKv4 SplitNCigarReads, and GATKv4 HaplotypeCaller.19 Variant Effect Predictor20 (Ensembl) was used to assess the effect of variants discovered in candidate genes to identify missense coding single-nucleotide polymorphisms.
For Sanger sequencing and restriction fragment length polymorphisms (RFLP) validation assays, each RNA sample was used to generate cDNA using MMLV reverse transcriptase (Sigma-Aldrich, St. Louis, Missouri) according to the manufacturer's instructions in a final 20 μL reaction containing 500 ng total RNA, 33 μM random nonamer primers, and 25 μM oligo-dT20 primers. The reaction was incubated at 37°C for 1 hour followed by 85°C for 10 minutes to inactivate the reverse transcriptase. Genomic DNA was isolated from ethylenediaminetetraacetic acid blood samples by the Cornell Veterinary Biobank using a standard salt precipitation technique, as previously reported.21
Polymerase chain reaction (PCR) primers were designed using Primer-BLAST at NCBI for HRAS flanking the Q61 position: forward primer ATCGGAAGCAAGTGGTCATCG, reverse primer ACTGGTGGATGTCCTCAAAGG; BRAF flanking the V600 position: forward primer CAGGGCATGGATTACTTACACGC, reverse primer TTGAAGGCTGCAAATTCTCCGTATC; and KRAS flanking the G12 and Q61 positions: forward primer GCCTGCTGAAAATGACTGAATATAAAC, reverse primer GCTAAGTCCTGAGCCTGTTTTG. Each PCR reaction contained 1x Q5 Hot Start High Fidelity Master (New England Biolabs), 25uM each PCR primer (IDT), and 1 μL cDNA (from tumour RNA) or 500 ng genomic DNA (from blood) in a 50 μL reaction volume. PCR reactions were cycled as follows: denaturation at 98°C/3 minutes; 35 cycles of 98°C/10 seconds—62°C/10 seconds—72°C/15 seconds [HRAS] or 30 seconds [BRAF, KRAS]; final extension 72°C/5 minutes. Reactions were cleaned up with a PCR purification kit (Qiagen buffers, Omega HiBind columns), eluted in 50 μL EB, and quantified with a Nanodrop. PCR amplicons were sequenced at the Cornell BRC Genomics Facility using 8 μL PCR product (120-400 ng) combined with 25 Pmol primer [HRAS and KRAS: reverse primer; BRAF: forward primer]. KB Basecaller software (Thermo Fisher) was used to compensate for mobility differences between dyes. Restriction digest reactions containing 45 ng PCR product, 1× buffer, 1 μL restriction enzyme (New England Biolabs) in total 20 μL reaction volume and were incubated at the recommended temperature for 20 minutes. Digested samples were run on a native 6% polyacrylamide gel electrophoresis gel at 100 V for 2 hours and stained with GelStar (Lonza).
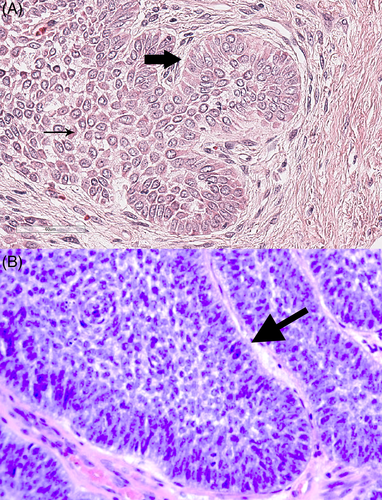
Analysis of RNA sequencing data for variants in SMO, BRAF, HRAS, KRAS, NRAS and FGFR2 identified reads that indicated a possible HRAS missense mutation (A → G) that would alter the protein sequence from glutamine (cAg) to arginine (cGg) at position 61 (p.Q61R mutation) in 11 of 12 CAA samples, while no oncogenic mutations were observed in the other candidate genes (see Appendix S1). The RNAseq reads for the CAA samples are available at the NCBI Sequence Read Archive repository with BioProject accession PRJNA533473. To validate the occurrence of the HRAS p.Q61R mutation in CAA, we designed a PCR assay for canine HRAS exon 2 flanking the variant site (amplicon size 168 bp) that can be used on either RNA (cDNA) or genomic DNA samples. The presence of the variant allele was assessed by two different approaches: Sanger sequencing and RFLP. The presence of the variant allele was detected by Sanger sequencing in cDNA from 15 of 16 (94%) CAA samples (inclusive of the 12 samples used for RNA sequencing plus four additional independent samples), as evidenced by a T nucleotide (antisense strand) as well as the wild-type C nucleotide (Figure 3 ; see also Appendix S1). To determine whether the mutant allele was present in other (non-tumour) tissues in some of the same dogs or arose within the tumour, we also sequenced genomic DNA isolated from paired blood samples from seven dogs. All seven dogs had only the wild-type allele, indicating a somatic mutation occurred in the tumour in each case.
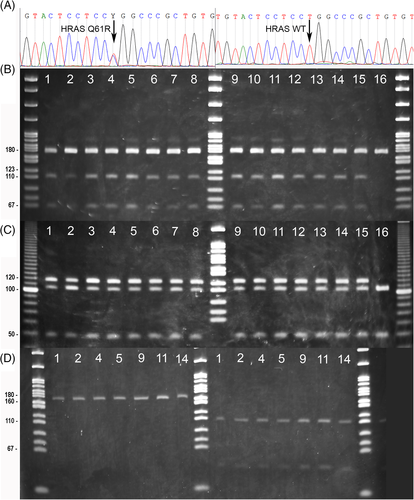
We further investigated these results using RFLP assays on all 16 CAA samples available for analysis. We developed RFLP assays for both the mutant and wild-type alleles: HpaII cuts only the mutant allele (CCGG) to generate bands of 106 bp and 62 bp; BstNI cuts the wild-type allele as well as an adjacent invariant site, resulting in 46 bp and 107 bp bands, whereas samples harbouring the mutant allele have a band at 122 bp (the invariant 15 bp fragment not visible on the gel). In all cases, the RFLP assays were internally consistent and agreed with the Sanger sequencing, confirming that all but one CAA sample (case No. 16) harbours the mutant allele. RFLP analysis (HpaII and BstN1 assays) in genomic DNA from seven paired blood samples confirmed all blood samples carry only the wild-type allele demonstrating that the HRAS p.Q61R mutation is somatic and not inherited. For BRAF and KRAS, which had the lowest read coverage in the RNAseq data, Sanger sequencing confirmed that no oncogenic variants were present in any of the CAA cases (see Appendix S1).
To confirm that the HRAS p.Q61R mutation was found at high frequency specifically in CAA cases, we performed Sanger sequencing on eight independent gingival OSCC and four normal gingival samples collected from healthy dogs. Oral squamous cell carcinoma was used for comparison because this tumour type represents the most common oral malignancy of epithelial origin in dogs, and shares some histological features with CAA.4, 22, 23 All OSCC samples had wild-type HRAS alleles, except case No. 22 which showed an HRAS p.Q61L allele (see Appendix S1), consistent with a previous report showing low frequency of this oncogenic mutation in this tumour type.24
The presence of a somatic HRAS p.Q61R mutation in 94% of the CAA tumour samples analysed in this study, combined with pathogenic similarities between CAA and AM, strongly implicate conserved oncogenic molecular mechanisms between canine and human cancers. The known clinical and histological similarities between CAA and AM,16 and now the observation of a shared oncogenic mutation, invokes the opportunity to leverage dogs as a natural model of this disease. Moreover, given that RAS mutations are also well-known drivers of several types of cancer in people,25 our findings suggest that dogs may potentially be used as translational models for targeted treatments of MAPK pathway mutated neoplasms. This is a significant finding considering that dog models offer many advantages over small and (other) large animal models (ie, primates) and can be particularly valuable for drug discovery and development purposes.26
The apparent prominent role of RAS mutations in tumorigenesis of odontogenic neoplasms is not unprecedented.15 Indeed, RAS mutant mice have been shown to develop odontogenic neoplasms.27 Additionally, most adenomatoid odontogenic tumours harbour RAS mutations.28 Finally, during the review process of this article, another study based on exome sequencing reported recurrent HRAS mutations in CAA.29
Despite the pathological similarities between CAA and AM, the data show a notable difference in the frequency of the HRAS p.Q61R mutation. Contrasting with our findings, only 20% of AM harbour RAS mutations, with HRAS p.Q61R occurring with a frequency of 6% or less (Figure 4).11-14 Similarly, while two-thirds or more of AM harbour a BRAF p.V600E mutation,11-14 the results of this and previous studies29, 30 show that homologous mutations are either not present in CAA or occur at low frequency.
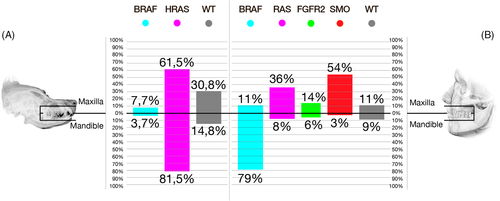
Nevertheless, given that both BRAF and RAS mutations can activate MAPK signalling,15 the difference in frequencies of BRAF and RAS mutations does not necessarily translate in differences in the biological mechanisms driving tumorigenesis. In fact, an HRAS mutant CAA cell line has been shown to be sensitive to targeted MAPK small-molecule inhibition, 29 further reinforcing the notion that dogs might represent a suitable model of disease. As well, our findings suggest that MAPK signalling is likely involved in both maxillary and mandibular CAA. This is consistent with MAPK activating mutations dominating oncogenesis of maxillary and mandibular AM, and SMO and other simultaneous mutations acting as secondary drivers.13
This study focused exclusively on candidate genes known to be mutated in AM,11-14 and it is possible that other oncogenic or tumour suppressor genes could be mutated in CAA, including non-coding RNAs.31 Furthermore, these data do not ascertain whether the HRAS p.Q61R mutation acts as a primary or secondary oncogenic driver in CAA. Therefore, additional studies are required, especially considering that the biological effects of RAS mutations (ie, proliferation vs senescence) depend on the level of expression of the mutated allele, among other variables.32 Genome-scale mutational analysis as well as molecular phenotyping will further elucidate the oncogenic mechanisms involved in CAA and clarify similarities and differences with AM.
The veterinary clinical and diagnostic significance of the current study should not be overlooked in light of its comparative and translational potential. Assays specific to the HRAS p.Q61R allele, such as those described here, may offer veterinary diagnostic potential to distinguish CAA from other oral tumours with overlapping histological features but different biological behaviour, including OSCC.4, 22, 23 Our confirmation of high frequency MAPK-activating HRAS alleles in 15 of 16 CAA cases justifies the investigation of precision-based therapies for companion dogs that have the potential to mediate disease progression as an alternative to current invasive and costly surgical treatments.
ACKNOWLEDGEMENTS
Christine Butler and Ann Tate provided technical help with experiments. This work was financially supported by the Foundation for Veterinary Dentistry (2016 Research Award). Cryopreserved samples and associated phenotypic data as well as genomic DNA isolated from paired blood samples were provided by the Cornell Veterinary Biobank, a resource built with the support of NIH grant R24 GM082910 and the Cornell University College of Veterinary Medicine. Figure 4 was designed with the assistance of AD Juha Kettunen, who is supported by the Maritza and Reino Salonen Foundation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The RNAseq reads for the CAA samples are available at the NCBI Sequence Read Archive repository with BioProject accession PRJNA533473: