Synthetic microRNA-205 exhibited tumour suppression in spontaneous canine malignant melanoma by intratumoral injection
Abstract
MicroRNAs (miRNA) are small, noncoding RNA molecules consisting of 18 to 25 nucleotides. Malignant melanomas (MMs) are one of the most common malignancies in both dogs and humans. We previously reported that chemically modified synthetic miRNA-205 (miR-205BP/S3) inhibits melanoma growth in vitro and in vivo. The present study aimed to evaluate the efficacy of intratumoral administration of synthetic miR-205 for spontaneous CMMs and to evaluate its potential as systemic therapy. Ten dogs with various stages of MM were treated with miR-205BP/S3 injected into tumours. Adverse effects (AEs) were assessed in accordance with the Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE) v1.1 guidelines. Five cases attained complete remission (CR), three attained stable disease (SD), and two cases displayed characteristics of progressive disease (PD). In all cases, no changes were observed in the blood parameters upon miRNA administration, and miR-205BP/S3 administration did not yield any side effects. The present results suggest that intratumoral administration of miR-205BP/S3 is a potentially applicable treatment for canine melanoma.
1 INTRODUCTION
MicroRNAs (miRNA) are small, non-coding RNA molecules of 18 to 25 nucleotides and are highly conserved across species.1, 2 In humans, over 3000 miRNAs have been predicted (miRBase; http://www.mirbase.org/). Approximately, 33% of all mRNAs are reportedly regulated by miRNAs, and increasing evidence indicates that they have critical roles in numerous cellular processes, including cell differentiation, proliferation, death, stress resistance and fat metabolism.2-4 Recent studies on cancer therapy using miRNA have reported significant advancements, for example, MRX34, a liposomal miR-34a mimic miR with tumour suppression activity, was analysed in a phase I clinical trial as potential cancer therapy.5
- The previous study reported that chemically modified synthetic miRNA-205 (syn-miR-205) inhibits melanoma growth in vitro and in vivo.14 Furthermore, we previously synthesized eight different types of synthetic miR-205s with different structures in the double-stranded form to analyse cell viability.14 In this previous study, the author used syn-miR-205 added an aromatic benzene-pyridine (BP) to the 3′ region of the double strands and via alteration of the passenger sequence (miR-205BP/S3), targeting E2F Transcription Factor 1 (E2F1) similar to pre-miR-205. Furthermore, miRNA modified chemically at the 3′-overhang region is resistant to RNase-mediated degradation, and when its passenger sequence is altered, the miRNA exhibits a more pronounced tumour-suppressive effect than unaltered wild-type miRNA because of increased affinity for Argonaute 2 (Ago 2) protein.15-17 Of these syn-miR-205s, miR-205BP/S3 displayed the most potent antitumor effect. Therefore, we used miR-205BP/S3 for clinical trials in the present study.
We tested whether the antitumor effect resulted from intratumoral administration in the spontaneous tumour of the dog. This study aimed to evaluate the efficacy of intratumoral administration of synthetic miR-205 for spontaneous CMMs and to evaluate its potential as systemic therapy.
2 MATERIAL AND METHODS
2.1 Study design
This study was conducted between March 2012 and December 2016 at the Veterinary Teaching Hospital of Gifu University, Japan, with approval from the Ethics Review Board of the Joint Faculty of Veterinary Medicine of Gifu University (approval number: E16003). Treatment was administered to dogs whose owners agreed for their participation in the study. All owners consented to the enrolment of their dogs in this clinical trial. When a lesion was not observed or when the tumour continued increasing without having an effect of miR-205BP/S3 or when the owner expressed disinterest in continuing the study, treatment was discontinued.
Inclusion criteria for dogs included herein were a histological diagnosis of CMMs. Furthermore, we enrolled dogs displaying tumour recurrence after surgery and/or radiotherapy and/or chemotherapy.
2.2 Synthesis of miR-205BP/S3
We used a synthetic miR-205s (Hokkaido System Sciences, Sapporo, Japan) with an aromatic BP analogue attached at their 3′-overhang region and with the passenger sequence of the mismatched region between passenger and guide sequences changed to matched ones. miR-205BP/S3 was generated as detailed in a previous report.14 To generate typical synthetic miR-205BPs, we selected eight different types.14 Of these, we used miR-205 (miR-205BP/S3) showing the most potent tumour suppression effect.
2.3 Treatment of chemically modified synthetic miR-205
miRNA treatment was administered alone for recurrent cases after surgery or radiotherapy. In cases 1 to 6, and case 10, miR-205BP/S3 (2 nmol) in 180 μL of Opti-MEM was mixed with 10 μL of Lipofectamine RNAiMAX (Invitrogen, Carlsbad, California), and the mixture was injected intra-tumourally using an insulin syringe after allowing them to stand at 15°C to 25°C for 10 minutes. In cases 7, 8, and 9, we injected a mixture of miR-205BP/S3 (20 μL) and 180 μL of Opti-MEM (Invitrogen) without Lipofectamine. miR-205BP/S3 was injected one or two times a week. The dose of miRNA was determined based on a previous report.14 We inserted a needle as much as possible into the middle of the tumour and injected miRNA there. miRNA was administered under sedation in all cases, except in cases 1 and 8. Medetomidine (0.03 mg/kg), midazolam (0.15 mg/kg), and butorphanol (0.1 mg/kg) were used as sedatives. The owners of the dogs were asked to visit the hospital once or twice a week, and only those cases were included for which a written agreement was obtained. No fees were charged from the owners for administering this treatment to their dogs, as the cost of treatment was borne out of the funding received for this trial study.
2.4 Assessment of study dogs
Adverse events (AE) were assessed in accordance with the Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE) v1.1 guidelines.18 AEs were monitored throughout the study through reports by owners, physical examination, haematology and blood biochemistry.
Tumour size was determined before treatment. Computer tomography (CT) was performed to determine the size of lung metastasis, and physical examination for external signs was performed using 2- or 3-dimensional callipers. Standard Response Evaluation Criteria in Solid Tumours (RECIST) were used for defining response.19 Complete remission (CR) was defined as the disappearance of all target lesions. Partial response (PR) was defined as a reduction of at least 30% in the sum of diameters of target lesions from baseline. Stable disease (SD) was defined as <30% decrease or >20% increase in sum of diameters of target lesions from smallest sum while on treatment. Progressive disease (PD) was defined as an increase in the sum of diameters of target lesions by at least 20% over the size present at entry on study, or the appearance of new lesions. Blood analysis was performed in all cases before and after miR-205BP/S3 administration, for complete blood count (CBC), blood urea nitrogen, Cre, alanine transaminase, aspartate transaminase and alkaline phosphatase. Routine urinalysis was not conducted in this study because of the following reasons: first, it was difficult to collect urine samples from the dogs consistently. Second, we had administered miR-205 to five healthy dogs in the past, and did not detect any signs of nephrotoxicity in them (Unpublished data).
2.5 Statistical analysis
The Kaplan-Meier method was used to calculate overall survival (OS) and progression-free survival (PFS) curves. All statistical analyses were performed using the EZR software, which is a graphical user interface for R, and is a modified version of R commander.20
3 RESULTS
3.1 Cases
In total, 10 dogs were included in this study, and their clinical characteristics are summarized in Table 1. The dogs were aged 9 to 17 years. The tumours in 10 dogs were classified in accordance with the TNM classification21, 22 as follows: two dogs had stage I, four dogs had stage II, three dogs had stage III, and one dog had stage IV disease (Table 1). Three cases of cancer recurrence were observed (cases 5, 8 and 9).
| Case no | Breed | Age (years) | Gender | Weight (kg) | Stage |
|---|---|---|---|---|---|
| Case 1 | Golden Retriever | 12 | F | 32 | III |
| Case 2 | Mix Breed | 12 | SF | 17 | III |
| Case 3 | Shetland Sheepdog | 9 | M | 15 | II |
| Case 4 | Miniature Dachshund | 13 | CM | 6 | II |
| Case 5 | Mix Breed | 12 | SF | 11 | II |
| Case 6 | Miniature Dachshund | 9 | M | 7 | I |
| Case 7 | Toy Poodle | 17 | F | 2 | II |
| Case 8 | Miniature Dachshund | 13 | SF | 5 | IV |
| Case 9 | Miniature Dachshund | 14 | SF | 3 | III |
| Case 10 | Miniature Dachshund | 12 | CM | 3 | I |
- Abbreviations: CM, castrated male; F, female; M, male; SF, spayed female.
- Stage: Clinical staging of dogs with melanoma in accordance with the WHO guidelines.
- Stage I: <2 cm in diameter, with no evidence of lymph node metastases.
- Stage II: 2 to 4 cm in diameter, with no evidence of lymph node metastasis.
- Stage III: >4 cm in diameter and/or lymph node metastasis.
- Stage IV: Any size with evidence of distant metastases.
3.2 Assessment of tumour location
Tumours targeted for treatment are summarized in Table 2.
| Case no | Tumours location | Period for administration (days) | Number of doses, times | Effect | Tumours characteristic |
|---|---|---|---|---|---|
| Case 1 | LN (mandibular) | 357 | 44 | CR | Metastases |
| Case 2 | LN (iliac) | 166 | 42 | SD | Metastases |
| Case 3 | Gum (mandibular) | 22 | 6 | PD | Primary tumour |
| Case 4 | Skin (mandibular) | 72 | 9 | CR | Primary tumour |
| Case 5 | Mandibular | 85 | 12 | CR | Primary tumour |
| Case 6 | Soft palate | 435 | 61 | CR | Primary tumour |
| Case 7 | Hard palate | 121 | 12 | CR | Primary tumour |
| Case 8 | Subcutaneous | 13 | 2 | PD | Metastases |
| Case 9 | Maxilla | 14 | 2 | SD | Primary tumour |
| Case 10 | Mandibular | 22 | 4 | SD | Primary tumour |
- Abbreviations: CR, complete response; LN, lymph node; PD, progressive disease PR, partial response; SD, stable disease.
3.3 Period of administration and number of doses
The period of administration and number of doses are summarized in Table 2.
3.4 Effects of miR-205BP/S3 administration
The effects of miR-205BP/S3 administration are summarized in Table 2. Five cases attained CR, three attained SD and two attained the PD state.
3.5 Clinical outcomes
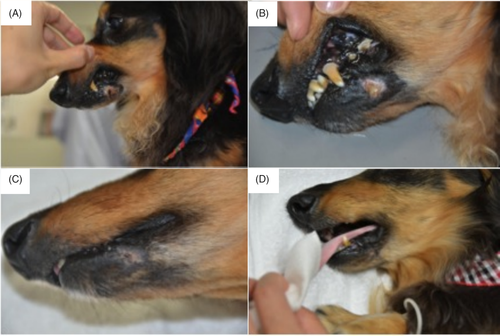
Upon follow-up examination of 7 of 10 cases (cases 1, 2, 4 and 5-8). The survival time (ST) from miR-205BP/S3 administration was 31 to 844 days (median survival time [MST]: 353 days). A representative episode of care is shown in Figure 1.
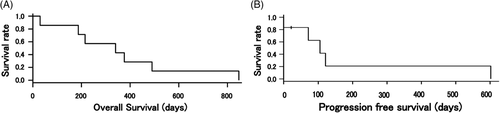
Kaplan-Meier curves for OS and PFS are shown in Figure 2. At the end of study, all seven cases that we were able to follow up were dead. The recurrence of tumour was found in five of the seven cases (cases 1, 5, 6, 7 and 8). The cause of death was metastases to lung in six of the seven cases. The cause of death of the remaining case was renal failure (case 7).
3.6 Adverse events of miR-205BP/S3 administration
miR administration did not yield any changes in blood parameters or adverse events in all cases.
4 DISCUSSION
This study revealed two important clinical findings. First, intratumoral administration of miR-205BP/S3 was effective in spontaneous CMMs. Second, the administration of miR-205BP/S3 did not result in severe side effects.
The previous study reported the high efficacy of miR-205BP/S3 in mice transplanted with human melanoma cells.14 In the present study, CR was attained in five of 10 (50%) cases in naturally occurring CMM, suggesting that miR-205BP/S3 positively affected not only human melanoma xenografts but also naturally occurring tumours. When we weigh the effect in the tumour transplantation mouse model and the effect for the spontaneous tumour, there is following difference. This refers to the uniformity observed among the model mice, as opposed to the differences in the overall status in dogs in the present study and differences in the behaviour of the tumour. Because the case of CR displayed the reduction of even a spontaneous tumour, indicating a 50% variety among stages III and I, further studies with a larger number of cases are required to confirm this finding. In the present study, CR was attained in five cases (50%) and intra-tumoural local administration miR-205BP/S3 was effective in spontaneous CMMs. Furthermore, the present results are essential for preliminary studies for systemic therapy using miR-205BP/S3 for CMMs.
The previous study provided a histopathological perspective of the side effects of mice receiving melanoma cell line transplants and treated with miR-205BP/S3.14 The study reported no side effects on the local skin or stroma around the tumour tissues. In this study, although histopathological analysis of side effects was not performed, macroscopic examination revealed the absence of side effects in the intratumoral injected tissues.
The present results revealed marked differences in every case upon administration of miR-205BP/S3. More specifically, we observed cases wherein CR was attained immediately several times after miR-205BP/S3 administrations (cases 1, 4, 5 and 7) or cases wherein a lesion progressed without any side effects after miR-205BP/S3 administration (cases 3 and 8). The previous study indicates that miR-205BP/S3 downregulates E2F1, vascular endothelial growth factor (VEGF) and B-cell CLL/lymphoma (BCL2) in melanoma cells and induces apoptosis in human malignant melanoma cell lines.14 E2F1 and VEGF were previously verified as targets of miR-205-5p in melanoma and breast cancer cells.9, 23 BCL2 is expressed downstream of E2F1. A difference may have resulted in the effect of miR-205BP/S3 owing to differences in these expression levels in each patient. However, we did not assess E2F1, VEGF and BCL2 expression levels in the local lesions; hence, further studies are required to verify these findings.
Furthermore, the present results indicate an effect in two cases (cases 7 and 9) (CR, SD) among three cases (cases 7, 8 and 9), wherein a transfection reagent was not used. The transfection reagent (Lipofectamine RNAiMAX) used herein was a simple substance; however, it reportedly exerts cytotoxic effects, and the likelihood that this cytotoxicity contributes to tumour suppression was considered. However, even the aforementioned two cases are considered to display an effect of miR-205BP/S3 administration, owing to visible post-administration effects. A previous study has reported that the antitumor effect of miRNA can be reinforced by chemically modifying it with benzene pyridine and by altering its passenger sequence.17 Systemic therapy using the combination of miRNA and lipofectamine may result in a side effect in the future because of the reported cytotoxic effects of lipofectamine. Therefore, it is desirable to administer miRNA therapy without lipofectamine. However, this can only be achieved if the antitumor effects of miRNA treatment can be augmented without the use of lipofectamine. Thus, further studies are required for examining such effects. Although some studies have been conducted investigating these effects, they are quite few in number and unlike the present study, do not include a pathological study of the lesions. Furthermore, the present study establishes that miR-205BP/S3 may be taken up by cells even without using a transfection reagent such as lipofectamine.
The post-surgery MST for dogs with oral melanoma is approximately 17 to 18, 5 to 6 and 3 months for animals with stages I, II and III of the disease, respectively.6 MST of dogs included in this study was 340 days.
Since only a few cases were studied, the MST of every stage was not calculated. Thus, more number of cases are required to evaluate the MST of each stage.
This study has the following limitations. The number of cases wherein miR-205BP/S3 was administered was limited. We examined the effect of intratumoral miR-205BP/S3 administration on 10 dogs with CMM; however, further studies are required with a larger number of cases to validate the present result. However, to date, few studies have attempted to determine miRNA expression in canine tissues. Therefore, the effects of systemic administration of miR-205BP/S3 in canine are unknown. We could not examine the side effects of systemic administration of miR-205BP/S3 for spontaneous CMMs, since, in the present study, intratumoral administration of miR-205BP/S3 for spontaneous CMM did not yield any clinical side effects. In addition, it is difficult to compare the side effects of a locally administered therapy with those of other systemic therapies. However, the data obtained in this study regarding the lack of side effects of the local administration of miRNA can become a reference for systemic therapeutic approaches in the future. Nonetheless, further studies are necessary to evaluate this apparent lack of side effects.
In conclusion, the present results indicate that intratumoral administration of miR-205BP/S3 was effective for spontaneous CMMs and did not result in any side effects. This study suggests that miR-205BP/S3 can be applied as a new therapeutic modality for melanoma through intratumoral administration. In a further study based on the present results, we will consider the clinical application of systemic therapy with miR-205BP/S3 for CMMs.