Nectin-4 and p63 immunohistochemical expression in canine prostate tumourigenesis
Abstract
Nectin-4 is an E-cadherin-based adherens junction protein of normal epithelial cells, as well as a potent mediator of anchorage-independent cancer colony formation. It is considered a tumour-associated histological and serological marker in various human cancers. The transcription factor p63 is a basal cell marker in the normal prostate, involved in cell adhesion, as well as in the formation and survival of circulating tumour cell clusters. The aim of this study was to evaluate Nectin-4 and p63 immunohistochemical expression in 42 canine prostate tissues including 2 normal prostates, 10 benign prostatic hyperplasias (BPHs), 30 prostatic carcinomas (PCs), 1 pulmonary and 1 lymph node metastasis. From normal to neoplastic tissues, Nectin-4 showed a progressive switching from membranous (m-Nectin-4) to cytoplasmic (c-Nectin-4), regardless of the histological subtypes, except for lack of expression in solid PCs. Metastatic cells exhibited both strong membranous and cytoplasmic positivity. c-Nectin-4 expression was significantly (P < 0.0001) increased in PCs/metastasis compared to BPHs cases and a decrease (P < 0.05) of nuclear p63 immunostaining was also detected in the two groups. Furthermore, data showed a significant association (P < 0.05) between p63 and m-Nectin-4 distribution, although their colocalization was detected only in scattered cells by double immunofluorescence. Our results suggest the involvement of m-Nectin-4 in canine prostate tumourigenesis and metastatic potential, while the exact role of c-Nectin-4 expression detectable in primary PCs requires further investigations.
1 INTRODUCTION
Aggressive cancer cells have a tendency to self-aggregate in order to survive and proliferate in the absence of an appropriate matrix anchorage.1 Clusters of circulating tumour cells (CTCs) have been identified in blood samples of several cancer-affected human patients, including those with prostate cancer, and therapies targeting such cell-cell contacts may represent a novel cancer therapeutic approach.2 Nectin-4, a component of the E-cadherin-based adherens junctions in epithelial cells, encoded by the Poliovirus-Receptor-Like 4 (PVRL4) gene, is a potent mediator of the anchorage-independent growth relying upon the formation of physical contacts between circulating cells.1 In humans, Nectin-4 is mainly expressed in the placenta and, to a lesser extent, in tonsils, oral mucosa, trachea, oesophagus, nasopharynx, prostate, lung and stomach.3-5 Given its role as an epithelial cell receptor for canine distemper virus, Nectin-4 expression has mainly been evaluated in relation to Morbillivirus infection in dogs,3, 6, 7 and its expression has been observed in the canine lung, kidney, intestine, urinary bladder,7 brain and placenta.3 Recently, the immunohistochemical expression of citoplasmic Nectin-4 (c-Nectin 4) has been detected in 4 out of 9 (45%) canine mammary tumour cell lines derived from three different dogs, as well as in paraffin sections of mammary adenocarcinoma,8 whereas lower Nectin-4 gene expression levels were observed in canine non-tonsillar oral squamous cell carcinoma and oral melanoma compared to normal gingival controls.9
In humans, Nectin-4 is a well-recognized, tumour-associated histological and serological marker for several types of adenocarcinoma (lung, breast, pancreas, ovary).10, 11 Nectin-4 expression is lost or reduced in many human cancer cell lines derived from melanoma, neuroblastoma, glioma, medulloblastoma, colon cancer, prostate cancer and renal cell carcinoma, as well as in related cancer tissue samples. It has also been reported to act as a tumour suppressor, especially in ductal breast carcinoma, colorectal adenocarcinoma and renal clear cell carcinoma.12, 13
Another interesting molecule that changes its expression during prostate tumourigenesis is the transcription factor p63, belonging to the p53 family. The ΔNp63 is the predominantly expressed isoform and it is normally detectable at high levels in the basal cells of the normal stratified and glandular epithelia.14 In the skin, the ΔNp63 isoform is essential for the maintenance of the progenitor population of the basal layer, while the TAp63 isoform is required to allow their complete differentiation in association with or subsequently to ΔNp63.15 Knockdown of p63 also causes down-regulation of cell adhesion-associated genes, resulting in cell detachment and anoikis in mammary epithelial cells and keratinocytes.16 On the other hand, over-expression of p63 up-regulate the expression of cell adhesion molecules, thus increasing cellular adhesion and enhancing CTCs formation and survival.17 The expression of both p63 isoforms is dysregulated in several human and canine tumours including prostate cancer.18-20 In this respect, several studies have investigated p63 expression in canine prostate carcinomas (PCs),19-23 revealing that p63+ canine PCs represent a very rare PC group showing a distinct phenotype compared to typical canine PCs.20 At the molecular lever, Nectin-4 and p63 are regulated by and regulate Interferon Regulatory Factor 6 (IRF6) protein in differentiated keratinocytes.24 In particular, p63 activates IRF6 transcription, while IRF6 gene depletion reduces Nectin-4 gene expression.24 Therefore, given the involvement of these proteins in regulating the expression of cell adhesion molecules, the evaluation of their expression and distribution in PCs may help to better understand their involvement in cell-to-cell contact during prostate cancer progression.
Thus, the aim of this study was to evaluate the immunohistochemical expression and cellular localisation of membranous and cytoplasmic Nectin-4 and p63 in normal, hyperplastic and neoplastic canine prostate tissues in order to investigate their possible role in the malignant transformation and invasive/metastatic properties of prostate cancer cells. In addition, since a diagnostic/prognostic marker of canine prostate cancer has not been identified so far, this preliminary study could provide the scientific basis for future investigations on Nectin-4 expression in prostate cancer tissues in association with follow-up information and the possibility to detect its soluble form in the serum of canine PC patients, which may help in filling this gap.
2 MATERIALS AND METHODS
Information on age, breed and castration status of the dogs is summarized in Supplementary tables n°1, n°2 and n°3.
2.1 Histological examination
In this study, 42 formalin-fixed, paraffin wax-embedded canine prostate samples were analysed, including 2 normal prostates, 10 benign prostatic hyperplasia (BPH) and 30 primary PCs. Two PCs were associated with pulmonary (1/2) and lymph node (1/2) metastases. All cases were classified according to the human WHO classification,25 recently adapted to canine PCs.26 Our study did not involve human participants or live animals and an ethics approval was not required.
2.2 Immunohistochemistry for Nectin-4 and p63
Immunohistochemistry was performed using the following primary antibodies (Abs): goat polyclonal anti-human Nectin-4 (1:70, AF2659, R&D Systems, Minneapolis, Minnesota) and mouse monoclonal anti-human p63 (1:400, clone 4A4; DAKO, Glostrup, Denmark), which identifies both p63 isoforms, that is, ΔNp63 and TAp63. Cross-reactivity with canine tissues for this primary Abs has been previously demonstrated.8, 19, 20 After deparaffinization and rehydration, antigen retrieval was performed submerging sections in 1 M Urea (pH 8.0) in a microwave for 15 minutes (3 cycles, 5 minutes/each). To reduce non-specific binding, slides were then incubated at room temperature with 5% non-fat dried milk, 5% BSA and 5% normal horse serum (Nectin-4) or normal goat serum (p63) for 15 minutes each, before overnight incubation with the specific primary Ab at 4°C. Sections were treated with 3% H2O2, in absolute methanol for 45 minutes, to inhibit endogenous peroxidase activity and then rinsed in 0.05 M Tris-buffered saline (TBS, pH 7.6) for 5 minutes. After incubation with secondary biotinylated horse anti-goat (Nectin-4) or horse anti-mouse (p63) (1:200; Vector Laboratories, Peterborough, UK) Ab for 30 minutes, the reaction was visualized using the Vectastain Elite ABC System (code PK 6200; Vector Laboratories, Peterborough, UK) for 30 minutes and 0.1% H2O2 in 3-3′-diaminobenzidine solution (code D5905, Sigma-Aldrich, St. Louis, Missouri) followed by Mayer's haematoxylin (Merck, Darmstadt, Germany) counterstaining. A negative control was performed in all instances by omitting the primary Ab and incubating tissue sections with TBS. The following positive controls were used: canine normal prostate for p6319 and canine normal lung for Nectin-4.7
2.3 Double immunofluorescence
Double immunofluorescence was also performed to investigate Nectin-4-p63 and Nectin-4-Laminin co-expression in normal prostates, BPHs and PCs. For Nectin-4-p63 analysis, tissue samples were treated as described for the immunohistochemical procedure using a mixture of primary Abs, applied overnight at 4°C. The first secondary biotinylated goat anti-goat Ab (for Nectin-4) (1:200 dilution; Vector Laboratories) was applied and incubated for 30 minutes at room temperature and slides were then treated with Texas Red-conjugated avidin (Vector Laboratories) diluted 1:100 in a buffer consisting of 0.1 M NaHCO3 and 0.15 M NaCl, pH 8.2-8.5, for 10 minutes at room temperature. An avidin/biotin blocking step was performed by incubating slides for 15 minutes with avidin and then biotin (Avidin/Biotin Blocking Kit; Vector Laboratories) at room temperature. Another secondary biotinylated goat anti-mouse Ab (for p63) (1:200 dilution; Vector Laboratories) was applied and incubated for 30 minutes at room temperature and the slides were then treated with fluorescein-conjugated avidin (1:100 dilution in 0.1 M NaHCO3, 0.15 M NaCl buffer, pH 8.2-8.5; Vector Laboratories) for 10 minutes at room temperature. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). For Nectin-4-laminin analysis, dewaxed and rehydrated 3-5 μm thick tissue sections were incubated with a primary antibodies mixture (Nectin-4 pAb 1:70; R&D Systems Inc., Minneapolis, Minnesota; Laminin pAb 1:100; DAKO) overnight at 4°C, followed by incubation with biotinylated secondary anti-goat (Nectin-4) and anti-rabbit (Laminin) antibodies (Vector Laboratories, UK) and avidin-conjugated fluorescein (Nectin-4) and Texas Red (Laminin) (Vector Laboratories, UK). Antigen retrieval was performed in urea buffer (pH 8.00) in a microwave for 20 minutes. Sections were mounted with Vectashield (Vector Laboratories).
2.4 Quantification of immunolabelling and statistical analysis
M-Nectin-4 (membranous) and c-Nectin-4 (cytoplasmic) were semiquantitatively assessed for each sample in 10 randomly selected high-power fields (400×) as follows: absent (0% positive cells), low (>0-<10%), moderate (≥10-<50%), high (≥50%-< 75%) and very high (≥75%). Labelling intensity for Nectin-4 was also graded as weak (+), moderate (++) or strong (+++). The number of p63-positive nuclei, for each sample, was calculated in 10 randomly selected high-power (400×) fields counting at least 1000 normal, hyperplastic or neoplastic epithelial cells, and expressed as a percentage. Positivity was evaluated in a double blinded study (Marcella Massimini and Leonardo Della Salda) for each case. Differences among BPHs and PCs/Metastasis tissues regarding Nectin-4 immunoreactivity and nuclear expression scores of p63 were assessed by the Fisher exact test. For Nectin-4, samples were divided into two categories based on the percentage of positive cells: <10% positive cells (absent and low immunoreactivity) vs >10% positive cells (moderate, high and very high immunoreactivity). For p63, the following groups were considered: <10% positive nuclei (absent and low number of positive nuclei) vs >10% positive nuclei (moderate and high number of positive nuclei)17 Differences in the percentage of p63 expression according to m-nectin-4 and c-nectin-4 distribution were investigated with a Kruskal-Wallis test followed by Dunn Multiple Comparison test. All statistical analyses were performed using GraphPad statistical software, with P < 0.05 considered to be significant.
3 RESULTS
3.1 Immunohistochemical expression of Nectin-4 and p63 in normal prostates and BPHs
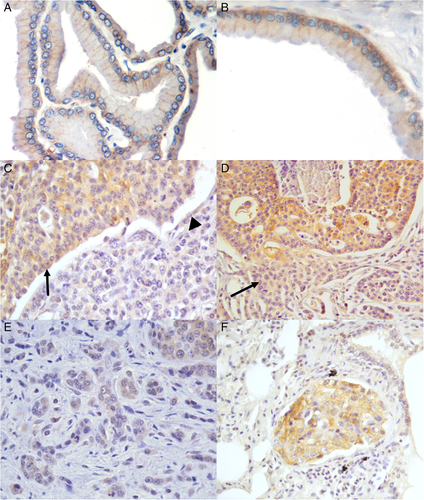
All normal (2/2) and hyperplastic (10/10) tissues showed a moderate (+/++) m-Nectin-4 expression, localized at the basolateral surface of the cells. Moreover, BPHs were frequently characterized by low (+) c-Nectin-4 staining, mainly localized at the basal side (Figure 1A,B).
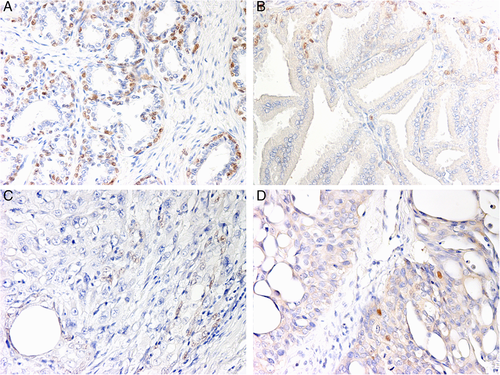
In normal prostates and BPHs, nuclear p63 expression was confined to the basal cells, forming a discontinuous layer along the basement membrane (Figure 2A,B). The percentage of positive nuclei in the two normal prostates was 35.6% and 37% respectively, while it ranged from 0% to 14.50% in BPHs samples, with an average of 6.70% (Table 1).
| Diagnosis | m-Nectin-4 | c-Nectin-4 | p63 | ||
|---|---|---|---|---|---|
| Distribution | Intensity | Distribution | |||
| 1 | Normal | Very high | + | Absent | 37% |
| 2 | Normal | Very high | + | Absent | 35.6% |
| Diagnosis | m-Nectin-4 | c-Nectin-4 | p63 | |||
|---|---|---|---|---|---|---|
| Distribution | Intensity | Distribution | Intensity | |||
| 1 | Benign prostatic hyperplasia, cystic | Very high | +/++ | Low | + | 0% |
| 2 | Benign prostatic hyperplasia, fibrosis | Very high | +/++ | Low | +/++ | 0% |
| 3 | Benign prostatic hyperplasia | Very high | +/++ | Low | + | 0% |
| 4 | Benign prostatic hyperplasia | Very high | +/++ | Low | +/++ | 7.5% |
| 5 | Benign prostatic hyperplasia | Very high | +/++ | Low | + | 5.34% |
| 6 | Benign prostatic hyperplasia | Very high | +/++ | Low | +/++ | 0% |
| 7 | Benign prostatic hyperplasia, squamous metaplasia | Very high | +/++ | Low | + | 9.34% |
| 8 | Benign prostatic hyperplasia | Very high | +/++ | Low | +/++ | 13.27% |
| 9 | Benign prostatic hyperplasia | Very high | +/++ | Low | + | 11.12% |
| 10 | Benign prostatic hyperplasia | Very high | +/++ | Low | +/++ | 14.5% |
- Abbreviations: BPH, benign prostatic hyperplasia.
- Nectin 4 expression was classified as membranous (localized at cell–cell boundaries) or cytoplasmic (uniformly distributed throughout the cytoplasm). Sample was grouped into five categories based on the number of positive cells for each type of Nectin 4 expression.
- Immunolabelling distribution: absent (0% labelled cells), low (>0-<10% labelled cells), moderate (≥10-<50% labelled cells), high (≥50%-< 75% labelled cells) and very high (≥75% labelled cells).
- Immunolabelling intensity: weak labelling (+), moderate labelling (++), strong labelling (+++).
3.2 Immunohistochemical expression of Nectin-4 and p63 in different histological subtypes of PCs
3.2.1 Cribriform PCs (6/30)
A variable, low to high c-Nectin expression was observed in cribriform PCs, whereas the membranous positive staining was generally high or very high (Figure 1C). Interestingly, a decreased expression of c- and m-Nectin-4 was detected in areas characterized by cribriform lesions in which neoplastic cells infiltrate the surrounding stroma (Figure 1D). The percentage of p63-positive cells ranged from 0.60% to 4.80%, with an average of 1.75% (Table 2).
| Diagnosis: carcinoma at single histotype (histological pattern)—small acinar /ductal | m-Nectin-4 | c-Nectin-4 | p63 | |||
|---|---|---|---|---|---|---|
| Distribution | Intensity | Distribution | Intensity | |||
| 1 | Small/acinar ductal PC | Moderate | ++ | Moderate | ++ | 4.3% |
| 2 | Small acinar/ductal PC | Moderate | ++ | Moderate | ++ | 7.3% |
| 3 | Small acinar/ductal PC | Moderate | ++ | Moderate | ++ | 5.6% |
| 4 | Small acinar/ductal PC | Absent | − | Low | + | 6.7% |
| 5 | Small acinar/ductal PC | High | +++ | High | +++ | 6.2% |
| 6 | Small/ductal PC | High | ++ | Low | + | 5.2% |
| Diagnosis: carcinoma at single histotype (histological pattern)—solid | m-Nectin-4 | c-Nectin-4 | p63 | |||
| Distribution | Intensity | Distribution | Intensity | |||
| 1 | Solid PC with scattered signet- ring cells, large areas of necrosis. | Absent | − | Low | + | 1.6% |
| 2 | Solid | Absent | − | Low | + | 0% |
| 3 | Solid with neoplastic cell from polygonal to spindle, multifocal necrosis, multinucleated giant cells, focal bone metaplasia | Absent | − | Absent | − | 0% |
| 4 | Solid with multifocal large signet-ring cells | Absent | − | Low | + | 0% |
| Diagnosis: carcinoma at single histotype (histological pattern)—cribriform | m-Nectin-4 | c-Nectin-4 | p63 | |||
| Distribution | Intensity | Distribution | Intensity | |||
| 1 | Cribriform PC with comedonecrosis and mineralisation; multifocal signet-ring cells | Absent | − | High | + | 4.8% |
| 2 | Cribriform PC with comedonecrosis with occasionalsignet ring cells | High | +++ | High | ++ | 0.8% |
| 3 | Cribriform + infiltrative aspects | High | ++ | Very High | +++ | 1.5% |
| 4 | Cribriform | Low | + | Very High | + | 1.6% |
| 5 | Cribriform | Low | ++ | Very High | + | 1.2% |
| 6 | Cribriform PC with comedonecrosis. | High | ++ | Moderate | ++ | 0.6% |
- Abbreviation: PC, prostatic carcinoma.
- Nectin 4 expression was classified as membranous (localized at cell–cell boundaries) or cytoplasmic (uniformly distributed throughout the cytoplasm). Samples were grouped into five categories based on the number of positive cells for each type of Nectin 4 expression.
- Immunolabelling distribution: absent (0% labelled cells), low (>0-<10% labelled cells), moderate (≥10-<50% labelled cells), high (≥50%-< 75% labelled cells) and very high (≥75% labelled cells).
- Immunolabelling intensity: weak labelling (+), moderate labelling (++), strong labelling (+++).
3.2.2 Solid undifferentiated PCs (4/30)
Neoplastic cells lacked both Nectin-4 and p63 expression in solid undifferentiated PCs (Figure 1C), with the exception of a single case characterized by scattered signet-ring cells and large areas of necrosis (Table 2).
3.2.3 Small acinar/ductal PCs (6/30)
A heterogeneous staining pattern was observed in small acinar/ductal samples showing moderate to high (++/+++) m-Nectin-4, with a single case exhibiting low membranous expression. Cytoplasmic labelling varied from <10% to ≥10-<50%, with a generally moderate intensity of staining (Figure 1E). P63-positive nuclei of neoplastic cells ranged from 4.30% to 7.30%, with an average of 5.88% (Table 2).
3.2.4 Mixed histological subtypes of PCs (14/30)
In mixed PCs, m-Nectin-4 expression of the different histotypes observed within each tumour reflected the pattern of expression already described for the individual tumours (Table 3). The staining was maintained in acinar and cribriform lesions, while lacking in solid areas. c-Nectin-4 was generally present in more than 75% cells, except for the solid type in which the signal was absent. Two cases (n. 2 and n. 12 in Table 3) contained multifocal sarcomatoid changes that showed a diffuse, low m-Nectin-4 and moderate c-Nectin-4 expression. In all mixed PCs, nuclear p63 immunolabelling was absent or detectable in a low number of cells (nuclear score < 10%) (Figure 2C,D), with only two cases -characterized by a papillary pattern- showing a high nuclear score (12.30%-19.60%) (Supplementary Figure 1, Supporting Information).
| Diagnosis: carcinomas at mixed histotypes (histological patterns) | m-Nectin-4 | c-Nectin-4 | p63 | |||
|---|---|---|---|---|---|---|
| Distribution | Intensity | Distribution | Intensity | |||
| 1 | - Cribriform - Small acinar/ductal - Solid Large areas of necrosis and haemorrhages |
Moderate Absent Absent |
++ − − |
Low Absent Absent |
+ − − |
0% 0% 0% |
| 2 | - Cribriform with central necrosis - Papillary - Multifocal sarcomatoid transformation Occasional signet ring cells |
High Absent Low |
+++ − + |
High High moderate |
++ ++ ++ |
0% 3,4% 0,6% |
| 3 | - Cribriform - Solid |
Moderate Absent |
+++ − |
Moderate Absent |
+ − |
1,5% 0% |
| 4 | - Papillary - Cribriform with occasional comedonecrosis - Small acinar/ductal Bone metaplasia |
High High Moderate |
++/+++ +++ ++ |
High High Moderate |
++/++ ++/++ ++ |
0% 0% 4,3% |
| 5 | - Multifocal small/acinar + mucinous carcinoma Perineureal invasion and mucinous fibroplasia |
Low |
++ |
High |
+ |
5,3% |
| 6 | - Cribriform - Papillary |
High Moderate |
+/++ + |
High High |
++ + |
3,3% 3,3% |
| 7 | - Cribriform with comedonecrosis - Small acinar/ductal |
Absent Moderate |
- + |
Very High Very high |
+++ +/++ |
2,4% 4% |
| 8 | - Cribriform without comedonecrosis - Solid - Small acinar Collagenous micronodules |
Moderate Absent Moderate |
+/+++ − + |
Very high Low Very High |
++/+++ + +/++ |
2,7% 0% 4,8% |
| 9 | - Cribriform - Solid Signet ring cells |
Moderate Absent |
++ − |
Very high High |
+++ + |
2,6% 0% |
| 10 | - Solid - Cribriform |
Absent Moderate | − +++ |
Low High |
+ ++/+++ |
0% 3% |
| 11 | - Solid - Small acinar |
Absent Absent |
− − |
Low High |
+ ++ |
0% 4,8% |
| 12 | - Small acinar/ductal - Solid - Sarcomatoid transformation Signet ring cell |
Moderate Absent Low |
+++ − + |
Moderate Low Moderate |
+ + ++ |
0% 0% 0% |
| 13 | - Small acinar/ductal - Papillary |
Low High |
+++ ++ |
Moderate Low |
+++ ++ |
0% 12,3% |
| 14 | - Cribriform - Papillary - Solid Perineural invasion |
High High Absent |
+++ +/+++ − |
Moderate Moderate Absent |
++ ++ − |
1,7% 19,6% 0% |
- Nectin 4 expression was classified as membranous (localized at cell–cell boundaries) or cytoplasmic (uniformly distributed throughout the cytoplasm). Sample was grouped into five categories based on the number of positive cells for each type of Nectin 4 expression.
- Immunolabelling distribution: absent (0% labelled cells), low (>0-<10% labelled cells), moderate (≥10-<50% labelled cells), high (≥50%-<75% labelled cells) and very high (≥75% labelled cells).
- Immunolabelling intensity: weak labelling (+), moderate labelling (++), strong labelling (+++).
3.3 Metastases and emboli
Metastatic lesions showed high (+++) m-Nectin-4 and moderate (++) c-Nectin-4 staining (Figure 1F). In the lymph node, clusters of metastatic cells with a strong membranous staining were observed within the medullary cords. Emboli in peritumoral lymphatic vessels observed in two PCs showed a strong c-Nectin-4 expression. Metastatic cells in the lung were p63-negative, while rare positive p63-positive nuclei (0.6%) were observed within the lymph node (Table 4).
| Diagnosis | m-Nectin-4 | c-Nectin-4 | p63 | |||
|---|---|---|---|---|---|---|
| Distribution | Intensity | Distribution | Intensity | |||
| 11a | Lung metastasis | Very high | +++ | Low | +/++ | 0% |
| 10a | Lymphatic vessels emboli | Very high | ++ | Very high | +++ | 0% |
| 14a | Lymphatic vessels emboli | Very high | +++ | Very high | ++ | 0% |
| 13a | Lymph node metastasis | Very high | +++ | Low | ++ | 0.9% |
- a Cases refer to the respective cases of mixed tumours.
3.4 Double immunofluorescence
Evaluation of m-Nectin-4-p63 co-expression revealed an alternate p63 distribution characterized by variable intensity of m-Nectin-4 (+/+++) in all cases, although colocalization of both proteins was only observed in scattered cells (Figure 3 and Supplementary Figures 2 and 3).
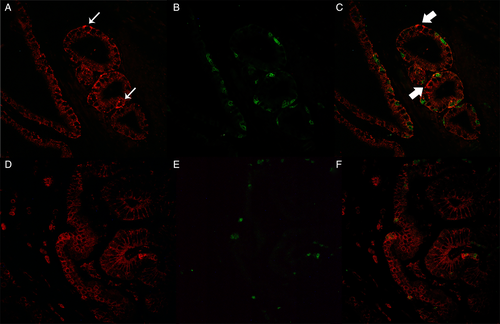
Nectin-4/laminin double staining showed an evident expression of m- and c-Nectin-4 in the basal cells of the cribriform structures, surrounded by a well-formed basal membrane characterized by an intense laminin staining, while the expression decreased in structures with a thin or absent basal membrane and in cells infiltrating the surrounding stroma.
3.5 Statistical analysis
C-Nectin-4 and p63 immunostaining showed a significant decrease (P < 0.0001 and P < 0.05 respectively) in PCs/metastasis when compared to BPH cases. Furthermore, the percentage of p63+ nuclei was significantly higher in samples with very-high m-Nectin-4 distribution than in all other samples (P < 0.05) (Supplementary Figure 4). On the other hand, a tendency towards an association between c-Nectin-4 immunostaining and decreased percentage of p63+ nuclei was only observed (Table 2).
4 DISCUSSION
Dogs with naturally occurring prostate cancer are relevant models for the disease in humans and pre-clinical studies of new diagnostic and therapeutic approaches in dogs may provide benefit for both species with prostate cancer.27 Murine, human and canine Nectin-4 protein shares high homology,3 and the critical domains for binding measles virus are completely conserved in the last two species.8
The present study focused on the significance of Nectin-4 expression in normal, hyperplastic and neoplastic canine prostatic tissues, suggesting that changes in Nectin-4 expression and function may be involved in prostate tumourigenesis and malignant progression. The first novel finding of this study was the detection of m-Nectin-4 expression in normal canine prostate tissue. In addition, Nectin-4 immunostaining switched from membranous in normal and BPHs samples to predominantly cytoplasmic in most of PCs, then disappearing in solid undifferentiated tumours. In this respect, the weak intensity of c-Nectin-4 expression detected in neoplastic cribriform areas characterized by stromal invasion and thin or discontinuous basal membrane, as well as its strong expression observed in metastases, is of particular interest. In fact, this finding could be in agreement with the loss of adhesion molecules facilitating the migratory phase of tumour cells and their re-expression during the typical clustering mode in metastasis of prostate tumours, although a higher number of normal and neoplastic cases are necessary to confirm this hypothesis.
To date, there are only two reports on the expression of Nectin-4 in several normal and neoplastic tissue in dogs, although there are some discrepancies in the expression patterns.7, 28 Cytoplasmic Nectin-4 has been found to be expressed in neoplastic canine mammary tissues,8 as well as in four canine mammary tumour cell lines, where it is likely correlated with malignancy. The latter study was carried out by using the same polyclonal antibody applied in the present investigation. In several human cancers,29 Nectin immunostaining has been described as predominantly cytoplasmic or both membranous and cytoplasmic,30 although the significance of this different distribution is still debated. M-Nectin-4 expression was significantly associated with a lower metastasis-free survival rate in breast cancer patients with luminal-A (oestrogen receptor-positive [ER+], and/or progesterone receptor-positive [PR+], human epidermal growth factor receptor 2 [HER−]) tumours, whereas high c-Nectin-4 expression was significantly associated with higher rates of disease-free survival and local relapse-free survival. On the other hand, the absence or a severe reduction of the cytoplasmic form in luminal-A tumours with undetectable cell membrane Nectin-4 has been associated with a higher risk of relapse.31
Two different studies31, 32 used the same commercial goat polyclonal antibody that we have applied in our samples, in both cases detecting Nectin-4 expression in the cytoplasm of breast cancer cells. However, M-Rabet et al (2017)33 recently tested this antibody on Nectin-4 mRNA negative triple-negative breast cancers detecting both cytoplasmic and nuclear staining by immunohistochemistry. Despite recognizing Nectin-4 by Western Blotting, this polyclonal antibody resulted in a high background signal on Nectin-4 mRNA negative breast cancer line cells, when compared to the human anti-Nectin 4 monoclonal antibody N41. We did not detect any nuclear positivities in our cases and, even if, according to the findings of M-Rabet et al (2017),33 the observed cytoplasmic expression may be argued as a background, this will not explain the different degree and intensity of immunoreactivity observed in each histotype examined, as well as the complete absence of cytoplasmic expression in solid tumours. These findings also suggest the importance of further investigating the prognostic significance of membranous and cytoplasmic Nectin-4 in canine prostate cancer.
Loss of junctional molecule expression (ie, E-Cadherin) in less differentiated invasive carcinomas is a well-known phenomenon, which occurs through multiple mechanisms, for example, complete or partial gene deletion, promoter inactivation by methylation, chromatin rearrangement34 and, as far as Nectin-4 is concerned, enzymatic cleavage of the membranous protein. In particular, the extracellular domain of Nectin-4 can be proteolytically cleaved to release a soluble fragment (sN4).35 The soluble form is generated by the activity of Tumour Necrosis Factor-α-Converting Enzyme-Metallopeptidase-Domain-17 (TACE-ADAM-17), that cuts the Nectin-4 protein ectodomain.32 To date, sN4 has been detected in the serum of cancer patients, suggesting its potential use to diagnose or predict cancer evolution, as described in ovarian,11, 37 lung38, 39 and breast cancers.36, 38 Since over-expression of TACE-ADAM-17 has been found in human prostatic tumour cell lines and tissue samples,40 and Nectin-4 expression has been detected in prostate cancer tissues, the serum levels of its soluble form may be of potential interest to be investigated in future studies, both in humans and dogs with prostate cancer. On the other hand, over-expression of m-Nectin-4 may induce lamellipodia formation through activation of small GTPase Rac1 (Ras-related C3 botulinum toxin substrate 1), enhancing migration on Matrigel of fibroblast-like cells, determining the formation of cell clusters, and favouring neoplastic invasion during metastasis.10 In our study, it is important to underline that both lymph node and pulmonary metastases showed tumour cells clusters with a strong m-Nectin-4 expression. Besides promoting anchorage-independent growth, Nectin-4 can also favour adhesion of cancer cells in different tissues: in vitro studies have demonstrated that blocking Nectin-4 with specific antibodies induces loss of neoplastic cells ability to adhere to pulmonary endothelial cells.1 It is likely that Nectin-4 can modulate a spectrum of still incompletely defined biological activities, depending on its level and intracellular localisation, both in normal and neoplastic tissues.31 Nectin-4 was also found to be mainly expressed in breast cancer cell lines with a luminal-like phenotype, as well as to be absent or weakly expressed in cells with a basal-like phenotype.41
As far as p63 is concerned, this molecule confirmed its expression pattern in normal and hyperplastic canine prostate, which is typically confined to basal cells, forming a discontinuous layer along the basement membrane,19 a finding in agreement with the discontinuity of the basal cell layer of canine prostate gland, differently from humans.42 On the other hand, nuclear p63 immunolabelling was frequently absent or detectable in a low percentage of neoplastic cells in PC cases, confirming that p63+ canine PCs represent a very rare PC subtype.20 Our study also investigated for the first time the possible relationship between p63 and Nectin-4 expression in canine prostate tissues. In this respect, even though Nectin-4-p63 coexpression by double immunofluorescence was rarely detectable, a significant association between very high m-nectin-4 distribution and high levels of p63+ nuclei was revealed, thus suggesting a possible, IRF6-dependent or -independent, link between Nectin-4 and p63 in the canine prostate.
5 CONCLUSIONS
In conclusion, this is the first study describing Nectin-4 expression in canine prostate tissue and correlating its expression with the basal cell marker p63. The significance of Nectin-4 cytoplasmic expression remains to be clarified, and the relation between p63 nuclear localization and m-nectin-4 distribution requires further investigations. Our results also pave the way for future studies directed to investigate the presence of Nectin-4 soluble form in the serum of canine PC patients, with the aim to compensate the lack of a veterinary diagnostic marker (biomarker) for canine prostate cancer.
ACKNOWLEDGEMENTS
The study was self-funded and partially supported by the Consorzio Interuniversitario Nazionale per la Bio-Oncologia (CINBO). In addition, the authors would like to thank Dr. Paola Fortugno (Istituto Dermopatico dell'Immacolata, Rome, Italy) for her invaluable technical support.
CONFLICT OF INTEREST
All the authors have no conflicts of interest to declare.