A comparison of 12- and 19-week CHOP protocols using non-randomized, contemporaneous controls
Abstract
This study is a concurrent comparison of two versions of CHOP protocols, a 19-week CHOP and a comparatively overall dose-intense 12-week CHOP. The 12-week protocol was designed to be 58% more dose intense than the 19-week protocol for both doxorubicin and cyclophosphamide; however, it was 21% less dose intense for vincristine (VCR). Forty-seven dogs were included for evaluation, and the characteristics of each population were similar. For dogs receiving the 19-week CHOP protocol, 89.5% experienced a complete response, with a median progression-free survival (PFS) of 245 days and median overall survival (OS) of 347 days. For dogs receiving the 12-week CHOP protocol, 89.3% experienced a complete response, with a median PFS of 141 days and median OS of 229 days. When evaluated by Log-rank analysis, the difference of PFS (P = 0.047) and OS (P = 0.013) between the groups were statistically significant. In summary, these data suggest that despite overall increased dose-intensity, dogs receiving treatment with a 12-week CHOP protocol experience less durable remission than our standard 19-week protocol in this population. Additional prospective investigation will be required to explore the implication that VCR dose intensity and/or shorter overall temporal drug exposure in this protocol may result in diminished efficacy.
1 INTRODUCTION
Multicentric lymphoma, the most common haematopoietic tumour in the canine species, is treated with multi-agent chemotherapy protocols to achieve best outcomes.1 The standard-of-care treatment in many institutions is the combination of vincristine (VCR), cyclophosphamide (CTX), and doxorubicin (DOX), with the CHOP protocol being the most widely utilized and described. Many permutations of this protocol have been designed, with the induction phase ranging in length from 12 to 25 weeks. Various protocols also utilize a maintenance phase following the completion of the induction phase.
The majority of studies use historical controls to make inferences regarding the success or failure of protocols. However, these studies are often limited by institutions using different staging techniques, classification of remission, definition of progressive disease, management of toxicities, criteria for dose delays and other treatment and disease-related parameters. This ultimately can lead to differences in interpretation of progression-free survival (PFS). A direct comparison of two protocols over the same period of time within a single institution may allow for a more accurate interpretation of whether one protocol is superior over another.
In the recent past, our institution has utilized a 19-week CHOP protocol (CHOP19) to treat patients with multicentric lymphoma. Patients treated with this protocol have benefitted from similar survival times as the 25-week CHOP protocol (CHOP25), and the shortened course of treatment may be more feasible for pet owners because of decreased temporal commitment.2, 3 In addition, the dose intensity (DI) of the chemotherapy drugs in CHOP19 compared to the CHOP25 may provide further benefit by challenging neoplastic cells with various chemotherapeutics sooner and more frequently, theoretically decreasing the time for development and replication of chemotherapy-resistant populations.4, 5
Simon et al6 reported a prospective study using a 12-week CHOP protocol (CHOP12), which resulted in complete remission (CR) rate of 76.3% and a median duration of first remission of 243 days. This has been the only study to describe a CHOP12 and was deemed to result in satisfactory outcomes with acceptable toxicity. In addition, Burton et al7 and Curran and Thamm8 have recently described a dose-intense 15-week CHOP protocol that has resulted in similar survival times compared to historical controls treated with more prolonged CHOP chemotherapy protocols. The appeal of a CHOP12 that results in similar survival time as a 19-week protocol are manifold, as a shorter protocol could result in increased DI of some chemotherapy drugs with no “holidays,” and would decrease the time commitment and financial burden of the standard-of-care protocol for owners.
In the present study, we compared the outcomes for two sets of patients contemporaneously treated at our institution, one with CHOP19 and the other with CHOP12. By evaluating the outcomes of each cohort, with the clinicians using similar criteria for toxicities, dose delays, time to progression and rescue protocols between each set of patients, we sought to determine if the outcomes of each group were equivalent or if one protocol was superior to the other in terms of patient outcome. Our hypothesis was that CHOP12, which was more dose intense for DOX and CTX, albeit at the expense of VCR DI and overall length of protocol, would be similarly tolerated in terms of adverse events (AEs) while providing equivalent objective response rates and PFS durations as our standard CHOP19.
2 MATERIALS AND METHODS
2.1 Patient selection
From December 2015 to December 2017 pet owners at our institution were offered CHOP19 or CHOP12 for dogs diagnosed with multicentric lymphoma. The decision as to which treatment protocol to pursue was left to the owners, with guidance from the attending veterinarian regarding associated costs and risks of the protocols.
This study was prospectively conceived and while data were collated and analysed retrospectively, the criteria for inclusion/exclusion were established a priori and the patients were treated contemporaneously. Patients were included in study analysis if they were diagnosed with multicentric nodal lymphoma through cytology or histopathology of peripheral lymph nodes, with further characterization through polymerase chain reaction for antigen receptor rearrangements (PARR) or flow cytometry if elected by the owner. Patients with lymphoma that were primarily extranodal or with debilitating systemic illness prior to lymphoma diagnosis (eg, severe renal or hepatic compromise) were excluded. Other inclusion criteria required that the patient had not received previous treatment with chemotherapy or corticosteroids. Information regarding response to therapy and patient outcomes were collected from our institution's medical records system and records from referral partners.
2.2 Data collection
Information collected from medical records included breed, gender, neuter status, age at diagnosis, weight at diagnosis, date diagnosed, date treatment initiated, diagnosis method, complete blood count and serum biochemistry parameters, and chest radiograph and abdominal ultrasound abnormalities, if performed. Data regarding induction protocols, date of relapse, rescue protocols and date and reason of euthanasia or death were recorded.
Diagnosis of intermediate or large cell lymphoma was based on interpretation of cytological or histopathological assessment by a board-certified pathologist. Flow cytometry or histopathology was pursued on patients with cytologically intermediate cell lymphoma that was concerning for an indolent lymphoma prior to delivery of a CHOP-based protocol. Patients diagnosed with or suspected of having small cell lymphoma were excluded, as were patients suspected to have lymphocytic or lymphoblastic leukaemia. Immunophenotype and method of determination were recorded if available. Methods of immunophenotyping included PARR, flow cytometry, immunohistochemistry and immunocytochemistry.
Abnormalities in haematology and serum biochemistry profiles were classified using the reference ranges determined by the diagnostic laboratories which performed the tests. The hematologic profiles performed following chemotherapy administration were assessed and recorded for the incidence and grade of neutropenia as defined by VCOG CTCAE 1.1.9 Non-hematologic AEs, such as gastrointestinal upset, were documented by owner-based descriptions and clinician observations during veterinary visits.
Stage and sub-stage were assessed using the World Health Organization clinical staging system. Abdominal ultrasound and bone marrow aspiration were not routinely performed during the study period. Presumption of stage V lymphoma was based on assessment of other clinical abnormalities (such as ocular or pulmonary involvement) or identification of circulating neoplastic cells on blood film evaluation by a board-certified clinical pathologist.
Patients were identified with intent-to-treat with either CHOP19 or CHOP12, with schedules as detailed in Table 1. Patients that had either progressive disease, were euthanized, or received rescue chemotherapy prior to completion of the first cycle were excluded. No maintenance chemotherapy was administered in any patients that attained remission following completion of the CHOP protocol. Patients that were enrolled in a clinical trial involving investigational compounds during the CHOP protocol were excluded.
| Week | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19-week CHOP | 1 | 2 | 3 | 4 | 6 | 7 | 8 | 9 | 11 | 12 | 13 | 14 | 16 | 17 | 18 | 19 |
| VCR 0.7 mg/m2 | X | X | X | X | X | X | X | X | ||||||||
| CTX 250 mg/m2 | X | X | X | X | ||||||||||||
| DOX 30 mg/m2 | X | X | X | X | ||||||||||||
| Prednisone | 2 mg/kg | 1.5 mg/kg | 1.0 mg/kg | 0.5 mg/kg | ||||||||||||
| 12-week CHOP | Week | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| VCR 0.7 mg/m2 | X | X | X | X | ||||||||
| CTX 250 mg/m2 | X | X | X | X | ||||||||
| DOX 30 mg/m2 | X | X | X | X | ||||||||
| Prednisone | 2 mg/kg | 1.5 mg/kg | 1.0 mg/kg | 0.5 mg/kg | ||||||||
- a Dogs less than 15 kg received doxorubicin at 25 mg/m2.
Chemotherapy was delayed 2 to 7 days if the neutrophil count at nadir was below 1500/μL, if platelet count was less than 50 000/μL, or if owners reported any toxicity that contraindicated treatment. Prophylactic oral enrofloxacin at 5 to 10 mg/kg every 24 hours for 5 days was implemented if the neutrophil count was below 1000/μL, and patients were hospitalized for supportive care if febrile or otherwise ill. Patients with a toxicity that resulted in a delay of a normally-scheduled chemotherapy administration had a 10% to 20% dose reduction of the limiting chemotherapy at the next administration, dependent on clinician discretion.
VCOG criteria10 were utilized to assess response to treatment, and was based upon physical examination at each visit, and additional tests if indicated. CR was defined as the lymph nodes returning to normal size with no other evidence of disease. Partial response (PR) was defined as a 30% decrease in the sum of the target lymph nodes. Progressive disease was considered increase of target lymph node size of 20% or greater beyond smallest recorded measurement, or the development of new lesions. Stable disease was classified as either no change in lymph node size or change of less than 30% decrease or 20% increase, with no new lesions developing. The initial assessment of treatment response was performed at 42 days as is mandated in the VCOG criteria.10
Following the diagnosis of progressive disease of lymphoma, rescue protocols were offered to the owners. Rescue protocols were determined based on attending clinician discretion for each patient. Repeated CHOP protocol was offered if the dog had experienced at least 3 months of remission following completion of the previous CHOP protocol. The success of each protocol and total number of protocols were recorded for each patient. Dogs that had received six doses of DOX were either switched to mitoxantrone for subsequent treatments, or concurrently administered dexrazoxane with DOX after the sixth dose.
Echocardiograms were not routinely performed for patients receiving DOX; dogs that had concern for decreased systolic function at initiation of CHOP protocol and that did not receive any doses of DOX were excluded from the present study. Patients diagnosed with the multi-drug resistance gene (MDR1) mutation were excluded from the present study data analysis because of differences in DI, as routine prophylactic dose reductions of 20% to 40% of VCR and DOX were performed at our institution for patients with MDR1 mutations.
2.3 DI calculations
DI of the cytotoxic chemotherapy drugs in CHOP12 was calculated as described by Longo et al11 in reference to CHOP19 (Table 2). Prednisone was excluded from DI calculations as the prednisone schedule was identical for each protocol with respect to both dose and temporal length of prednisone treatment. The standard planned total administration dose for each cytotoxic chemotherapy drug was calculated and divided by the total number of weeks of the protocol, which provided an mg/m2/week. The DI of each drug in CHOP12 was divided by the DI of each drug in CHOP19 to provide a ratio for relative DI. Overall DI was then calculated by adding the DI for each drug and divided by the total number of drugs. Delivered DI was calculated for each patient population by calculating the actual delivered mg/m2 of each chemotherapeutic drug for each week of the CHOP protocol and dividing this by the total number of weeks required for each respective patient to complete their CHOP protocol. Average and relative DI was stated as a mean ± SD.
| 19-week CHOP | Calculated mg/m2/week | Calculated relative DI | Delivered mg/m2/week* | Delivered relative DI* |
|---|---|---|---|---|
| VCR 0.7 mg/m2 | 0.29 | 1.0 | 0.274 ± 0.03 | 0.931 ± 0.11 |
| CTX 250 mg/m2 | 52.6 | 1.0 | 49.05 ± 4.9 | 0.932 ± 0.09 |
| DOX 30 mg/m2 | 6.3 | 1.0 | 5.86 ± 0.58 | 0.928 ± 0.09 |
| Overall DI | — | 1.0 | — | 0.930 ± 0.09 |
| 12-week CHOP | Mg/m2/week | Relative DI | Delivered mg/m2/week | Delivered relative DI |
|---|---|---|---|---|
| VCR 0.7 mg/m2 | 0.23 | 0.79 | 0.210 ± 0.02 | 0.711 ± 0.08 |
| CTX 250 mg/m2 | 83.33 | 1.58 | 73.45 ± 9.6 | 1.40 ± 0.18 |
| DOX 30 mg/m2 | 10 | 1.58 | 8.53 ± 1.2 | 1.35 ± 0.19 |
| Overall DI | — | 1.32 | — | 1.15 ± 0.14 |
- Abbreviations: CTX, cyclophosphamide; DI, dose intensity; DOX, doxorubicin; VCR, vincristine.
- * All values in the column are significantly different (P < 0.0001) for CHOP19 compared to CHOP12.
2.4 Data analysis
Data collected from medical records were contained in a spreadsheet for analysis. Continuous data were expressed as medians, ranges and means ± SD (where appropriate), and categorical data were expressed as frequencies and percentages. PFS, the primary endpoint of this study, was defined as the time interval from date of initiation of chemotherapy treatment to the date of relapse or progression of lymphoma, or death from any cause. OS, a secondary endpoint, was defined as the time from date of initiation of chemotherapy treatment to the date of death from any cause. Dogs that remained alive at the end of the follow-up period or were lost to follow-up were censored at the date of last contact.
Kaplan-Meier estimation was used to demonstrate and display patients' PFS and OS probabilities. The Log-Rank test was used to analyse the PFS and OS between groups. Based on literature review, we anticipated an approximately 8-month PFS in CHOP19 group. True non-inferiority trials generally require very large numbers to be adequately powered12 and, as such, for this exploratory analysis a power calculation was designed to detect a 15% difference in the 8-month progression-free rate between CHOP19 group and CHOP12 group; that is, 50% vs 65% were found to be free from progression at 8 months, respectively. It was felt that a 15% difference in temporal efficacy would be a reasonable limit of clinical relevance. Based on this calculation, 28 dogs in each group would detect a 15% difference with 80% power and a P-value of 0.05. Objective response rate was defined as the combination of patients that experienced complete or partial response. Cox proportional hazards regression was utilized to assess patient characteristics and treatment data in relation to PFS and OS. Categorical data were compared between groups using a two-sided Fisher's exact test, whereas continuous data (eg, age, body weight) was compared between groups using the unpaired two-tailed Students t test after first passing a Kolmogorov-Smirnov normality test. All statistical analyses were performed with a commercial software package (Prism v8.0, Graph-Pad Software, LaJolla, California). A P-value of ≤0.05 was considered statistically significant.
3 RESULTS
3.1 Patient characteristics
From December 2015 to December 2017, 63 patients were treated with a CHOP protocol; however, only 47 patients were eligible for inclusion in this study. Excluded patients either had primarily extranodal lymphoma (such as hepatosplenic or gastrointestinal forms), severe pre-existing co-morbidity, an MDR1 mutation, or did not receive standard chemotherapy drugs of the CHOP protocol (eg, mitoxantrone instead of DOX). Twenty-eight patients were treated with CHOP12, and 19 patients were treated with CHOP19. Patient characteristics are summarized and statistically compared between groups in Table 3.
| Variable | 19-week CHOP | 12-week CHOP | P-value |
|---|---|---|---|
| Best response | 0.93 | ||
| Complete remission | 89.5% | 89.3% | |
| Partial response | 5.3% | 3.6% | |
| No response | 5.3% | 7.1% | |
| Body weight (kg) | 0.47 | ||
| Median | 34.5 | 28 | |
| Mean | 31.4 | 28 | |
| Range | 5.1-78.9 | 4.4-54.6 | |
| Age (years) | 0.88 | ||
| Median | 8.3 | 7.5 | |
| Mean | 7.8 | 7.9 | |
| Range | 3.5-10.8 | 3.5-13.3 | |
| Immunophenotype | (n = 17/19) | (n = 23/28) | >0.99 |
| B-cell | 76.5% | 78.3% | |
| T-cell | 23.5% | 21.7% | |
| Sub-stage b | 21.1% | 32.1% | 0.51 |
| Stage V | 10.5% | 28.6% | 0.17 |
| Anaemia | 15.8% | 21.4% | 0.72 |
| Hypercalcemia | 11.8% | 14.3% | >0.99 |
| Increased alkaline phosphatase | 21.1% | 35.7% | 0.34 |
| Increased aminotransferase | 26.3% | 35.7% | 0.54 |
| Received rescue or reinduction | 70.6% | 59.3% | 0.53 |
| Rescue/reinduction | 0.092 | ||
Protocol CHOP |
46% |
13% |
|
| CCNU/L-spar | 54% | 87% |
|
Immunophenotype was determined in 40 dogs (85.1%). Seventeen (89.5%) dogs in CHOP19 had immunophenotyping performed, and 4 of 17 (23.5%) were T-cell. Thirteen of 17 dogs had flow cytometry performed (12 B-cell, 1 T-cell), 1 of 17 had PARR performed (1 B cell), and 3 of 17 had ICC performed (1 B-cell, 2 T-cell). Twenty-three (82%) of the CHOP12 dogs had immunophenotyping performed, and 5 of 23 (21.3%) were T-cell. Fifteen of 23 had flow cytometry performed (13 B-cell, 2 T-cell), 2 of 23 had histopathology performed (2 B cell), 5 of 23 had ICC performed (3 B-cell, 2 T-cell), and 1 of 23 had PARR performed (1 T-cell).
Full WHO clinical staging (ie, thoracic and abdominal imaging, bone marrow aspirate) was not routinely performed. In CHOP19 cohort, 100% of the patients had CBC, chemistry panel, and urinalysis performed, 89.5% had chest radiographs performed, and 26.3% had an abdominal ultrasound reported. No patients had abdominal radiographs or bone marrow aspiration performed. All dogs in this study were classified as at least stage III. At presentation, 2 of 19 (10.5%) were considered stage V, and 5 of 19 (26.3%) were classified as sub-stage b. Among the three patients classified as stage V, one had circulating lymphoblasts present on the blood film, one had pulmonary infiltrates, and one had pulmonary and ocular infiltration consistent with lymphoma.
In the CHOP12 cohort, 100% of patients had a CBC, chemistry panel and urinalysis performed, 82% had chest radiographs performed, 7% had an abdominal ultrasound performed, and no patients had a bone marrow aspiration nor abdominal radiographs reported. At presentation, 8 of 28 (28.6%) dogs were considered stage V and 9 of 28 (32.1%) dogs were considered sub-stage b. Of the dogs classified as stage V, four dogs had circulating blasts, two dogs had ocular involvement, one dog had pulmonary infiltrates, and one dog had pulmonary infiltrates with neoplastic pleural effusion.
3.2 Protocols
The 19- and 12-week proposed schedules are detailed in Table 1. None of the dogs in the present study discontinued or changed treatment protocols for reasons other than lymphoma progression. Among dogs in CHOP19, 17 of 19 (89.5%) dogs completed the protocol; two had progressive disease while receiving treatment. Nine of 19 dogs completed the protocol in a 19-week period; average length to completion of CHOP19 was 19.9 weeks, with an SD of 1.3 weeks. Seventeen of 19 dogs had progressive disease by the end of data collection; 13 of 17 (76.5%) of these underwent further treatment with chemotherapy. Overall, 13 dogs in the 19-week group underwent a range of 0 to 3 rescue protocols (median = 1; mean = 1.05). Six of 13 dogs received repeated CHOP19 as a rescue, with mitoxantrone substituted at starting at the second cycle of repeated CHOP, and 7 of 13 dogs received CCNU/L-asparaginase as a first-line rescue therapy. Later rescue protocols included single-agent rabacfosadine (three dogs), CCNU/L-asparaginase (three dogs), single-agent DOX (one dog), and single-agent vinblastine (one dog).
Twenty-one of 28 (75%) dogs completed CHOP12; seven dogs had disease progression while receiving treatment. Six out of 28 dogs completed the protocol as scheduled in a 12-week period; average length to completion of CHOP12 was 13.4 weeks, with an SD of 1.4 weeks. Twenty-seven of 28 dogs had the progressive disease of by the end of the data collection, and 16 of 27 (60%) pursued rescue chemotherapy. Overall, dogs in the 12-week group underwent a range of 0 to 4 protocols (median = 1; mean = 1.14). First line rescue protocols included CHOP12 (2/16) and CCNU/L-asparaginase (14/16), and later rescue protocols included single-agent mitoxantrone (6), single-agent rabacfosadine (4 dogs), single-agent vinblastine (3), CCNU/L-asparaginase (1) and single-agent DOX (1).
The proportion of dogs that received rescue or reinduction chemotherapy after progression was not statistically different between the two protocols (Table 3; P = 0.53). Number of rescue/reinduction protocols per each cohort was compared by the Students t test (P = 0.53) and found not to be statistically different.
3.3 DI calculations and delivery
The targeted DI was calculated for CHOP19 for each drug and the overall protocol and compared with the DI of CHOP12. The DI of CHOP12 was calculated to be 1.32 in comparison to CHOP19, with the VCR DI being lower (0.79) and CTX and DOX DI being higher (1.58 for each). The actual delivered DI was then calculated for each drug in each protocol, and again compared with the target of CHOP19 (Table 2). The overall delivered DI of CHOP19 was 0.930, with the DI for VCR (0.931), CTX (0.932), and DOX (0.928) being lower than the baseline of the calculated CHOP19 DI. The overall delivered DI of CHOP12 was 1.15, with the delivered VCR DI being lower (0.711) and the CTX and DOX delivered DI again being higher (1.40 and 1.35, respectively). The differences in DI for the overall protocol and each individual cytotoxic chemotherapy were found to be statistically significant (P < 0.0001) between CHOP12 and CHOP19 (Table 2).
3.4 Adverse events
There were 143 AEs recorded; 53 for the 19 dogs in CHOP19, and 90 for the 28 dogs in CHOP12. AEs are summarized and graded in Table 4. One of 19 dogs in CHOP19 required hospitalization because of febrile neutropenia following chemotherapy administration; this dog had suspected hepatic dysfunction secondary to lymphoma at the initiation of treatment and despite a lowered dose of VCR (0.4 mg/m2), required hospitalization 6 days following administration. This dog obtained CR and liver values returned to normal; the dog tolerated all future doses of full-dose VCR appropriately. Nine of 19 dogs had dose reductions of chemotherapy; four had DOX dose reduction, and five dogs each had reduction of CTX and VCR. Nine dogs did not have any treatment delays, six had one treatment delay, two had two treatment delays, and two had three delays.
| Toxicity | 19-week protocol | 12-week protocol | P-value |
|---|---|---|---|
| Dogs experiencing any adverse event | 14/19 (73.7%) | 22/28 (79%) | 0.74 |
| Dogs experiencing a dose limiting adverse event | 9/19 (47.3%) | 18/28 (64%) | 0.37 |
| Dogs with any neutropenia | 13/19 (68.4%) | 19/28 (68%) | >0.99 |
| Grade I | 16 events8/19 dogs (42.1%) | 17 events11/28 dogs (39.3%) | >0.99 |
| Grade II | 9 events7/19 dogs (17.5%) | 14 events12/28 dogs (42.9%) | 0.77 |
| Grade III | 6 events4/19 dogs (21.1%) | 12 events9/28 dogs (32%) | 0.18 |
| Grade IV | 3 events2/19 dogs (10.5%) | 10 events7/28 dogs (25%) | 0.28 |
| Dogs with Grade III/IV neutropenia | 5/19 (26.3%) | 11/28 (40%) | 0.53 |
| Thrombocytopenia | 3 events3/19 dogs (15.8%) | 8 events7/28 dogs (25%) | 0.72 |
| Vomiting | 9 events6/19 dogs (31.6%) | 8 events5/28 dogs (17.9%) | 0.31 |
| Diarrhoea | 7 events5/19 dogs (26.3%) | 14 events7/28 dogs (25%) | >0.99 |
| Lethargy | 1 events1/19 dogs (5.3%) | 7 events5/28 dogs (17.9%) | 0.38 |
Three of 20 dogs in CHOP12 required hospitalization because of febrile neutropenia following chemotherapy administration; one of these three dogs had received CTX with a referring veterinarian despite displaying a Grade III afebrile neutropenia, and the other two patients were hospitalized following DOX treatment. Eighteen of 28 dogs had dose reductions of chemotherapy; 12 had DOX dose reduction, and eight had reduction of CTX, and six had reduction of VCR. Nine dogs did not have any treatment delays, 10 had one treatment delay, four had two treatment delays, one had three delays, and four had four delays.
3.5 Outcomes
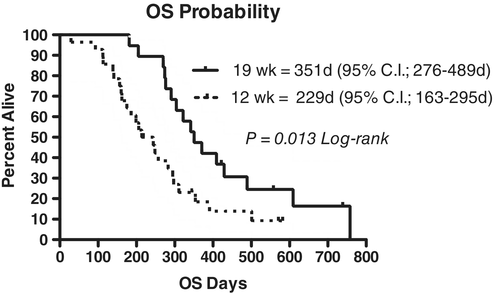
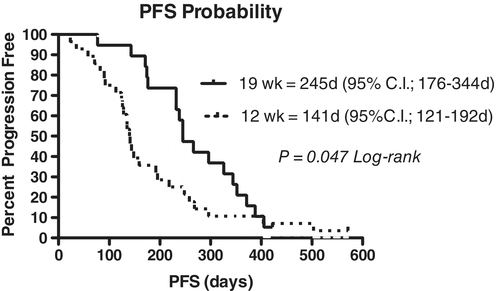
Of the CHOP19 group, 17 of 19 (89.5%) had a complete response, 1 of 19 (5.3%) had a partial response and 1 of 19 (5.3%) failed to achieve a response. The median PFS for the dogs in this group was 245 days (95% confidence interval [CI]: 176-344 days), and the median overall survival (OS) was 351 days (95% CI: 276-489 days). Three dogs remained in remission at the time of study data collection at 322, 422 and 558 days, and two dogs had relapsed but remained alive at the time of study completion. All other dogs in the study died because of lymphoma.
Of the CHOP12 group, 25 of 28 (89.3%) had a complete response, 1 of 28 (3.6%) had a partial response and 2 of 28 (7.1%) failed to achieve a response. The median PFS for the dogs in this group was 141 days (95% CI: 121-192 days), and the median OS was 229 days (95% CI: 163-295 days). One dog died of unrelated causes (biopsy-confirmed osteosarcoma with pathologic fracture) at 571 days and remained in clinical remission for the lymphoma at the time of euthanasia, and one dog came out of remission at 218 days and was lost to follow-up following that time. All other dogs died because of lymphoma.
Comparison of the CHOP19 and CHOP12 PFS and OS are presented in Figures 1 and 2. Dogs receiving CHOP19 had longer PFS (P = 0.047) and OS (P = 0.013) when compared to CHOP12.
Univariate analysis was performed on all patients together to determine potential prognostic factors for PFS and OS, and statistically significant factors are summarized in Table 5. The small sample size for these variables precluded meaningful multivariate analysis.
| Variable | Median progression-free survival (days) | P-value | Median overall survival (days) | P-value | |
|---|---|---|---|---|---|
| Anaemia | Yes | 171 | 0.120 | 199 | 0.020 |
| No | 226 | 297 | |||
| Hypercalcemia | Yes | 109 | <0.001 | 184 | 0.107 |
| No | 232 | 295 | |||
| Increased alkaline phosphatase | Yes | 174 | 0.246 | 257 | 0.028 |
| No | 232 | 311 | |||
| Increased aminotransferase | Yes | 148 | 0.021 | 257 | 0.017 |
| No | 242 | 304 | |||
| Immunophenotype | T-cell | 128 | 0.014 | 257 | 0.107 |
| B-cell | 245 | 297 |
Performance of T- and B-cell lymphoma in each group were additionally compared individually. The number of T-cell lymphomas in the present study was likely too low (four in CHOP19, five in CHOP12) for meaningful analysis, however, the median PFS of the CHOP12 group was 126 days and median PFS of the CHOP19 group was 159 days (P = 0.140). Among B-cell lymphomas, the CHOP12 group had a median PFS of 168 days and the CHOP19 group had a median PFS of 296 days (P = 0.191).
4 DISCUSSION
The present study provided a direct, albeit non-randomized, comparison between the traditional 19-week CHOP protocol and a 12-week CHOP protocol in a contemporary group of dogs treated at a single institution. Intuitively, a comparison using groups treated by the same clinicians over the same timeframe may introduce less bias than occurs using historical controls of patient outcomes because of the use of similar criteria for treatment, AE interpretation, and progression of disease. Furthermore, similar staging modalities were used for each group; abdominal ultrasound and bone marrow were not routinely recommended because of evidence that stage migration does not lead to significant differences in patient outcome.13 Neoplastic lymphocytosis with or without thrombocytopenia, ocular involvement, pulmonary infiltrates and other clinicopathological anomalies at diagnosis were used to suggest overt stage V disease.
The basic premise of our study was that by significantly increasing the DI of DOX and CTX in CHOP12, we would maintain similar efficacy as CHOP19 despite a decrease in VCR DI and a shorter treatment interval. This premise was based on the fact that cytotoxic chemotherapeutic agents generally have linear dose-response curves. In addition, excluding treatment delays inherent to the CHOP19 protocol during the weeks following DOX treatments and overall increased DI would be more likely to result in higher likelihood of high-grade neutropenic episodes and necessary treatment delays, which has been found to correlate with improved patient outcome. 4, 5, 7 Using a neutrophil count of 1500 cells/μL and above to permit chemotherapy administration was described by Fournier et al14 as a clinically appropriate cut-off that would permit the most condensed length of each overall treatment in each protocol, and thus, provide the highest potential DI. Furthermore, it is generally believed that anthracyclines are the most efficacious component of CHOP protocols3, 15 and we felt that the increased DI of DOX in our CHOP12 would offset any diminished efficacy associated with the lower VCR DI. While our “delivered” DI of DOX and CTX was not as high as planned in our CHOP12 owing to AEs inherent in CHOP-based treatment, we still achieved 35% DOX and 40% CTX dose intensification over our target DI for the standard 19-week protocol. In fact, comparing actual “delivered” DI between groups, dogs in CHOP12 received 45.6% and 49.7% higher DI for DOX and CTX, respectively (P < 0.0001). However, with CHOP12, VCR was 30.5% less dose intense (P < 0.0001) than CHOP19. Ultimately, our data did not support our basic premise; while overall response rates were nearly identical between CHOP12 and CHOP19, the durability of response (ie, PFS) was significantly shorter for CHOP12. The PFS, overall survival and incidence of AE for the current CHOP19 was similar to previous reports of DOX-based protocols.3, 8, 16-19 However, both PFS (P = 0.047) and OS (P = 0.013) were found to be statistically shorter in dogs receiving CHOP12, compared with dogs receiving CHOP19 (Figures 1 and 2). The median PFS and OS of our CHOP12 group was more analogous to reported single-agent DOX chemotherapy protocols20-22 than reports involving iterations of multiagent DOX-based protocols.3, 8, 16-19, 23
While a power calculation demonstrated that 28 dogs in each treatment group would be sufficient to show a 15% difference in progression-free rate at 8 months, after 2 years of accruals, we became concerned that the CHOP12 group was underperforming and performed an interim analysis was completed which confirmed dogs treated with CHOP12 had decreased PFS and OS compared to those treated with CHOP19 and accrual was stopped.
While our study design does not allow a clear identification of the cause for the shorter PFS observed in our CHOP12 group, we can speculate on potential explanations as only two known variables were, by design, different between the two protocols. First, the DI of the three major components (DOX, CTX and VCR) were different but only VCR was of lower DI. This suggests that VCR DI may be more important for maintaining durability of PFS than we had previously thought. There is some support for this line of reasoning based on work by Sato et al who showed, through molecular analysis, that VCR provides strong cytoreductive activity in canine patients receiving CHOP treatment for lymphoma.24 Thus, the increase of DOX and CTX DI at the expense of VCR DI in our study could have consequences for duration of PFS. This is not, however, supported by outcomes reported in Simons CHOP12 protocol, which has an identical reduction in VCR DI without a seeming loss in durability of outcomes.6 The Simon study reported a first remission duration (FRD) of 243 days, which is longer than our observed PFS of 141 days. The Simon study differed from our study in that it utilized L-asparaginase at the beginning of treatment, although in multiple studies25, 26 this has not been shown to contribute to duration of remission with more standard-length protocols. Also, FRDs are generally longer than PFS endpoints as the former does not include ‘death from any cause’ as an event. Ultimately, prospective randomized trials that vary the DI of just one, rather than several, cytotoxic agents in a protocol will be necessary to determine the relative effect of each.
The other known variable designed to be different between CHOP12 and CHOP19 is the temporal length of treatment and as such is a second potential explanation for poor outcomes. CHOP19 includes an additional 49 days of treatment, albeit at a lower DI. As we rarely achieve molecular CRs in dogs with lymphoma27-32 and the vast majority of dogs eventually relapse when their chemotherapy-achieved minimal residual disease burdens progress to clinically measurable disease, one could theorize that the longer period of active treatment may, in essence, delay this return to a clinically measurable burden. While a less durable PFS in patients that spend less time undergoing chemotherapy is inconsistent with studies suggesting that maintenance chemotherapy following more conventional CHOP protocols is not advantageous,16, 33-35 perhaps there is a minimum temporal threshold where this holds true and CHOP12 does not meet this threshold. However, the median PFS of the CHOP19 group was 104 days longer than the CHOP12 group, which is twice the length of the 49 days of additional treatment, suggesting there is an alternative or additional factor involved.
Finally, the non-randomized design of our study, with owners choosing which protocol to pursue, could have introduced some as yet unidentified bias in case selection resulting in the differences observed. PFS is the preferred endpoint of this study as OS is more likely to be biased in veterinary patients with lymphoma owing to client-driven decisions on retreating (ie, reinduction/rescue) once progression has occurred and variability in client euthanasia decisions. Regarding the former, there was no statistical difference found in either the likelihood nor the number of additional chemotherapy protocols received after first progression in our two protocol groups suggesting this bias did not exist. However, nearly half of CHOP19 dogs went on to receive CHOP reinduction or rescue, whereas the vast majority of CHOP12 dogs had CCNU/L-asparaginase rescue, although this difference did not quite achieve significance (Table 3). This difference may have been driven by financial limitations, as the CCNU/L-asparaginase protocol is less costly and time-intensive than the CHOP protocol at our institution. The CHOP protocol was only recommended as a rescue to clients if the patient has been in remission for greater than 3 months after the completion of the initial CHOP protocol, which was more frequently achieved with the CHOP19 group than the CHOP12 group. These differences in rescue/reinduction protocols, while having no bearing on PFS, could have affected OS.
The second part of our hypothesis was that CHOP12 would be similarly tolerated as CHOP 19 with respect to AEs. This was confirmed (Table 4) as the relative rate, grade and characteristic of AEs were indeed similar. While dogs receiving CHOP12 were numerically more likely to have a dose-limiting AE and a higher grade of neutropenia, this was not statistically different from CHOP19.
As expected, dogs with T-cell lymphoma had significantly shorter PFS compared to dogs with B-cell lymphoma (Table 5). While the numerical differences in PFS were maintained when these comparisons were made within treatment groups, they did not achieve statistically significant difference likely because of Type II error. At our institution, all dogs, regardless of immunophenotype, are offered CHOP as the initial standard-of-care protocol. Therefore, in light of equal proportions of T-cell lymphoma in each treatment group, we chose to include both immunophenotypes in the population under study. This also reflects the clinically-relevant scenario for those owners who decline immunophenotyping for various reasons. Patients suspected or definitively diagnosed to have small cell or indolent lymphoma were excluded from the present study as the CHOP protocol is not utilized for these patients at our institution. Although the definitive subcategory of T-cell lymphoma was not always known, several methods were used for immunophenotyping and those cases initially suspected to be indolent based on clinicopathological findings had further characterization through either flow cytometry or immunohistochemistry that were consistent with typical findings of aggressive lymphoma.
The small number of patients in this study precluded the use of meaningful multivariate analysis. Univariate analysis found that presence of hypercalcemia (P < 0.001), T-cell immunophenotype and increased alanine aminotransferase (ALT) (P = 0.029) were associated with decreased PFS, while anaemia (P = 0.023), increased alkaline phosphatase (P = 0.038), and increased ALT (P = 0.017) were associated with decreased OS. Increased ALT was the only value that was associated with both shorter PFS and OS, which could be because of more significant infiltration of the liver by neoplastic cells, meaning a higher disease burden at the time of diagnosis. Anaemia has been previously described as a prognostic indicator for poor OS but not for PFS,36 which is consistent with our findings.
There were several limitations to this study. The number of patients in each group were small; however, because of significantly poorer outcomes found among CHOP12 patients when compared with CHOP19 patients during interim analysis, continued expansion of the cohorts was not performed. The study participants were not randomized, and clients elected which treatment group they wished for their dogs to enter which can create unrecognized bias. Thorough WHO staging (eg, bone marrow, abdominal imaging and abdominal cytology) was not performed in all patients, although this was a more real-world scenario and was unlikely to result in significant bias as previously discussed.
In conclusion, while CHOP12 is overall well tolerated, in this patient population it underperformed with respect to PFS durability when compared to CHOP19. CHOP19 should remain the preferred protocol over CHOP12 when pet owners seek the longest initial remission from a multiagent chemotherapy protocol.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.