Full-left-full-right split liver transplantation for adult recipients: a systematic review and meta-analysis
Summary
Full-left-full-right split liver transplantation (FSLT) for adult recipients, may increase the availability of liver grafts, reduce waitlist time, and benefit recipients with below-average body weight. However, FSLT may lead to impaired graft and patient survival. This study aims to assess outcomes after FSLT. Five databases were searched to identify studies concerning FSLT. Incidences of complications, graft- and patient survival were assessed. Discrete data were pooled with random-effect models. Graft and patient survival after FSLT were compared with whole liver transplantation (WLT) according to the inverse variance method. Vascular complications were reported in 25/273 patients after FSLT (Pooled proportion: 6.9%, 95%CI: 3.1–10.7%, I2: 36%). Biliary complications were reported in 84/308 patients after FSLT (Pooled proportion: 25.6%, 95%CI: 19–32%, I2: 44%). Pooled proportions of graft and patient survival after 3 years follow-up were 72.8% (95%CI: 67.2–78.5, n = 231) and 77.3% (95%CI: 66.7–85.8, n = 331), respectively. Compared with WLT, FSLT was associated with increased graft loss (pooled HR: 2.12, 95%CI: 1.24–3.61, P = 0.006, n = 189) and patient mortality (pooled HR: 1.81, 95%CI: 1.17–2.81, P = 0.008, n = 289). FSLT was associated with high incidences of vascular and biliary complications. Nevertheless, long-term patient and graft survival appear acceptable and justify transplant benefit in selected patients.
Introduction
Full-left-full-right split liver transplantation (FSLT) is a procedure whereby the donor liver is split into two hemi-liver grafts, consisting respectively of segment I–IV (left lobe) and segment V–VIII (right lobe) [1, 2]. Since one liver graft can be shared between two adult recipients, FSLT may increase the number of available grafts and reduce waitlist time and mortality [3-5]. This technique may especially favor smaller adolescent and adult patients, who are disadvantaged on the waiting list to receive a timely size-matched graft [6, 7].
The first FSLT was reported by Bismuth et al. in 1989 [1]. One cadaveric liver graft was used for the transplantation of two adult patients suffering from fulminant hepatitis, and both patients died after transplantation. Since 1989, numerous case reports and case series of successful FSLT for two adult recipients have been published. But safety and feasibility of FSLT remains a topic of debate as the procedure is both technically challenging and requires almost simultaneous increased resource availability [8]. Vasculature of the graft needs to be shared to achieve adequate in and outflow for both hemi-liver grafts. The anatomy of the hepatic artery and the middle hepatic vein needs to be carefully assessed and may require reconstruction. In addition, adequate biliary drainage for all liver segments of both grafts is imperative. Due to these technical difficulties, FSLT patients may be at increased risk for vascular and biliary complications as compared with whole liver transplantation (WLT). Another concern relates to providing an adequate graft volume for both recipients, which may need particular attention for the left hemi-liver to prevent a low graft-to-recipient weight ratio [9]. Finally, FSLT requires almost immediate availability of skilled personnel to perform three complex surgical procedures simultaneously.
Although numerous series and several comparative studies on FSLT have been published, data involving larger sample sizes is lacking. To assess the feasibility of FSLT, data on the outcomes of both hemi-liver grafts is imperative. Therefore, the primary objective of this systematic review and meta-analysis is to assess graft and patient survival after FSLT in a pooled analysis and compare outcomes to WLT. In addition, the occurrence of postoperative complications after FSLT will be assessed.
Methods
The study protocol was prospectively registered in the PROSPERO database (CRD42017060013) [10]. The manuscript was written according to the Cochrane handbook for interventional systematic reviews and the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [11, 12].
Search strategy
The Embase, Medline Ovid, Cochrane, Web of Science and Google Scholar databases were searched for studies reporting on FSLT transplantation through January 2021. The full search syntax is presented in the supplement. Full text studies reporting on FSLT with or without a control group were included. Abstracts, reviews, case reports, letters and editorials, pediatric-only studies, and studies not written in English were excluded. Studies were first evaluated for inclusion based on title and abstract. Selected abstracts were subsequently included based on the full text record. Study selection was performed by two independent reviewers (DS and ABvD). Article selection was finalized based on mutual consensus. Manual cross-referencing was performed to identify additional relevant studies. Studies presenting data on FSLT for adult recipients were included; studies including incidental pediatric patients due to shared adult pediatric FSLT were included as well. If multiple articles were reported on the same source population of patients (duplicate cases), the most recent publication presenting relevant outcomes was included.
Quality assessment
Quality assessment was performed independently by two reviewers according to the validated checklist of Downs and Black [13]. The Downs and Black checklist consists of 27 items grouped in five subscales (quality of reporting, external validity, potential for bias, confounding, and power analysis). The maximum score is 32 points. Item 27 concerning the study power was modified, as it usually does not translate well to niche subjects with small patient populations. We anticipated a majority of observational studies in which it would be rarely feasible to reach a power of >80%. Instead of the original 5-point scale, we assigned a score of ‘1’ to studies without a power calculation, 3 if a power calculation was present, and 5 if the study power was adequate.
Data extraction
Data were extracted with the use of standardized forms by two independent reviewers. Available baseline and outcome characteristics were extracted for the FSLT group and if available for the reference group. Extracted baseline characteristics comprise number of patients in each group, age, weight, model for end-stage liver disease (MELD) score, in situ split liver, and ex situ split liver. Extracted outcomes comprise patient survival, graft survival, vascular complications, biliary complications, primary non-function, and small for size syndrome. Aggregated data were collected as reported. If not reported, survival data were extracted from graphs according to methods described by Tierney et al. with the use of specialized and previously validated software [14, 15].
Statistical analysis
Available data on actual 90-day, 1-year, 2-year, and 3-year graft- and patient survival after FSLT as well as data on postoperative complications were pooled with the use of R-statistics and ‘OpenMetaAnalyst’ [16]. Binary random-effects models were used, heterogeneity was quantified according to the Der Simonian Laird method. Pooled proportions were presented with corresponding 95% confidence intervals (95%CI). Patient-and graft survival from studies comparing FSLT to WLT were compared according to the generic inverse variance method with the use of Revman 5, according to methods described by Tierney et al. [15]. Hazard ratios were presented with corresponding 95%CIs and visually in forest plots. The occurrence of vascular and biliary complications was compared between FSLT and WLT with the use of random-effects models. Odds ratios (OR) with corresponding 95%CIs were visually presented in forest plots. Between-study heterogeneity on presented outcomes was assessed with the I2 statistic. A p-value <0.05 was considered significant.
Results
Literature search results
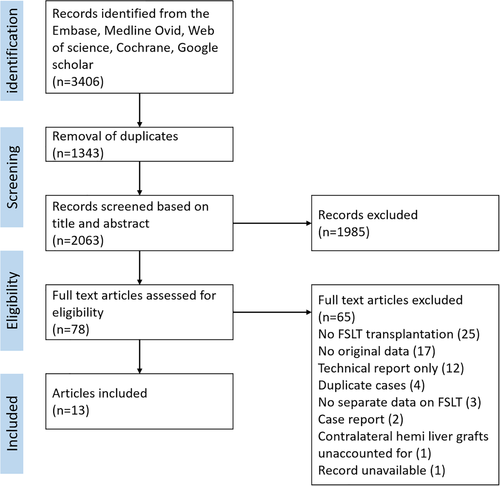
The PRISMA flow chart is presented in Fig. 1. The initial search returned 2063 original records, a total of 78 selected articles were assessed based on full text. Finally, 13 articles were included [2, 8, 9, 17-26]. Four articles were excluded, since a more recent article reported similar cases [27-30]. One large registry-based study reported outcomes of 651 right hemi-liver grafts, but included only the outcomes of 117 left hemi-liver grafts without presenting reasons for exclusion. This study was excluded, since data on the majority of left hemi-liver grafts were unavailable [31]. The study of Chan et al. and Lee et al. reported different outcomes for the same cohort of patients and were both included [9, 24]. Quality assessment of included studies is summarized in Table 1. All studies were of moderate quality, scoring a median of 16 out of 32 points (range 14–19 points). Seven studies compared FSLT with a cohort of WLT recipients and two studies to a cohort of living donor liver transplantation (LDLT) recipients [8, 9, 17-20, 22, 24]. Comparative quantitative meta-analysis was possible in three studies concerning the occurrence of vascular and biliary complications [17, 19, 20], six studies provided adequate data to compare patient survival, and five studies provided appropriate data to compare graft survival after FSLT versus WLT [8, 9, 17, 18, 20, 22]. Baseline characteristics of included studies are summarized in Table 2, donor recipient or graft-to-recipient weight ratios are presented in Table 3. In total, 397 hemi-liver grafts (195 left and 202 right lobes) were included in the present study. In total, data of 26 hemi-liver grafts out of five studies were not reported [9, 18-22]. Of these, 21 hemi-liver grafts were transplanted in another center, resulting in loss of data [9, 18-20]. Two hemi-liver grafts were discarded due to technical concerns [19, 20]. One study did not report the data of a hemi-liver graft that was used for repeat liver transplantation [22]. In one other study, it remained unclear why two hemi-liver grafts were missing in reported data [21].
| References | Reporting | External validity | Internal validity (risk of bias) | Internal validity (confounding) | Power | Total points |
|---|---|---|---|---|---|---|
| Aseni et al. 2014 | 9 | 3 | 3 | 2 | 1 | 19 |
| Azoulay et al. 2001 | 9 | 3 | 4 | 2 | 1 | 18 |
| Giacomoni et al. 2008 | 7 | 3 | 4 | 2 | 1 | 17 |
| Lee et al. 2013 | 7 | 3 | 4 | 2 | 1 | 17 |
| Vagefi et al. 2013 | 7 | 3 | 4 | 2 | 1 | 17 |
| Broering et al. 2005 | 6 | 3 | 4 | 2 | 1 | 16 |
| Hashimoto et al. 2014 | 6 | 3 | 4 | 2 | 1 | 16 |
| Humar et al. 2008 | 6 | 3 | 4 | 2 | 1 | 16 |
| Zamir et al. 2002 | 6 | 3 | 4 | 1 | 1 | 15 |
| Colledan et al. 2001 | 6 | 3 | 2 | 2 | 1 | 14 |
| Jung et al. 2017 | 6 | 3 | 4 | 1 | 1 | 15 |
| Herden et al 2018 | 8 | 3 | 4 | 2 | 1 | 18 |
| Chan et al. 2019 | 6 | 3 | 4 | 2 | 1 | 16 |
| Maximum score | 11 | 3 | 7 | 6 | 5 | 32 |
| Study | n | Age (years) | Weight (kg) | MELD score | In situ Split | Ex situ split | CIT (minutes) | Missing hemi-liver grafts, n (%) |
|---|---|---|---|---|---|---|---|---|
| Full-left full-right split liver transplantation | ||||||||
| Colledan 2001 | 8 | 36.5 (10–56) | 54 (34–79) | NA | 6 | 2 | 430 (285–840) | 0 |
| Zamir 2002 | 6 | 35 (17–47) | 58 (50–67) | NA | 6 | 0 | 420 (360–840) | 0 |
| Vagefi 2014 | 18 | 48.5 (2–66) | 54 (14–119) | 28.5 (10–40) | 0 | 18 |
L: 585 (216–912) R:588 (366–804) |
0 |
| Jung 2017 | 16 | 51.5 (25–64) | 56.9 (37.8–97.1) | 19 (10–33) | 16 | 0 | 270 (55–544) | 0 |
| Herden 2018 |
A: 27 P: 17 |
50 (23–68) 9 (1–17) |
64 (45–97 29 (9–60) |
6 (6–11) 8 (6–40) |
NA | NA | 616 (193–1137) | L: 2 |
| Giacomoni 2008* | 16 | 49 (32–61) |
L: 62.4 (56–74) R:65.2 (61–76) |
L: 20 (10–21) R: 21.5(15–35) |
16 | 0 |
L: 460 (360–780) R: 410 (124–760) |
R:2 L: 4 |
| Azoulay 2001* | 34 | 47 (25–65) | 61 (35–97) | NA | 4 | 30 |
L: 515 (85–883) R: 678 (30–953) |
0 |
| Broering 2005* | 35 |
L: 12(3–64) R:48 (31–65) |
L: 35 (13–61) R:70 (45–97) |
NA | 7 | 12 |
L: 503 (288–703) R: 665 (193–933) |
R: 3 |
| Aseni 2014* | 64 | 47.5 (6–66) | 58 (31–76) | 16 | NA | NA | 420 (90–840) | 0 |
| Hashimoto 2014* | 25 | 56 (13–75) | 58 (25–101) | NA | 23 | 2 | 390 (248–592) | 6 |
| Chan 2019* | 100 | 50 (39–65) | NA | 19 (7–45) | 53 | 1 | (69–779) | 8 |
| Humar 2008* | 31 | 52 (34–68) | NA | 18 (12–39) | 31 | 0 | 492 | 1 |
| Lee 2013* | 42 |
L: 48 (33–63) R: 44 (33–60) |
L: 52 (39–70) R: 73 (50–86) |
22 (14–40) | 42 | 0 |
L: 765 (182–1054) R: 711 (231–10) |
0 |
| Study | n | Age (years) | Weight (kg) | MELD score | CIT | Matched cohort |
|---|---|---|---|---|---|---|
| Comparative group: Whole liver transplantation | ||||||
| Giacomoni 2008 | 232 | NA | NA | NA | NA | No |
| Azoulay 2001 | 88 | 52 (18–69) | 73 (43–110) | NA | 508 (153–960) | No |
| Broering 2005 | 207 | 30 (3–65) | NA | NA | NA | No |
| Aseni 2014 | 1199 | 52 (7–70) | 73 (31–130) | 17 | 480 (66–1320) | Yes |
| Hashimoto 2014 | 121 | 55 (18–77) | 88 (41–160) | 21 (10–40) | 421(150–840) | Yes |
| Humar 2008 | 284 | 51 (18–73) | NA | 26 (14–20) | 420 | No |
| Chan 2019 | 165 | NA | NA | NA | NA | NA |
| Comparative group: living donor liver transplantation | ||||||
| Humar 2008 | 69 | 49.7 (22–68) | NA | 17 (10–26) | <30 | No |
| Lee 2013 | 282 | 54 (19–69) | NA | 16 (11–21.3) | NA | No |
- A, adult; CIT, cold ischemia time; L, left; MELD, model for end-stage liver disease; NA, not available; P, pediatric; R, right.
- When available continuous variables are presented as median (range).
- * Comparative studies.
| Study | Donor recipient weight ratio | Graft vs recipient weight ratio | Total liver volume (graft) vs standardized liver volume (recipient) |
|---|---|---|---|
| Colledan 2001 | 1.4 (0.82–1.67) | NA | NA |
| Zamir 2002 | 1.8 (1.67–2.63) | NA | NA |
| Vagefi 2014 | 1.8 (0.91–3.78) | NA | NA |
| Jung 2017 | 1.3 (0.66–2.33) | NA | NA |
| Azoulay 2001 |
R: 1.06 (0.83–2.28) L: 1.55 (1.15–2.18) |
NA | NA |
| Aseni 2014 | 1.40 (0.80–3.30) | NA | NA |
| Giacomoni 2008 | NA |
R: 0.84 (0.72–1.12) L: 0.82 (0.76–1.04) |
NA |
| Hashimoto 2014 | NA |
R: 1.62 (0.74–2.54) L: 1.13 (0.93–3.12) |
NA |
| Chan 2019 | NA | 1.0 (0.6–2.0) | NA |
| Broering 2005 | NA | NA |
R: 0.70 (0.49–0.84) L: 0.60 (0.34–1.20) |
| Herden 2018 | NA | ||
| Humar 2008 | NA | NA | NA |
- NA, not available, median and range are given.
Complications after FSLT
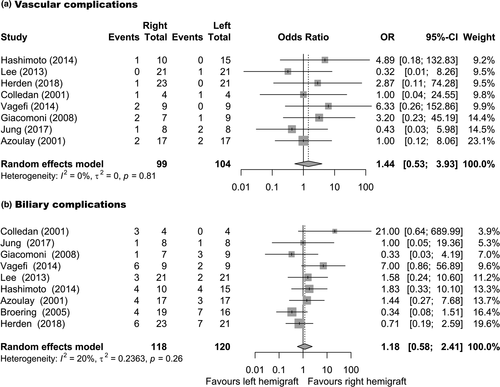
Reported frequencies of complications after FSLT are summarized in Table 4, Specific types of complications are summarized in the supplement. Vascular complications were reported in 25/273 patients in 10 studies (Pooled proportion: 6.9%, 95%CI: 3.1–10.7%, I2: 36%, Figure S1) [2, 8, 17, 19-21, 23-26]. Biliary complications were reported in 84/308 patients in 11 studies (Pooled proportion: 25.6%, 95%CI: 19–32%, I2: 44%, Figure S1) [2, 8, 17-21, 23-26]. Primary non-function was reported in 15/248 patients in nine studies (Pooled proportion: 5.7%, 95%CI: 2.9–8.6%, I2: 0%, Figure S2) [2, 8, 17, 18, 20, 23-26]. Small for size syndrome was reported in 6/264 patients reported in 10 studies (Pooled proportion: 2.1%, 95%CI: 0.4–3.9%, I2: 0%, Figure S2) [2, 8, 17-20, 23-26]. Three studies compared the occurrence of vascular and biliary complications after FSLT to WLT (n = 75). The incidence of vascular complications was higher after FSLT compared with WLT (Pooled OR: 5.7, 95%CI: 1.95–16.8, P = 0.001, I2: 0%, Fig. 2a) [17, 19, 20]. Similarly, the incidence of biliary complications was higher after FSLT (Pooled OR: 3.0, 95%CI: 1.6–5.9, P < 0.001, I2: 0%, Fig. 2b) [17, 19, 20]. When comparing the frequency of vascular complications in recipients of left and right hemi-livers, no significant difference was present for left versus right FSLT (Pooled OR: 1.44, 95%CI: 0.53–3.93, P = 0.475, I2: 0%, Fig. 3a) [2, 17, 19-21, 23, 24]. Similarly, no significant difference was present in the frequency of biliary complications after left versus right FSLT (Pooled OR: 1.18, 95%CI: 0.58–2.41, P = 0.647, I2: 20%, Fig. 3b) [2, 17-21, 23-25].
| Study | Number of hemi-grafts* | Vascular complications | Biliary complications | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Left | Right | Total | Left | Right | Total | Left | Right | |
| Zamir 2002 | 6 | 3 | 3 | 0 | 0 | 0 | 2 (33) | 1 (33) | 1 (33) |
| Colledan 2001 | 8 | 4 | 4 | 2 (25) | 1 (25) | 1 (25) | 3 (38) | 3 (75) | 0 |
| Jung 2017 | 16 | 8 | 8 | 3 (19) | 1 (17) | 2 (33) | 2 (13) | 1 (17) | 1 (17) |
| Giacomoni 2008 | 16 | 7 | 9 | 3 (19) | 2 (29) | 1 (11) | 4 (25) | 1 (14) | 3 (33) |
| Vagefi 2014 | 18 | 9 | 9 | 2 (11) | 2 (22) | 0 | 7 (39) | 6 (67) | 2 (22) |
| Hashimoto 2014 | 25 | 10 | 15 | 1 (4) | 1 (10) | 0 | 8 (32) | 4 (40) | 4 (27) |
| Humar 2008 | 31 | 15 | 16 | NA | NA | NA | NA | NA | NA |
| Azoulay 2001 | 34 | 17 | 17 | 4 (12) | 2 (12) | 2 (12) | 6 (18) | 4 (24) | 3 (18) |
| Broering 2005 | 35 | 19 | 16 | NA | NA | NA | 10 (29) | 4 (21) | 7 (44) |
| Lee 2013† | 42 | 21 | 21 | 1 (2) | 0 | 1 (5) | 5 (12) | 3 (14) | 2 (10) |
| Herden 2018 | 44 | 23 | 21 | 1 (2) | 1 (4) | 0 | 13 (30) | 6 (26) | 7 (33) |
| Aseni 2014 | 64 | 32 | 32 | 8 (13) | NA | NA | 24 (38) | NA | NA |
| Chan 2019† | 100 | 48 | 52 | NA | NA | NA | NA | NA | NA |
| Total (n) | 397 | 195 | 202 | 25 | 84 | ||||
| Pooled proportion (%) | 6.9% | 25.6% | |||||||
| 95%CI | 3.1–10.7% | 18.9–32.2% | |||||||
| Study | Primary non-function | Small for size syndrome | Re-transplantation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Left | Right | Total | Left | Right | Total | Left | Right | |
| Zamir 2002 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Colledan 2001 | 1 (13) | 0 | 1 (25) | 0 | 0 | 0 | 2 (25) | 1 (25) | 1 (25) |
| Jung 2017 | 1 (6) | 1 (17) | 0 | 0 | 0 | 0 | 1 (6) | 1 (17) | 0 |
| Giacomoni 2008 | NA | NA | NA | 1 (6) | NA | NA | 0 | 0 | 0 |
| Vagefi 2014 | 0 | 0 | 0 | 1 (6) | 1 (11) | 0 | 1 (6) | 1 (11) | 0 |
| Hashimoto 2014 | 2 (8) | 1 (10) | 1 (7) | 0 | 0 | 0 | 2 (8) | 1 (10) | 1 (7) |
| Humar 2008 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Azoulay 2001 | 3 (9) | 3 (18) | 0 | 0 | 0 | 0 | 4 (12) | 4 (24) | 0 |
| Broering 2005 | 2 (6) | 2 (11) | 0 | 0 | 0 | 0 | 4 (11) | 2 (11) | 2 (13) |
| Lee 2013† | 3 (7) | 3 (14) | 0 | 0 | 0 | 0 | NA | NA | NA |
| Herden 2018 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Aseni 2014 | 3 (5) | NA | NA | 4 (9) | NA | NA | 3 (5) | NA | NA |
| Chan 2029† | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Total | 15 | 6 | 17 | ||||||
| Pooled proportion (%) | 5.7% | 2.1% | 6.4% | ||||||
| 95%CI | 2.9–8.6% | 0.4–3.9% | 3.2–9.6% | ||||||
- NA, not available. Absolute numbers and (%) are given.
- * Number of transplanted hemi-grafts is reported.
- † Studies representing in part the same patients. Exact types of complications are specified in the supplement. Pooled proportions are calculated with random-effect models. Pooled proportions are not calculated for the subgroups of left or right FSLT.

Graft and patient survival after FSLT
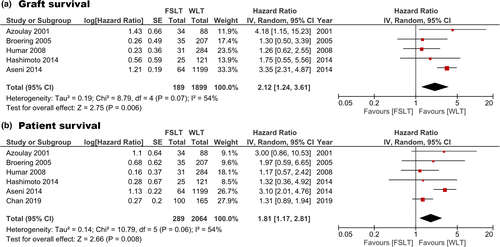
Graft and patient survival rates for each study are summarized in Tables 5 and 6. Graft and patient survival for recipients of specifically a left or right hemi-liver graft is summarized in Table 7. Re-transplantation was reported in 17/222 patients in nine studies (Pooled proportion: 6.4%, 95%CI: 3.2–9.6%, I2: 0%, Figure S3) [2, 8, 17-20, 23, 25, 26]. Reported 90-day graft survival ranged between 72 and 89% (n = 249). Reported 1-year graft survival ranged between 63 and 80% (n = 265). The estimated pooled 3-year graft survival was 72.8% (95%CI: 67.2–78.5% I2: 0%, n = 231). Reported 90-day patient survival ranged between 76.2 and 96% (n = 365). Reported 1-year patient survival ranged between 68.3 and 88.1% (n = 365). The estimated pooled 3-year patient survival was 77.3% (95%CI: 68.7–85.8% I2: 49%, n = 331). Patient and graft survival after FSLT could be compared with WLT with data from six and five studies respectively. FSLT was associated with increased graft loss (pooled HR: 2.12, 95%CI: 1.24–3.61, P = 0.006, I2: 54% Fig. 4a) and patient loss (pooled HR: 1.81, 95%CI: 1.17–2.81, P = 0.008, Fig. 4b) [8, 9, 17, 18, 20, 22]. Two studies compared FSLT with LDLT. Lee et al. [24] reported 90-day, 1 year, and 5-year graft survival rates after FSLT (n = 42) of 76.2%, 71.4%, and 69% compared with 89.3%, 79.9%, and 70.4%, respectively, after LDLT (n = 282, P = 0.489). Humar et al. [22] reported 3-year graft survival rate of 74% after FSLT (n = 31) versus 89% after LDLT (n = 69) (P = 0.05).
| Study | n | Follow-up, median (range) | Proportion (%) graft survival | |||||
|---|---|---|---|---|---|---|---|---|
| 3 months | 1 year | 2 year | 3 year | 5 year | 10 year | |||
| Aseni 2014 | 64 | – | 72 | 63.3 | 62 | 62 | 58.7 | – |
| Herden 2018 | 44 | 91(34–202) | 88.3 | 83.4 | 80.5 | 80.5 | 77.3 | 59.6 |
| Broering 2005 | 35 | 27.4 (1–68.3) | 88.4 | 80 | 74 | 74 | – | – |
| Azoulay 2001 | 34 | – | 81.5 | 74.5 | 58.5 | – | – | |
| Humar 2008 | 31 | 43.6 (6–90) | 89 | 76 | 74 | 74 | – | – |
| Hashimoto 2014 | 25 | 54 (1–113) | 88 | 80 | 80 | 80 | 80 | – |
| Vagefi 2014 | 18 | – | – | – | – | – | – | 70 |
| Jung 2017 | 16 | 113.2 (0.7–158.8) | 80 | 68.8 | 68.8 | 68.8 | 68.8 | – |
| Giacomoni 2008 | 16 | 55.1 (1–102.83) | – | 69 | 69 | 69 | 69 | – |
| Pooled proportion (95% CI) | 85 (79–90) | 75.7 (70.3–81) | 71.3 (65.6–77.0) | 72.8 (67.2–78.5) | – | – | ||
| I^2 | 24.99 | 8.233 | 9.283 | 0 | – | – | ||
| P (for I^2) | 0.238 | 0.367 | 0.358 | 0.512 | – | – | ||
- Percentages in italic were not reported but derived from charts.
| Study | n | Follow-up, median (range) | Proportion (%) patient survival | |||||
|---|---|---|---|---|---|---|---|---|
| 3 months | 1 year | 2 year | 3 year | 5 year | 10 year | |||
| Chan 2019 | 100 | 81 | 68.3 | 65 | 60.5 | 59 | ||
| Aseni 2014 | 64 | – | 80 | 73.2 | 74 | 69 | 63.3 | – |
| Herden 2018 | 44 | 91(34–202) | 93 | 88.1 | 88.1 | 88.1 | 88.1 | 74.5 |
| Broering 2005 | 35 | 27.4 (1–68.3) | 94.2 | 86 | 86 | 86 | – | – |
| Azoulay 2001 | 34 | – | 87 | 81 | 69 | – | – | – |
| Humar 2008 | 31 | 43.6 (6–90) | 90 | 80 | 74 | 74 | – | – |
| Hashimoto 2014 | 25 | 54 (1–113) | 96 | 88 | 88 | 88 | 88 | – |
| Vagefi 2014 | 18 | – | – | – | – | – | – | 76 |
| Jung 2017 | 16 | 113.2 (0.7–158.8) | 81.3 | 81.3 | 81.3 | 81.3 | 81.3 | – |
| Giacomoni 2008 | 16 | 55.1 (1–102.83) | – | 69 | 69 | 69 | 69 | – |
| Pooled proportion (95% CI) | 88.9 (84.2–93.6) | 80 (74.3–85.7) | 77.5 (70.7–84.3) | 77.3 (68.7–85.8) | – | – | ||
| I^2 | 51.6 | 46.05 | 59.51 | 49 | – | – | ||
| P (for I^2) | 0.044 | 0.063 | 0.011 | <0.001 | – | – | ||
- Percentages in italic were not reported but derived from charts.
| Patient survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Left/right | n | Follow-up, median(range)* | Proportion (%) survival | |||||
| 3 months | 1 year | 2 year | 3 year | 5 year | 10 year | ||||
| Chan 2019 | Left | 48 | 17.2 (0–196.4) | 66 | – | – | – | – | – |
| Right | 52 | 90 | – | – | – | – | – | ||
| Aseni 2014 | Left | 32 | – | 81 | 74 | 70 | 70 | 67.2 | – |
| Right | 32 | – | 71 | 68 | 64 | 64 | 59.4 | ||
| Herden 2018 | Left | 23 | 91(34–202) | 100 | 90.7 | 90.7 | 90.7 | 90.7 | 90.7 |
| Right | 21 | 100 | 85.2 | 85.2 | 85.2 | 85.2 | 56.8 | ||
| Broering 2005 | Left | 19 | 27.4 (1–68.3) | 94.7 | 89.5 | 89.5 | 89.5 | – | – |
| Right | 16 | 93.7 | 87.5 | 87.5 | 87.5 | – | – | ||
| Azoulay 2001 | Left | 17 | – | – | 87.5 | 64.2 | – | – | – |
| Right | 17 | – | – | 74.2 | 74.2 | – | – | – | |
| Hashimoto 2014 | Left | 10 | 54 (1–113) | 90 | 80 | 80 | 80 | 80 | – |
| Right | 15 | 100 | 93 | 93 | 93 | 93 | – | ||
| Vagefi 2014 | Left | 9 | – | – | – | – | – | – | 78 |
| Right | 9 | – | – | – | – | – | – | 74 | |
| Giacomoni 2008 | Left | 7 | 55.1 (1–102.83) | – | 71 | 71 | 71 | 71 | – |
| Right | 9 | – | 67 | 67 | 67 | 67 | – | ||
| Graft survival | |||||||||
| Aseni 2014 | Left | 32 | – | 66 | 56.8 | 56.8 | 56.8 | 56.8 | – |
| Right | 32 | – | 75 | 68 | 65 | 65 | 60.7 | – | |
| Herden 2018 | left | 23 | 91(34–202) | – | 79.8 | 73.3 | 73.3 | 73.3 | 36.8 |
| Right | 21 | – | 86.7 | 80.5 | 80.5 | 80.5 | 80.5 | ||
| Broering 2005 | Left | 19 | 27.4 (1–68.3) | 93.7 | 75 | 75 | 63 | – | – |
| Right | 16 | 84 | 84 | 84 | 84 | – | – | ||
| Azoulay 2001 | Left | 17 | – | 87 | 74.2 | 74.2 | – | – | – |
| Right | 17 | – | 75 | 75 | 43 | – | – | – | |
| Hashimoto 2014 | Left | 10 | 54 (1–113) | 93 | 86.7 | 86.7 | 86.7 | 86.7 | – |
| Right | 15 | 80 | 70 | 70 | 70 | 70 | – | ||
| Vagefi 2014 | Left | 9 | – | – | – | – | – | – | 74 |
| Right | 9 | – | – | – | – | – | – | 66 | |
| Giacomoni 2008 | Left | 7 | 55.1 (1–102.83) | – | 67 | 67 | 67 | 67 | – |
| Right | 9 | – | 71 | 71 | 71 | 71 | – | ||
- Percentages in italic were not reported but derived from charts.
- * Not available separately for left and right groups.
Discussion
This is the first systematic review summarizing outcomes of FSLT for two adult recipients. Based on the present literature, FSLT is associated with increased odds for both vascular and biliary complications compared with WLT. Absolute incidences of vascular and biliary complications were approximately 6.9% and 26%. Additionally, patient and graft survival after FSLT were impaired as compared with WLT and LDLT recipients. However, estimated long-term (3 year) patient- and graft survival were approximately 78.8% and 72.8%, respectively, which would be supportive of a transplant benefit for most waitlisted patients [9, 22, 24].
Graft and patient survival after FSLT appear mainly compromised in the early post-operative period, 90-day and 1-year outcomes appear to show the largest differences between WLT and FSLT. This early effect is probably related to increased incidence of postoperative complications. The technical challenges associated with FSLT could lead to more vascular and biliary complications, which is in keeping with the outcomes of the meta-analysis. Nevertheless, the complication, graft loss and mortality incidence after FSLT do not exceed percentages that are being reported for other accepted liver transplant options such as donation after cardiac death (DCD) or marginal donation after brain death (DBD) grafts, and outcomes after repeat liver transplantation. The incidence of biliary complications after DCD donation may be up to 34% [32-34]. Nevertheless, it should be considered that grafts used for FSLT are usually of good quality and do not compare directly with extended criteria grafts. The majority of studies did not correct for confounding factors when comparing results after FSLT and WLT, therefore, we may assume these populations differ significantly. Likely some degree of donor and patient selection was present, leading to better graft quality in the FSLT group. Therefore, risks associated with FSLT may be slightly underestimated as compared with adequately matched WLT grafts, and it is not possible to quantify the effect of these confounding factors based on present aggregated data. Although FSLT grafts may encounter longer cold ischemia times, inferior results after FSLT are potentially more frequently related to technical issues rather than graft function. Evolution of machine perfusion may open up new avenues to decrease exposure to ischemia and improve ex situ splitting technique in FSLT [35, 36].
Small for size syndrome was only rarely reported by included studies, likely resulting from mostly adequate size matching based on reported donor to recipient weight ratios and low median body weight of FSLT recipients. FLST was only incidentally applied in larger adults.
Although the survival benefit of FSLT needs to be carefully balanced against the associated risks, survival after FSLT may not be inferior to other accepted graft-to-recipient matches. FSLT may in particular benefit small adult recipients, who are reported to be disadvantaged on the waitlist with longer waiting times, resulting in progression of liver disease, increased waitlist morality, and drop-out [6, 7, 34, 37-43]. At the same time, patient physiology and anatomy need to support the additional risks of complications and the technical complexity of split liver transplantation. We would therefore not recommend the use of full split liver grafts for repeat liver transplantation and in very frail patients. Similarly, while in certain situations, use of FSLT for fulminant liver failure has facilitated life-saving super-urgent liver transplantation when the chances of receiving another offer have become very slim, this needs to be carefully balanced against the potential availability of a full liver graft and severity of the critical illness [1, 44].
Additionally, the use of FSLT may benefit the entire waitlist population, as two patients on the waitlist benefit from one graft. Sharing between multiple centers in a national or regional allocation program may be the most likely way to implement more widespread use of FSLT grafts and alleviate logistic pressures of performing FLST at one center. Previous studies estimated up to 12% of offered grafts may be suitable for splitting, either for FSLT or for a left lateral segment for a child combined with extended right graft for an adult [4, 5]. In practice, allocation algorithms as well as logistics and learning curve may currently favor splitting for an adult and a pediatric recipient [45]. The North-Italian experience is an example of sharing hemi-liver grafts in a regional allocation program [8]. Grafts were matched to recipients with a computerized algorithm and pediatric recipients took priority. This commendable effort lead to 64 FSLTs in a 12-year study period among seven collaborative centers, signifying the challenges of implementation of FSLT in an allocation program, and securing sufficient exposure and experience with the procedure to build technical confidence and trust between centers. The risk-benefit balance may differ locally and per allocation system. Currently, the risk-benefit based on this meta-analysis does not seem to favor introduction as a standard procedure, but rather selective use based on careful patient selection potentially in a smaller network of centers to condense availability of technical experience and logistic resources. Designated centers may benefit from experience in pediatric and/or living donor liver transplantation. The risk balance in emerging systems of deceased donation where living donation is the main alternative needs to be established separately.
Furthermore, introduction of FSLT would need to be balanced against other alternatives to increasing the donor organ pool for smaller adult recipients, such as machine perfusion, applying opt-out system in organ donation, considering options for living donation, and rationalizing acceptance of a wider margin for size-matching grafts as well as adapting implantation techniques when facing a potential acceptable size-mismatch (e.g. classical piggyback instead of modified piggyback).
To date one large registry-based study on FSLT has been performed, but was excluded from the present analysis because data on >80% of contralateral hemi-liver grafts were not reported [31]. One technical alternative to transplant a hemi-liver is a reduced graft, in which vascular and biliary structures of the whole liver graft are not split but kept with the hemi-liver, deeming the contralateral lobe unsuitable for transplantation. We could therefore not ascertain that all reported hemi-liver grafts in the registry database would be full split liver grafts. More importantly, significant under-reporting of majority of contralateral outcomes may cause substantial selection bias. In contrast, the majority of studies included in the present meta-analysis included outcomes of both hemi-liver grafts, and only two hemi-liver grafts were reported to be discarded, allowing for a balanced interpretation of results.
Although a formal statistical testing to compare the survival in left versus right hemi graft recipients was not possible, most studies reported comparable cumulative survival proportions. Only in the study by Chan et al., patient survival in left graft recipients was substantially impaired compared with right graft recipients at 90 days [9]. No detailed data were reported on the causes of graft dysfunction in recipients of a left hemi graft who succumbed in the first 90 days. In studies reporting outcomes on vascular and biliary complications for left and right FSLT recipients, no significant differences were present.
Additional limitations of the present review include substantial but acceptable heterogeneity in outcomes based on the I2 statistic. Heterogeneous baseline characteristics were reported in the included study populations. Differences in surgical techniques, in situ vs. ex situ split, local use or sharing between centers, and demographics of the populations at hand could explain different outcomes of included studies. Other contributing factors may be the inclusion era and surgical experience at the time. However, these factors could not be taken into account as only aggregated data were available for pooled analysis. Survival data and hazard ratios were calculated by methods described by Tierney et al. [15] and were thus in part derived from graphically presented data which may be less accurate. However, inaccuracy in survival data derived from calculation errors will likely be relatively minor and not greatly influence the magnitude of effect estimates [46]. Moreover, the direction of effect estimates in present analysis was consistent across different studies. For survival analysis, the HRs do not provide the true relative risks for this specific intervention, given graft and patient loss occurred predominantly early, the hazard is likely not proportional over time. Therefore, the HRs presented in this study may be an underestimation early after transplantation, but are likely overestimated for later years. Additionally, in most included studies, the reported numbers at risk may diminish substantially during follow-up, making long-term survival data less accurate. Although, 13 studies presented adequate data on the topic, the total number of patients analyzed for each comparison remained relatively low. It is important to stress that a meta-analysis may never be better than the studies it is based on. In this case, all data were observational and included studies were of low to moderate methodologic quality.
Conclusion
FSLT is associated with increased odds for biliary and vascular complications as compared with WLT. Additionally, FSLT was associated with impaired graft and patient survival compared with WLT. However, long-term patient and graft survival appear acceptable to justify a transplant benefit in selected patients. FSLT may be a feasible technique in specialized centers as an additional option to reduce waiting time and waitlist mortality.
Authorship
All authors contributed to the conception and design of the study. DS, ABvD: extracted data, performed the initial analysis and drafted the manuscript. WGP, DFM, MTPRP, HH: critically revised the manuscript.
Funding
The authors have declared no funding.
Conflict of interest
The authors declare no conflict of interest related to the submitted work.
Acknowledgements
We would like to thank Wichor Bramer for his expert assistance with the literature search.




