C3d-positive donor-specific antibodies have a role in pretransplant risk stratification of cross-match-positive HLA-incompatible renal transplantation: United Kingdom multicentre study
Abstract
Anti-HLA-antibody characteristics aid to risk-stratify patients and improve long-term renal graft outcomes. Complement activation by donor-specific antibody (DSA) is an important characteristic that may determine renal allograft outcome. There is heterogeneity in graft outcomes within the moderate to high immunological risk cases (cross-match-positive). We explored the role of C3d-positive DSAs in sub-stratification of cross-match-positive cases and relate to the graft outcomes. We investigated 139 cross-match-positive living-donor renal transplant recipients from four transplant centres in the United Kingdom. C3d assay was performed on serum samples obtained at pretreatment (predesensitization) and Day 14 post-transplant. C3d-positive DSAs were found in 52 (37%) patients at pretreatment and in 37 (27%) patients at Day 14 post-transplant. Median follow-up of patients was 48 months (IQR 20.47–77.57). In the multivariable analysis, pretreatment C3d-positive DSA was independently associated with reduced overall graft survival, the hazard ratio of 3.29 (95% CI 1.37–7.86). The relative risk of death-censored five-year graft failure was 2.83 (95% CI 1.56–5.13). Patients with both pretreatment and Day 14 C3d-positive DSAs had the worst five-year graft survival at 45.5% compared with 87.2% in both pretreatment and Day 14 C3d-negative DSA patients with the relative risk of death-censored five-year graft failure was 4.26 (95% CI 1.79, 10.09). In this multicentre study, we have demonstrated for the first time the utility of C3d analysis as a distinctive biomarker to sub-stratify the risk of poor graft outcome in cross-match-positive living-donor renal transplantation.
Introduction
It is known for the past five decades that the presence of complement-activating anti-graft antibodies results in reduced graft survival and precludes transplantation [1]. Early attempts at renal transplantation across antibody barriers have improved access to transplantation for highly sensitized patients [2-4]. Refinement of desensitization protocols has led to better survival for these patients compared with remaining on dialysis [5-8]. However, even with significant progress in desensitization immunotherapies, highly sensitized patients wait longer. Approximately thirty per cent of patients on the waiting list for deceased-donor organs are highly sensitized and have calculated panel reactive antibodies (cPRA)>20% [9]. Recipients who have undergone HLA-incompatible transplants are at higher risk for both acute antibody-mediated rejection (ABMR) and shortened graft survival compared with those who have received compatible or ABO-incompatible transplants. Despite advances in immune-monitoring technologies, uncertainties with pretransplant risk stratification prevail. CDC-positive cross-match is associated with the highest risk of both an ABMR and graft failure. The risk is lesser if the DSA is detectable only by FC cross-match and the risk falls further in cross-match-negative cases, where DSA is detected by single-antigen bead (SAB) analysis [10-12]. Graft failure is usually related to chronic antibody-mediated injury[11, 13-15].
Numerous investigations have begun to establish the role of DSA in mediating renal allograft rejection and graft survival [14, 16, 17]. We have previously shown that following HLA-incompatible transplantation, anamnestic responses peak at around 14 days post-transplant and the risk of acute ABMR is higher with DSA MFI> 7000 [18]. Anti-HLA subclasses IgG1 and IgG3 strongly activate complement and have the potential to serve as predictors of rejection and allograft survival [19-21]. The field of antibody testing has evolved, and it is possible to study the complement proteins and split products on solid-phase assays using isolated HLA proteins [22-24]. The advancements in immune monitoring emphasize the need to investigate the role of DSA that activates the complement cascade in the highly sensitized patients with positive cross-match, both pretreatment and following early transplantation. Awareness of the presence of complement-activating DSA could potentially enable effective risk stratification of cross-match-positive patients as well as the prediction of ABMR. However, studies in standard or low immunological risk (cross-match-negative) patients, complement-fixing assays have shown conflicting results in risk stratification for ABMR and graft survival[23, 25-30]. In this multicentre study, we investigated the role of pretreatment and early post-transplant complement-activating DSA (as measured by C3d assay) to aid in further risk stratification of moderate to high immunological risk (positive cross-match) cases, for renal allograft survival.
Concise materials and methods
Study design
This analysis comprises a United Kingdom multicentre retrospective cohort study of patients who underwent HLA-antibody-incompatible renal transplants at four University Hospital centres: University Hospitals Coventry and Warwickshire (UHCW); Guy's Hospital, London; University Hospital of Wales (UHW, Cardiff), and Leeds Teaching Hospitals (LTH). Ethics committee approved the study (Ethics reference number is CREC-055/01/03 and 13/WM/0090). Clinical and research activities abide by the 2013 Declaration of Helsinki and the 2008 Declaration of Istanbul.
All of the following inclusion criteria were met the following: recipients of living-donor HLA-antibody-incompatible transplants with pretransplant plasmapheresis; CDC and/or FC cross-match positive and DSA positive by SAB before desensitization therapy.
The exclusion criteria were the following: recipients with DSA detected by SAB alone (i.e. cross-match negative) that did not require pretransplant plasmapheresis; dual HLA and ABO-incompatible renal transplants; paediatric renal transplant recipients (age < 18 years); and primary nonfunction of the graft.
One hundred and ninety-nine recipients received antibody-incompatible renal transplants between 2005 and 2015 at the four transplant centres, of whom 139 met the inclusion criteria and comprised the final study cohort. We analysed pretreatment (pretransplant sample before plasmapheresis and/or IVIG) and Day 14 samples [18, 31-33]. Pretreatment serum samples were available for 139 recipients, but post-transplant Day 14 samples were only available for 111 recipients.
HLA typing and antibody testing
Histocompatibility and immunogenetics laboratory performed the HLA typing and serological testing. HLA types were determined by DNA-based methods for HLA-A, B, C, DR, DPB and DQB alleles. CDC (without AHG enhancement) and FC assays were performed as published in our previous study [13]. CDC cross-match was performed only in three of the four centres. All the stored pretreatment serum samples were tested for IgG antibodies at a final dilution of 1:5 as per the manufacturer guidelines (Lifecodes, Immucor, UK) and other published literature [28, 34]. This technique reduced the commonly encountered problem of high-dose hook effect [34]. The detailed method is described in the Appendix S1. Data acquired with Luminex version 2.3 were analysed with MatchIT software provided by the manufacturer.
C3d analysis
The C3d analysis was performed with the C3d assay kit (Lifecodes®, Immucor). Both pretreatment and Day 14 samples were tested for C3d deposition on the HLA single-antigen beads as per manufacturer guidelines. C3d data acquired with Luminex version 2.3 were analysed with MatchIT software provided by the manufacturer.
Desensitization protocol
Patients with positive CDC and/or positive FC cross-match against their donors typically underwent between three and seven sessions of plasmapheresis with or without IVIG to render them CDC and/or FC cross-match negative at the time of the transplant. Desensitization protocols, induction agent and maintenance immunosuppression followed at the four centres are summarized in Table S2.
Rejection episodes were diagnosed with renal biopsy findings in line with contemporary Banff criteria [35-37]. In eight cases where the biopsy was precluded for reasons such as anticoagulation, patient refusal, the diagnosis of ABMR was based on clinical findings (drop in urine output with rising creatinine) and the laboratory data (rapidly rising DSA levels). ABMR episodes within the first-month post-transplant were considered as early ABMR. Rejection episodes were treated initially with intravenous methylprednisolone. Other treatment for ABMR included rATG (21 patients), plasmapheresis and IVIG (3 patients), and two recipients with refractory ABMR received rituximab and eculizumab.
Statistical analysis
Statistical analysis was performed using the Pearson chi-square test for categorical variables. Normality of data was tested using the Shapiro–Wilk test. Independent samples t-test and Mann–Whitney U-test were used for continuous data depending on the distribution of data. Multivariable analysis for medium-term survival outcome was carried out using Cox proportional hazard models. We chose three different models to determine the independent role of C3d DSA after adjusting for variables. IgG DSA variable was studied differently in the models (In Model 1 – continuous variable, Models 2 and 3 – as a categorical variable with different cut-offs of 8000 and 5000). The IgG DSA arbitrary cut-offs were based on association with FC or CDC reactivity at our centres. IgG DSA cut-off ≥ 8000MFI is associated with higher positive predictive value for positive CDC cross-match [38, 39]
Kaplan–Meier survival analysis and adjusted death-censored graft survival analysis were carried out after adjusting for age, gender, duration on dialysis, mismatch, previous transplantation and ABMR. Groups were compared based on the log-rank test. For clinical relevance, relative risks for death-censored five-year graft failures were computed using an online calculator for effect size measures [40]. The null hypothesis of no difference between the groups of interest was tested at the 5% significance level. Calculations were performed using SPSS V24 (Chicago, IL).
Results
Characteristics of the study population
The characteristics of the 139 recipients are presented in Table 1. The median follow-up time was 48 months (IQR 20.47–77.57). Fifty-two (37%) pretreatment and thirty-seven (27%) post-transplant cases had C3d-positive DSA. Characteristics that were associated with a higher proportion of C3d-positive DSAs included younger age, male gender, longer dialysis duration and patients with previous transplants. There were no significant differences between the groups with regard to immunosuppression at induction apart from a higher proportion of cases with C3d-positive DSA receiving rATG, but overall numbers were low. Pretreatment C3d-positive DSA was associated with higher IgG DSA MFI and was predominantly HLA class II antibodies. Thirty-five grafts failed over the entire period of whom pretreatment C3d-positive DSA was present in twenty-two cases (Table S3). Six patients died with functioning grafts during the follow-up. None of these patients had pretreatment C3d-positive DSA. Only one patient had Day 14 C3d-positive DSA.
| Characteristics (n) | All patients | Pretreatment C3d positive | Pretreatment C3d negative | P-value* |
|---|---|---|---|---|
| Number of patients | 139 | 52/139 | 87/139 | |
| Age, mean (SD) | 42 (11.38) | 38 (9.7) | 44 (11.8) | 0.003 |
| Gender, male, n (%) | 50 (36) | 25 (48) | 25 (29) | 0.021 |
| Previous transplantation, n (%) | 88 (63) | 40 (77) | 48 (55) | 0.01 |
|
Dialysis years, median (IQR) mean rank |
8 (3–16) |
14.5 (8–18.75) 80.15 |
7 (3 −15) 63.93 |
0.02 |
|
Mismatch, (A, B and DR) median (IQR) mean rank |
3 (2–4) |
3 (2–4.75) 78.12 |
3 (2–4) 5.15 |
0.06 |
|
DR mismatch, median (IQR) mean rank |
1 (1–1) |
1 (1–1) 75.44 |
1 (1–1) 66.75 |
0.15 |
| Basiliximab (%) | 92 (66) | 32 (62) | 60 (69) | 0.48 |
| Alemtuzumab (%) | 33 (24) | 11 (21) | 22 (25) | 0.70 |
| rATG (%) | 14 (10) | 9 (17) | 5 (6) | 0.06 |
|
Median highest IgG MFI, (IQR) Mean rank |
7192 (3713–12278 |
12679 (10003–16254) 100.92 |
4949 (2328–7912) 51.52 |
<0.001 |
| DSA class I only, n (%) | 54 (39) | 7 (13) | 47 (54) | <0.001 |
| DSA class II only, n (%) | 27 (19) | 16 (31) | 11 (13) | 0.014 |
| Class I + II, n (%) | 58 (42) | 29 (56) | 29(33) | 0.013 |
| Day 14 C3d + DSA, n (%) | 37(34) | 23(44) | 14(16) | <0.001 |
| Early ABMR, n (%) | 49 (35) | 21 (40) | 28 (32) | 0.36 |
| ACR, n (%) | 16 (11) | 5 (10) | 13 (15) | 0.44 |
| Mixed rejection, n (%) | 10 (7) | 3 (6) | 7 (8) | 0.74 |
| Graft loss, n (%) | 35(25) | 22(42) | 13 (15) | <0.001 |
- * Statistical tests used: age-independent samples t-test, gender, previous transplants, immunosuppression, DSA class, ABMR, ACR and graft loss—Pearson chi-square test. Dialysis years, mismatch and IgG DSA MFI—Mann–Whitney U-test.
C3d analysis and renal allograft survival
Over the study period, thirty-five (25%) grafts were lost. Twenty-nine cases had renal biopsy features consistent with chronic antibody-mediated rejection. Other causes included acute ABMR (one case); recurrence of glomerular disease (four cases); and sepsis (one case). Twenty-nine grafts failed at five years. The relative risk of death-censored five-year graft failure in pretreatment C3d-positive cases was 2.52 (95% CI 1.29–4.91). The difference in survival proportions for C3d-positive DSA group at five years was − 0.39 (95% CI − 1, 0.686).
The association of pretreatment C3d-positive DSA with renal allograft survival was investigated in a multivariable Cox proportional hazard analysis, using the three models, as shown in Table 2. In Model 1 with IgG values taken as a continuous variable, the presence of pretreatment C3d-positive DSA was associated with worse graft survival, hazard ratio 3.29 95%CI 1.37–7.86 (P = 0.007) (Table 2) (Fig. 1).
| Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95% CI | P-value | Variable | Hazard ratio | 95% CI | P-value | Variable | Hazard ratio | 95% CI | P-value |
| Age | 1 | 0.97–1.03 | 0.84 | Age | 1 | 0.97–1.04 | 0.78 | Age | 1.006 | 0.97–1.04 | 0.737 |
| Gender, female | 1.88 | 0.87–4.11 | 0.11 | Gender, female | 1.97 | 0.91–.27 | 0.09 | Gender, female | 1.909 | 0.887–4.11 | 0.098 |
| Previous transplants | 7.03 | 2.24–22.12 | 0.001 | Previous transplants | 7.15 | 2.24–22.80 | 0.001 | Previous transplants | 7.335 | 2.30 –23.32 | 0.001 |
| ESRD duration | 0.93 | 0.89–0.98 | 0.014 | ESRD duration | 0.94 | 0.89–0.98 | 0.015 | ESRD duration | 0.935 | 0.89–0.99 | 0.014 |
| Total mismatch | 0.94 | 0.73–1.23 | 0.66 | Total mismatch | 0.96 | 0.74–1.25 | 0.77 | Total mismatch | 0.953 | 0.73–1.24 | 0.719 |
| Early AMR | 1.36 | 0.68–2.73 | 0.38 | Early AMR | 1.31 | 0.65–2.62 | 0.45 | Early AMR | 1.389 | 0.69–2.79 | 0.355 |
| IgG DSA highest MFI | 1 | 0.99–1.00 | 0.69 | IgG DSA MFI ≥ 8000 | 1.56 | 0.69–3.50 | 0.284 | IgG DSA MFI ≥ 5000 | 1.288 | 0.51–3.23 | 0.589 |
| Pretreatment C3d-positive DSA | 3.29 | 1.37–7.86 | 0.007 | Pretreatment C3d-positive DSA | 2.98 | 1.29–6.88 | 0.011 | Pretreatment C3d-positive DSA | 3.319 | 1.45–7.58 | 0.004 |

In model 2, IgG DSA MFI was categorized into ≥ 8000 and < 8000. Sixty-five patients had pretreatment IgG DSA MFI> 8000, of whom forty-three were C3d-positive. Of seventy-four patients who had IgG DSA MFI levels < 8000, only nine were C3d-positive. Pretreatment C3d-positive DSA cases were associated with lower graft survival compared with C3d-negative DSA cases, hazard ratio 2.98 (95%CI 1.29–6.88) (P = 0.011) (Table 2).
In Model 3, IgG DSA MFI was categorized into ≥ 5000 and < 5000. Ninety-one patients had pretreatment IgG DSA MFI> 5000 of whom forty-eight were C3d-positive, and forty-eight patients had an IgG DSA MFI < 5000 of which four were C3d-positive. Pretreatment C3d-positive DSA cases were associated with lower graft survival compared with C3d-negative DSA cases, hazard ratio 3.31 95% CI 1.45–7.58 (P = 0.004) (Table 2).
In all the three models, pretreatment C3d-positive DSAs were independently associated with poor renal allograft survival. IgG DSA was not significantly related to graft survival in any of these models. Previous kidney transplantation and ESRD duration were other independent significant factors.
Subgroup analysis
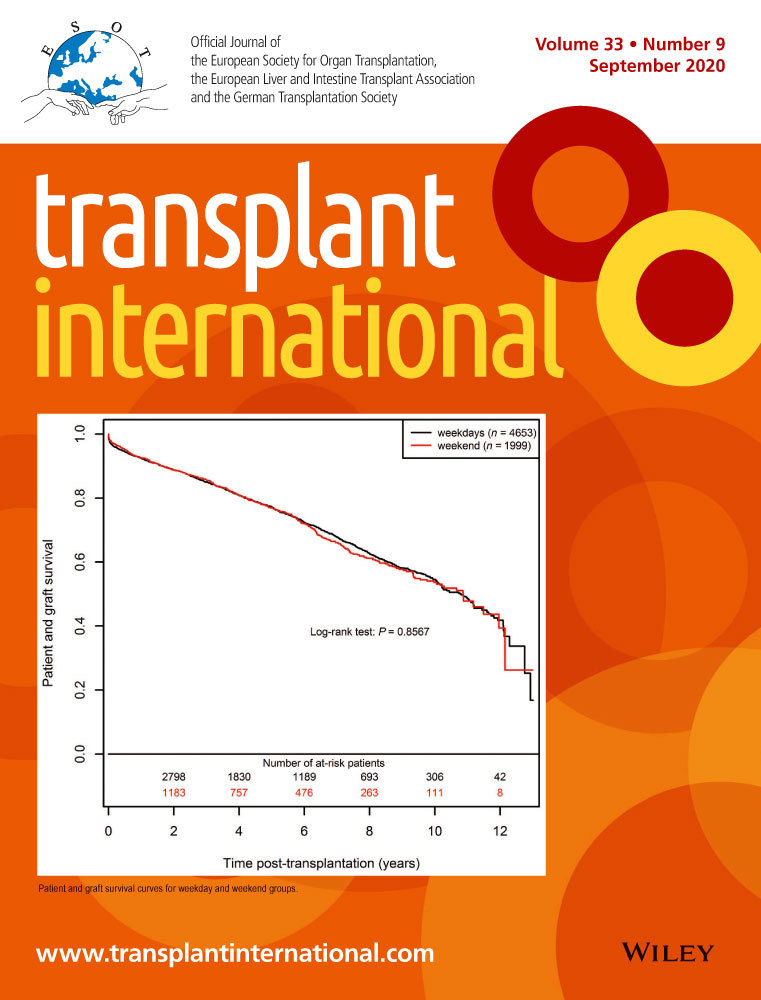
C3d-positive DSA and graft survival in flow cross-match-positive patients
Of 139 cases, twenty-six were CDC-positive. Forty cases did not have CDC tested; hence, we looked at seventy-three cases that were only FC positive as a subgroup to explore sub-stratification using C3d. Eleven grafts failed over the study period in this group. C3d-positive DSA presents in seventeen cases.
The death-censored graft survival at five years in C3d-positive DSA cases were lower at 64.5% compared with 90.7% in C3d-negative DSA cases. The relative risk of five-year graft failure was 3.74 (95%CI 1.12, 12.49). The difference in survival proportions for C3d-positive DSA group at five years was − 0.25 (−0.53, 0.014).
In the multivariate cox proportional hazards analysis (Model 1), C3d-positive DSA cases were associated with lower graft survival compared with C3d-negative DSA cases. The hazard ratio was 9.8 (95%CI 1.6, 59.4). (Fig. 2).
Graft survival according to pretreatment C3d-positive DSA HLA class
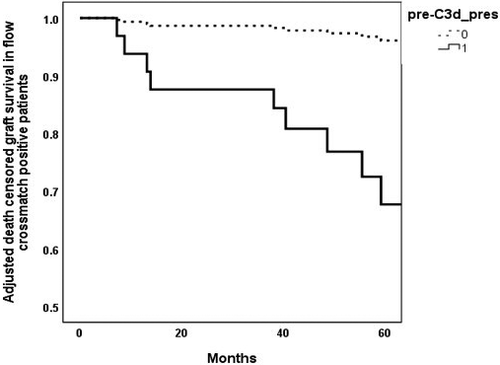
Of 139 cases, IgG HLA class I DSAs were present in one hundred and eleven cases, and IgG HLA class II DSAs were present in eighty-six cases. Sixty-one cases had both HLA classes I and II IgG DSAs. Fifty-two cases had either HLA class I, HLA class II or both HLA classes of pretreatment C3d-positive DSAs. HLA class I C3d-positive DSA only were present in fifteen cases, HLA class II C3d-positive DSA only was present in twenty-nine cases and both HLA classes I and II C3d-positive DSAs were present in four cases. Of the fifteen HLA class I C3d-positive DSA cases, four grafts failed over five years.
In Kaplan–Meier survival analysis, death-censored graft survival at five years in cases with pretreatment HLA class I C3d-positive DSA was marginally lower at 73.3% compared with 75.5% in HLA class I C3d-negative DSA cases (Fig. 3). The relative risk of death-censored five-year graft failure was 1.06 (95%CI 0.33, 3.42).
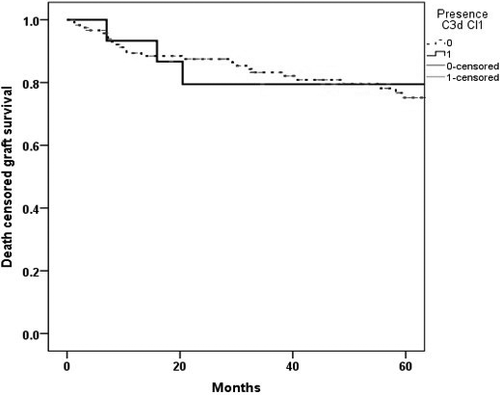
In Kaplan–Meier survival analysis, the death-censored graft survival probability at five years in the class II C3d-positive DSA cases was 60.7% compared with 80.6% in class II C3d-negative DSA cases (Fig. 4) with the relative risk of 2.02 (95% CI 1.04–3.94).
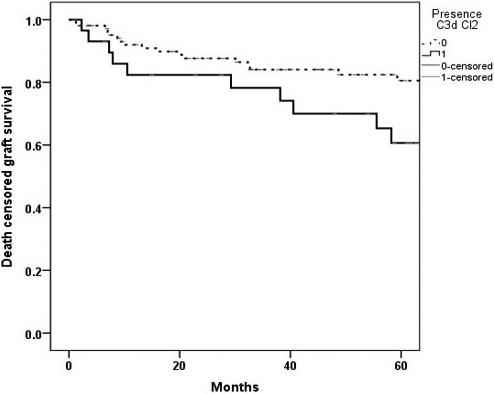
In Kaplan–Meier survival analysis, the death-censored graft survival probability at five years with both class I and II C3d + DSA was lower at 50% compared with 80.7% in rest of the cases (Fig. 5)with the relative risk of 2.05 (95%CI 0.73, 5.78).
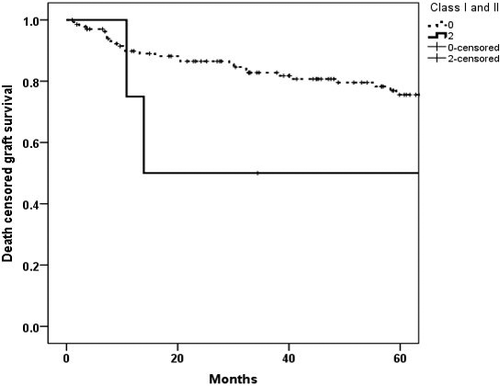
Persistent C3d-positive DSAs and death-censored graft survival analysis
One hundred eleven cases with both pretreatment and Day 14 samples were studied. C3d status at pretreatment and Day 14 were as follows. Fifty-seven cases had C3d-negative DSA for both pretreatment and on Day 14 sample; twenty-four cases had both pretreatment and Day 14 C3d-positive DSA; Sixteen cases had pretreatment C3d-positive DSA but negative on Day 14, and fourteen cases had negative pretreatment but C3d-positive DSA on Day 14.
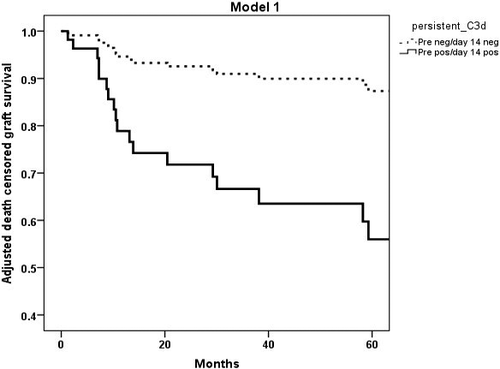
In the Kaplan–Meier survival analysis, death-censored five-year graft survival was worse in cases with both pretreatment and Day 14 C3d-positive DSAs (43.5%) compared with cases with both pretreatment and Day 14 C3d-negative DSAs (87.2%) (log-rank P = <0.001) (Figure S1).
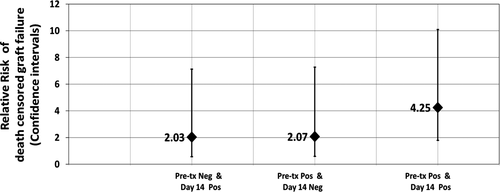
In the death-censored five-year graft survival analysis, cases with pretreatment and Day 14 C3d-positive DSAs were associated with worse five-year graft survival of 45.5% compared with 87.2% in cases with pretreatment and Day14 C3d-negative DSAs (Fig. 7). The relative risk of graft failure was 4.25 (95%CI 1.79, 10.09).
The relative risk of death-censored five-year graft failure, according to C3d status, is presented in Fig. 6. There were mainly four categories. In cases with pretreatment C3d-positive DSA and Day 14 C3d-negative DSA compared to cases with pretreatment and Day 14, C3d-negative DSA was 2.07 (95%CI 0.59, 7.28); the relative risk of five-year graft failure in cases with pretreatment C3d-negative and Day 14 C3d-positive DSA compared to cases with pretreatment and Day 14 C3d-negative DSA was 2.03 (95%CI 0.58, 7.12).
In a multivariable Cox proportional hazard analysis (Model1), cases with pretreatment and Day 14 C3d-positive DSA were associated with lower renal allograft survival compared with pretreatment and Day 14 C3d-negative DSA cases. The hazard ratio was 4.56 (95%CI 1.46–14.39, P = 0.009) (Table 3). Models 2 and 3 had similar results (data not included, available if necessary).
| Variable | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Age | 0.80 | 0.09–6.80 | 0.840 |
| Gender, female | 1.45 | 0.55–3.81 | 0.45 |
| Previous transplants | 4.55 | 1.19–17.41 | 0.027 |
| ESRD duration | 0.09 | 0.01–0.63 | 0.016 |
| Total mismatch | 0.16 | 0.01–2.23 | 0.18 |
| Early AMR | 0.91 | 0.39–2.12 | 0.83 |
| IgG DSA highest MFI | 2.6 | 0.41–16.65 | 0.31 |
| Both pretreatment and Day14 C3d + DSA | 4.56 | 1.46–14.40 | 0.009 |
Discussion
This multicentre study illustrates the role of C3d assay in the sub-stratification of graft survival risk in cross-match-positive cases, for the first time. C3d assay was able to clearly define groups with better outcome among moderate to high immunological risk cases (Fig. 6). The best graft survival is seen in patients with C3d-negative DSA at both pretreatment and Day 14 (Fig. 7), which is comparable to five-year graft survivals in deceased-donor transplants in the United Kingdom. However, marginally lower than standard living-donor transplants [9, 41, 42]. The most inferior graft survival was found for those with C3d-positive DSAs in both pretreatment and Day 14 sera. Thus, the lower risk cases can be predicted before the transplant. In contrast, the highest risk cases can be assessed if there is additional testing in the early post-transplant period, long before the actual time of failure, which indicates there is a potential for intervention in the early post-transplant period.
Previous studies have only explored the role of complement activation DSA assays in a predominantly lower immunological risk group (DSA alone positive with cross-match-negative cases). In a single-centre study, of 68 highly sensitized patients (twenty-one CDC cross-match-positive patients) looked at the pretreatment risk stratification using in vitro C4d deposition on SAB. Presence of pretransplant C4d-positive DSAs was associated with acute AMR. One-, three- and five-year death-censored graft survival was also significantly lower in the C4d-positive DSA patients compared with C4d-negative DSA patients [43]. Similarly, other studies have shown that in cross-match negative, sensitized patients with complement-activating DSAs, as measured by C1q binding at the time of transplantation and/or post-transplantation, are associated with poor renal allograft survival [25, 44, 45]. A large study by Kamburova et al. concluded that the presence of pretransplant C3d-positive DSAs was associated with reduced renal allograft survival but did not reach statistical significance [28]. Possible reason could include the study cohort that comprised of cross-match-negative DSA-positive cases (different from our study cohort). Another possible explanation is consideration of only pretransplant status of the DSAs. In other studies on paediatric renal transplant population, complement-activating DSAs in the post-transplant period are a risk factor for graft failure [46, 47].
Positive complement activation assays of DSAs utilized at other time points (such as graft dysfunction or ABMR) following transplantation were also associated with inferior graft outcomes. Two comparable studies have indicated that testing for complement-activating DSA (C1q binding and C3d activation assays) at the time of AMR predicts graft survival [23, 25]. Similarly, a recently published study from the deteriorating kidney allograft function (DeKAF) investigators, in a cohort of standard risk renal transplants, the results of a C3d assay performed at the time of development of DSA and graft dysfunction, predicted a higher risk of graft failure in C3d-positive DSA group compared with C3d-negative DSA group [27].
Thus, a common thread in these studies is that the combination of pre- and postrenal transplantation testing for complement-activating DSA can be of predictive value, and their use, particularly in immunologically high-risk cases, is becoming compelling. Which measure of in vivo complement-activating potential, C1q binding or C3d generation is superior, if at all, remains to be proven; the study by Kim et al. and Lee et al. [24, 48] suggests the latter. Each assay measures different DSA properties; C1q binding being dependant on Fc density on the antibody target, while C3d generation quantifies the full activation potential of the DSA (including Fc cross-linking). C3 activation is the pivotal reaction of the complement cascade, which leads to the production of inflammatory mediators and direct tissue damage. As such, the C3d assay would appear to be the more relevant measure of DSA pathogenicity, which is why we chose it for this study.
Also, we did not find a significant linear correlation between C3d and higher IgG MFIs as compared to other studies using C1q and IgG MFI [49, 50] and C3d and IgG MFI [38] (Figure S2). There remains to be proven what an MFI value means for complement assay (either C1q or C3d), as a higher MFI for complement split product may not necessarily correlate in a linear fashion to the quantity similar to IgG MFI. Thus, in this study cohort, we have used only categorical/qualitative results.
It is established that complement-activating antibodies, as detected by complement-dependent cytotoxicity cross-match, are associated with a worse graft survival [1]. However, the CDC assay is not always specific and can identify other, non-HLA complement-activating antibodies [50, 51]. The availability of viable donor cells still limits the CDC assay, and it is not always practical to perform this assay on multiple occasions in the pretransplant and post-transplant periods. Correlation of complement-activating DSA assays with CDC/FC cross-matches is of interest. In recently published studies that included samples from single centre of this cohort, a positive C3d assay was shown to correlate with high specificity, and a positive predictive value with positive FC cross-match. However, sensitivity and negative predictive values were low [38, 39]. A negative C3d assay had a higher negative predictive value for CDC, and this could be a useful surrogate marker for risk stratification, as negative CDC cross-match is generally required at the time of transplantation.
The flow cytometry cross-matches, although more sensitive than CDC, have similar limitations to the CDC assay. Typically, a positive pretransplantation cross-match is discouraged due to the high risk of rejection [52] and reduced graft survival [10, 11]. Subgroup analysis of FC cross-match patients in this study showed pretreatment C3d-positive DSAs were associated with lower graft survival (Fig. 2). The findings are not entirely surprising as one of the earlier studies that looked at the utility of C4d-positive DSA in a cohort of highly sensitized patients, and the presence of pretransplant C4d-positive DSA in CDC cross-match patients was associated with worse graft survival compared with C4d-negative cohort [53]. The above findings demonstrate the potential utility of C3d testing in cross-match-positive patients.
In the subgroup analysis of graft survival based on HLA class I and class II, cases with both HLA classes C3d-positive DSAs had lower graft survival as compared to other cases. The presence of only HLA class II C3d-positive DSAs reached statistical significance. Limited number of studies have looked at the HLA class of complement-activating DSAs, and majority of these studies are limited by relatively low numbers of patients. In a recent study, de novo class II C3d-positive DSAs were associated with higher rejection episodes and significantly lower graft survival [24]. A study that looked at the effect of C4d-positive DSA class on ABMR found no significant difference [43]. However, a previous study from the same group found lower graft survival in the presence of HLA class I C4d-positive DSA but not with HLA class II C4d-positive DSA [53]. Studies that have looked at HLA class difference of DSAs on allograft survival have not found statistical significance. However, recent studies have demonstrated lower graft survival with post-transplant de novo complement-activating HLA class II DSAs. [25, 54, 55].
The strengths of our study include multicentre validation of the C3d assay in a large cohort of cross-match-positive patients. Also, patients received relatively homogenous maintenance immunosuppression. However, this is a retrospective study and post-transplant Day 14 samples were not available for all 139 cases. Heterogeneous induction regimen may have affected the long-term outcomes, although there was no statistical significance between the groups (Table S4). It is also not possible to extrapolate optimal treatment strategies for early post-transplant rising DSA levels with no graft dysfunction, as protocol biopsies were not performed. Further mechanistic studies are required to explore the potential for therapeutic options in the early post-transplant period. One of the pathways studied includes, inhibiting the functions of active complement fragments such as C3d, which has a role in augmenting B-cell-mediated alloimmunity. [56]. Complement regulatory proteins, both membrane-bound and circulating, play a role in the susceptibility of microvascular endothelial cells to complement-mediated damage. Augmenting the expression of CD55, CD46 or CD59 on endothelial cells may reduce the cytotoxicity of complement-activating antibodies [57, 58]. There are potential diagnostic and therapeutic implications for identifying critical pathological pathways, including complement activation[59, 60]. We speculate that rendering the C3d negative before a transplant can result in better graft survival.
In conclusion, C3d assay enabled differentiation of IgG antibodies with varying pathogenicity. Thus, it has a potential role as an additional biomarker in further risk assessment of transplant patients, especially in cases with preformed DSAs and positive cross-match results against their potential donors. Further prospective controlled studies are required in high immunological risk patients to establish the role of this approach.
Authorship
SD, DB, OS, DM, RH and SG: participated in research design. AB, LH, KC and EB: participated in performance of the research. AB, NK, DB, SD and OS: participated in the analysis of the data. NK, SD and AB: performed statistical analyses. AB, NK, OS, NSK, SG, DB, AS, BC, AD, DZ, RMH, DM and SD: drafted and revised the paper. AB, OS, NK, SG, DB, NSK, SF, CI, AS, RB, MWS, BC, KC, TR, RE, EB, LH, AD, DZ, RMH, DM and SD: approved the final version of the manuscript.
Funding
The Transplant Research and Development Fund, University Hospitals Coventry and Warwickshire NHS Trust, Coventry, UK, and Viapath Histocompatibility and Immunogenetics Laboratory, London.
Conflict of interest
Authors declare no conflicts of interest.
Acknowledgements
We would like to acknowledge the time and efforts of patients, clinical staff, renal research nurses, renal transplant co-ordinators and clinical scientists from the participating centres.