Higher calcineurin inhibitor levels predict better kidney graft survival in patients with de novo donor-specific anti-HLA antibodies: a cohort study
Marc-Antoine Béland
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorIsabelle Lapointe
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorRéal Noël
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorIsabelle Côté
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorEric Wagner
Immunology and Histocompatibility Laboratory, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorJulie Riopel
Department of Pathology, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorEva Latulippe
Department of Pathology, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorOlivier Désy
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorStéphanie Béland
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorCiara N. Magee
Department of Nephrology & Renal Transplantation, UCL Centre for Nephrology, Royal Free London NHS Foundation Trust, London, UK
Search for more papers by this authorIsabelle Houde
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorCorresponding Author
Sacha A. De Serres
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Correspondence
Sacha A. De Serres MD SM FRCPC, Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, 11 Cote du Palais, Quebec, QC, Canada, G1R 2J6.
Tel.: 418-691-5464;
fax: 418-691-5757;
e-mail: [email protected]
Search for more papers by this authorMarc-Antoine Béland
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorIsabelle Lapointe
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorRéal Noël
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorIsabelle Côté
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorEric Wagner
Immunology and Histocompatibility Laboratory, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorJulie Riopel
Department of Pathology, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorEva Latulippe
Department of Pathology, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorOlivier Désy
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorStéphanie Béland
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorCiara N. Magee
Department of Nephrology & Renal Transplantation, UCL Centre for Nephrology, Royal Free London NHS Foundation Trust, London, UK
Search for more papers by this authorIsabelle Houde
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Search for more papers by this authorCorresponding Author
Sacha A. De Serres
Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, Quebec, QC, Canada
Correspondence
Sacha A. De Serres MD SM FRCPC, Transplantation Unit, Renal Division, Department of Medicine, Faculty of Medicine, University Health Center of Quebec, Laval University, 11 Cote du Palais, Quebec, QC, Canada, G1R 2J6.
Tel.: 418-691-5464;
fax: 418-691-5757;
e-mail: [email protected]
Search for more papers by this authorSummary
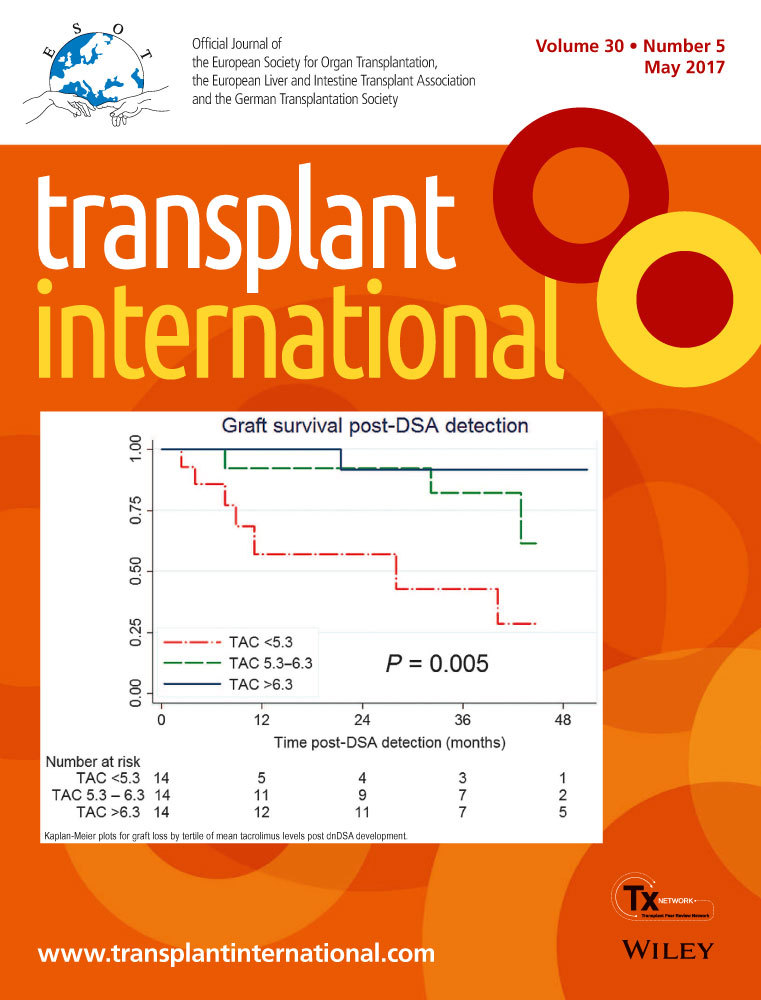
The development of de novo anti-HLA donor-specific antibodies (dnDSA) is associated with poorer outcomes in kidney transplant recipients. Despite this, antibody screening post-transplant is not widespread, largely because the optimal management of patients with dnDSA remains undetermined. We hypothesized that in this population, calcineurin inhibitor blood levels would be an independent predictor of graft loss. We analyzed a cohort of unsensitized patients for whom anti-HLA antibody screening was performed prospectively post-transplant. During the screening period between January 2005 and April 2016, 42 patients developed dnDSA. There was no difference in the clinical characteristics or the histological scores of patients biopsied for clinical indication versus those biopsied solely due to detection of dnDSA. Cox modeling revealed a strong relationship between mean tacrolimus levels following dnDSA detection and graft loss, with a hazard ratio of 0.49 (95% CI, 0.33–0.75), which persisted following adjustment for established independent predictors (HR, 0.52, 95% CI, 0.30–0.89). Kaplan–Meier analysis by tertiles of tacrolimus levels and receiver operating curve analysis concurred to show that a threshold of 5.3 ng/ml could be predictive of graft loss. These data suggest that anti-HLA antibody monitoring post-transplant could guide maintenance immunosuppression and improve graft outcomes.
Supporting Information
| Filename | Description |
|---|---|
| tri12934-sup-0001-FigS1-S3.pdfPDF document, 116.3 KB |
Figure S1. (a) Kaplan–Meier plots for graft loss by quartiles of mean tacrolimus levels post-dnDSA development. Comparison was assessed using log-rank test. (b) ROC curve analysis using graft survival as the binary event and mean tacrolimus levels as the exposure. Figure S2. Dot plot of tacrolimus values used for the sensitivity analysis (mean of all the levels available between month 1 and 24 post-dnDSA detection) versus values used in the main analysis (mean of the levels at month 1, 3, 6, 12 and 24 post-dnDSA detection). Figure S3. Dot plot of the mean tacrolimus T0 levels (ng/dL) computed using all values available in the database vs. mean tacrolimus T0 levles (ng/dL) computed using the values available at the following defined timepoints: 1, 3, 6, 12 and 24 months after dnDSA detection.. |
| tri12934-sup-0002-TableS1-S2.docxWord document, 15.3 KB |
Table S1. Univariate and multivariate risk estimates for graft loss associated with tacrolimus levels post dnDSA detection. Table S2. Univariate and multivariate risk estimates for graft loss associated with tacrolimus levels post dnDSA detection. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 2012; 12: 1157.
- 2Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant 2014; 14: 255.
- 3Lefaucheur C, Nochy D, Andrade J, et al. Comparison of combination plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant 2009; 9: 1099.
- 4Lesage J, Noël R, Lapointe I, et al. Donor-specific antibodies, C4d and their relationship with the prognosis of transplant glomerulopathy. Transplantation 2015; 99: 69.
- 5Ekberg H, Grinyo J, Nashan B, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. Am J Transplant 2007; 7: 560.
- 6Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007; 357: 2562.
- 7Abramowicz D, Del Carmen Rial M, Vitko S, et al. Cyclosporine withdrawal from a mycophenolate mofetil-containing immunosuppressive regimen: results of a five-year, prospective, randomized study. J Am Soc Nephrol 2005; 16: 2234.
- 8Roodnat JI, Hilbrands LB, Hene RJ, et al. 15-year follow-up of a multicenter, randomized, calcineurin inhibitor withdrawal study in kidney transplantation. Transplantation 2014; 98: 47.
- 9Vincenti F, Ramos E, Brattstrom C, et al. Multicenter trial exploring calcineurin inhibitors avoidance in renal transplantation. Transplantation 2001; 71: 1282.
- 10Sigal NH, Dumont FJ. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol 1992; 10: 519.
- 11Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713.
- 12Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008; 8: 753.
- 13Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 2014; 14: 272.
- 14Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant 2015; 15: 2921.
- 15Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol 2007; 2: 374.
- 16Kiberd BA, Miller A, Martin S, Tennankore KK. De novo donor-specific human leukocyte antigen antibody screening in kidney transplant recipients after the first year posttransplantation: a medical decision analysis. Am J Transplant 2016; 16: 3212.
- 17Dugast E, Soulillou JP, Foucher Y, et al. Failure of calcineurin inhibitor (tacrolimus) weaning randomized trial in long-term stable kidney transplant recipients. Am J Transplant 2016; 16: 3255.
- 18Tan HP, Donaldson J, Basu A, et al. Two hundred living donor kidney transplantations under alemtuzumab induction and tacrolimus monotherapy: 3-year follow-up. Am J Transplant 2009; 9: 355.
- 19Moreso F, Carrera M, Goma M, et al. Early subclinical rejection as a risk factor for late chronic humoral rejection. Transplantation 2012; 93: 41.
- 20Goldfeld AE, Tsai E, Kincaid R, et al. Calcineurin mediates human tumor necrosis factor alpha gene induction in stimulated T and B cells. J Exp Med 1994; 180: 763.
- 21De Bruyne R, Bogaert D, De Ruyck N, et al. Calcineurin inhibitors dampen humoral immunity by acting directly on naive B cells. Clin Exp Immunol 2015; 180: 542.
- 22Matignon M, Muthukumar T, Seshan SV, Suthanthiran M, Hartono C. Concurrent acute cellular rejection is an independent risk factor for renal allograft failure in patients with C4d-positive antibody-mediated rejection. Transplantation 2012; 94: 603.
- 23Rodrigo E, Segundo DS, Fernandez-Fresnedo G, et al. Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development. Transplantation 2016; 100: 2479.