Use of an anti-D-alloimmunization kinetics model to correct the interval censored D-alloimmunization rate following red blood cell transfusions
Emil Ainsworth Jochumsen and Kathleen Selleng contributed equally to this study and are both considered to be first authors.
Abstract
Introduction
The rate of D-alloimmunization amongst RhD-negative recipients of RhD-positive red blood cell (RBC) transfusions is not certain. Recipients with a short duration between the index RhD-positive transfusion and the last antibody detection test that did not show anti-D might become D-alloimmunized in the future. A regression model was developed to predict how often such patients might develop D-alloimmunization in the future to help account for the immunohematological uncertainty that accompanies having short serological follow up periods.
Methods
Using the published literature on recipients who were intentionally transfused with RhD-positive RBCs and serially followed with antibody screens, as well as unpublished datasets, a regression model was constructed to demonstrate the timing of D-alloimmunization for recipients who became D-alloimmunized within 6 months following the index transfusion. The model was then applied to a series of RhD-negative hospitalized recipients of at least one unit of RhD-positive RBCs who did not become D-alloimmunized but who had fewer than 6 months of serological follow up to weight their contribution to the D-alloimmunization rate.
Results
Overall, the rate of D-alloimmunization was 21/105 (20.0%). There were 39 patients whose last documented antibody screen was performed between 14 days and 6 months after the index RhD-positive transfusion, and these patients were entered into the weighted model. After applying the model, the D-alloimmunization rate rose to 26.3%.
Conclusion
Using a weighted model can help reduce the immunohematological uncertainty that accompanies the inclusion of patients with relatively short serological follow up in studies of RBC alloimmunization.
Abbreviations
-
- HDFN
-
- hemolytic disease of the fetus and newborn
-
- Ig
-
- immunoglobulin
-
- IT
-
- information technology
-
- LTOWB
-
- low titer group O whole blood
-
- RADAR
-
- foRmation of Anti D After Rhogam
-
- RBC
-
- red blood cell
-
- RhIg
-
- Rh immunoglobulin
1 INTRODUCTION
The rate of D-alloimmunization following the transfusion of RhD-positive red blood cells (RBC) to RhD-negative individuals has been studied for many years. Early investigations that involved administering small quantities of RhD-positive RBCs to healthy volunteers found D-alloimmunization rates of between approximately 50% and 90%.1 These studies were performed largely to determine the optimal dose and frequency of administering RhD-positive RBCs to volunteers for the purpose of producing Rh immunoglobulin (Ig). Although these high D-alloimmunization rates were derived from healthy volunteers, the routine clinical practice at many hospitals was to administer only RhD-negative RBCs to RhD-negative patients and those whose RhD-types were unknown when urgent transfusions were required. This was especially true for females of childbearing potential because of the possibility that D-alloimmunization could cause hemolytic disease of the fetus and newborn (HDFN) in a future pregnancy.2
However, the practice of administering RhD-positive RBCs to RhD-negative individuals, despite the risk of alloimmunization, can occur in massive or emergency transfusion situations.3-5 Recent interest in the consequences of transfusing RhD-positive RBCs to RhD-negative patients has intensified, particularly with the resurgence of civilian low titer group O whole blood (LTOWB) transfusions.6-9 It has been recently recognized that the rate of D-alloimmunization following the transfusion of RhD-positive RBCs to hospitalized RhD-negative patients is much lower than the seroconversion rate amongst healthy volunteers.1 Many of the studies of hospitalized transfusion recipients reported D-alloimmunization rates between 20% and 30%.1 However, in many of these studies the recipients were not serially examined for D-alloimmunization following their RhD-positive exposure. The term serological follow up is used to describe the time from the index RhD-positive RBC transfusion in an RhD-negative recipient until the last antibody detection test (herein referred to as an antibody screen) that did not demonstrate anti-D (the antibody against the RhD-antigen). Thus, in studies of the D-alloimmunization rate, RhD-negative recipients who had one antibody screen performed a few weeks after their RhD-positive exposure that did not show anti-D, that is, short serological follow up, were treated with the same weight in the study as patients who also did not become D-alloimmunized but who had multiple antibody screens performed over a longer period of time, that is, longer serological follow up. As the patient's serological follow up increases without the detection of anti-D, the greater the probability becomes that the patient will not go on to develop anti-D.
When studying the rate of D-alloimmunization amongst RhD-negative patients who received RhD-positive RBCs or LTOWB (herein collectively referred to as RBCs), a patient who produces anti-D has clearly achieved the endpoint of the study. However, there is uncertainty about how to handle those recipients who had not become D-alloimmunized during the study. Including a participant amongst the non-alloimmunized study participants who became D-alloimmunized after the study period had ended would lead to underestimating the true rate of D-alloimmunization because that participant was effectively a false negative. Since it is less certain that a patient with shorter serological follow up will truly remain non-D-alloimmunized compared to a patient with longer serological follow up, adjusting for differences in the length of serologic follow up could be helpful in obtaining a more accurate D-alloimmunization rate.
To address this issue, a regression model was developed to weight the contribution of non-D alloimmunized patients to the overall D-alloimmunization rate according to the length of their serological follow up. This model was then applied to adjust the D-alloimmunization rate amongst transfused but not D-alloimmunized RhD-negative patients for the length of each patient's serological follow up in order to obtain a potentially more accurate D-alloimmunization rate.
2 STUDY DESIGN AND METHODS
2.1 Weighted model of the kinetics of D-alloimmunization
To build the weighted model, it was necessary to determine as accurately as possible how long after the index RhD-positive transfusion that anti-D was detected in RhD-negative recipients. A literature search without date limitations was conducted on PubMed using the search terms RhD (alloimmuni* OR immuni*) “transfusion” NOT(sickle) NOT(pregnant*) NOT(platelet*) to identify studies in English on the timing of D-alloimmunization following RhD-positive RBC transfusion to RhD-negative recipients. The references of the identified studies were also used to locate additional studies that were not found in the PubMed search. Recipient data were included in the model if the studies met the following criteria: (1) Minimum serological follow-up period of 6 months, (2) Transfusion of RhD-positive RBCs or LTOWB, (3) No limit on the volume of RhD-positive transfusion, but transfusions must have occurred as a single event, that is, not repeated low volume RhD-positive transfusions that were intended to provoke an immune response, and (4) Exclusion of RhD-negative recipients who were severely iatrogenically immunocompromised, such as stem cell and solid organ transplant recipients. The recipients in these studies were grouped by the volume of RhD-positive RBCs that were administered and the nature of the recipients as follows: 0.5–5 mL RhD-positive RBC (volunteers), at least one RhD-positive RBC unit (volunteers), and at least one RhD-positive RBC unit (patients).
In addition to the data collected from the published literature, several sites that participated in the foRmation of Anti D After Rhogam (RADAR) study,10 a multicenter study that investigated the causes of anti-D-alloimmunization, provided data as well as a single center that systematically investigated the rate of D-alloimmunization after emergency transfusion in RhD-unknown recipients11; this center contributed data on RhD-negative patients who were transfused between January 2000 and August 2024. The data provided by the RADAR study sites and the single center included the timing and results of all antibody screen tests performed on RhD-negative recipients after the index RhD-positive transfusion, and for those who became D-alloimmunized, the length of time between the index RhD-positive transfusion and the detection of the anti-D. To include these patients in the model, a logistic regression of the data was performed because antibody screens on these patients were not necessarily performed on a regular basis. Based on the regression models, the predicted proportion of anti-D detections per month following RhD-positive RBC exposure was calculated. These regressions were performed using Prism 10 (GraphPad, Boston, MA, USA).
For all of the recipients who were included in the weighted model, that is, volunteer study subjects from the literature, patients from the RADAR study, and the single center dataset, starting at the time of the index RhD-positive transfusion, the results of the recipients' antibody screens were recorded by 30-day periods (herein defined as months) for 6 months (180 days) following the index transfusion. For those who became D-alloimmunized, the number of days between the index RhD-positive transfusion the detection of the anti-D was also calculated as was the date of the last antibody screen that did not contain anti-D. For each month, the weight was calculated by adding the number of D-alloimmunized recipients that were first detected in that month plus the D-alloimmunized recipients that had been detected in the previous months divided by the total number of D-alloimmunized patients at end of the sixth month. Thus, for the model it was assumed that patients with a negative antibody screen at month 6 would not go on to develop anti-D in the future, and only data up to month 6 was included in building the model. Any patient who developed anti-D within 14 days of the index RhD-positive transfusion was excluded as these could potentially represent anamnestic (secondary) immune responses.
The results of the antibody screen testing (presence or absence of anti-D) from all of the included recipients were then aggregated and analyzed using a logistic growth regression by the non-linear least squared method.
2.2 Applying the weighted model to the D-alloimmunization rate at a large regional hospital
Using data from the transfusion information technology (IT) system at a large hospital in the Region of Southern Denmark (ProSang, Omda, Oslo, Norway), all RhD-negative patients who received ≥1 unit of RhD-positive RBCs between January 1, 2008 and December 31, 2023 were retrospectively identified. LTOWB is not utilized in this region. The results and dates of all antibody screen tests that were performed on these patients after the index RhD-positive transfusion were recorded. Patients whose last antibody screens that did not show anti-D were performed within 14 days of the index RhD-positive RBC transfusion were excluded because of the insufficient length of serological follow up to detect a primary immune response. Patients whose anti-D was detected within 14 days of the RhD-positive exposure were also excluded to reduce the probability that an anamnestic anti-D would be included amongst the recipients with a primary anti-D. Patients with anti-D detected prior to transfusion and those with known Rh immunoglobulin (RhIg) administration were also excluded.
Antibody screen tests at this hospital were typically performed using automated or manual gel-column agglutination assays although less commonly manual techniques such as LISS tube testing could also have been performed.
To determine the certainty with which the patient's last recorded antibody screen reflects their true D-alloimmunization status (i.e., a screen that did not detect anti-D indicates that they will not become D-alloimmunized at a later date), the patients were divided into two groups:
Group 1: Last antibody screen performed >180 days after the index RhD-positive RBC transfusion.
Group 2: Last antibody screen performed between days 14 and 180 after the index RhD-positive RBC transfusion.
Group 1 patients were weighted at 100% meaning that for those with a screen that did not reveal anti-D performed >180 days after the index transfusion, their result was treated as a true negative. Thus, the weighted model was not applied to patients in Group 1 because the certainty of their negative antibody screen was assumed. The weighted model was applied to the patients in Group 2 according to the timing of their last antibody screen result that did not show anti-D relative to the index transfusion. Patients in Group 2 who became D-alloimmunized between 14 and 180 days after the index transfusion were treated as having a true positive result. Thus, the purpose of the weighted model was to predict which patients in Group 2 whose last antibody screen that did not reveal anti-D was performed between days 14 and 180 following the index RhD-positive transfusion might go on to develop anti-D if they had longer serological follow up. Data analysis and application of the weighted model was done using R (version 4.3.0) in RStudio (Posit, Boston, MA, USA).
The data collection protocol was approved by the administration of the Region of Southern Denmark (OP_1070 Transfusionskvalitetsregistret i Region Syddanmark).
3 RESULTS
3.1 Weighted model of the kinetics of D-alloimmunization
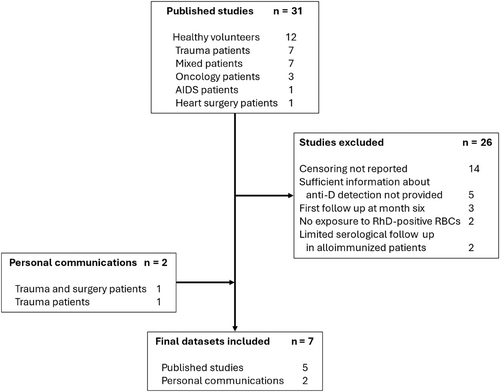
Figure 1 illustrates the results of the literature search. Of the 31 identified studies, 5 studies contained patient data that could be used to build the model. In addition, patient datasets were obtained from of the RADAR study participants and from a separate single center (Table 1).10, 11 In total, 419 recipients were included in the model including 126 who were D-alloimmunized.
| Year | Reference | Study population | Exposure | Study design | Immunized subjects, n (% alloimmunized in each study) | Total number of immunized and non-immunized recipients (n) |
|---|---|---|---|---|---|---|
| 1970 | 12 | Healthy volunteers | 0.5 or 5 mL RBC | Prospective | 9 (81) | 11 |
| 1971 | 13 | Healthy volunteers | 0.5 mL RBC | Prospective | 8 (89) | 9 |
| 1971 | 14 | Healthy volunteers | ≥1 RBC units | Prospective | 18 (82) | 22 |
| 1981 | 15 | Healthy volunteers | 1 mL RBC | Prospective | 4 (33) | 12 |
| 1981 | 16 | Healthy volunteers | ≥1 RBC units | Prospectivea | 21 (75) | 28 |
| Per. Com. | Single center dataset with serially screened recipients | Patients | ≥1 RBC units | Prospectiveb | 41 (13) | 312 |
| Per. Com. | RADAR study sites | Patients | ≥1 RBC or LTOWB units | Retrospectivec | 25d | 25 D-alloimmunizedd |
- Abbreviations: LTOWB, low titer group O whole blood; Per. Com., Personal communication; RBC, red blood cell.
- a To fit data from this study to the weighted model, the monthly distribution was randomly sampled using an R-simulation, assuming a normal distribution around the reported average time to first detection of anti-D, with a standard deviation estimated as ¼ of the reported range.
- b Recipients not tested for alloimmunization at month 4 and 5.
- c Monthly frequencies based on logistic regression analysis of data.
- d The total number of RhD-negative recipients is not known because the data supplied from the RADAR study sites only included D-alloimmunized patients, therefore, the rate of D-alloimmunization cannot be calculated.
Figure 2 presents the results of the weighted model, which demonstrates the timing of anti-D production amongst RhD-negative recipients of RhD-positive RBCs who became D-alloimmunized. For example, the model predicts that a patient who is destined to become D-alloimmunized has an approximately 70% chance that their anti-D will develop within the first 3 months after the RhD-positive transfusion. Note that the model does not predict which patients will become D-alloimmunized, rather it demonstrates the timing of D-alloimmunization amongst those who will make the antibody.
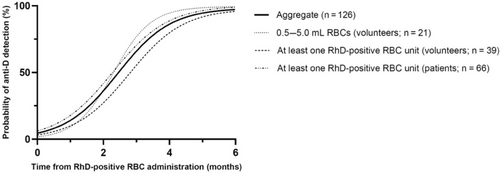
The aggregate regression of the three RhD-negative recipient subgroups, that is, 0.5–5 mL RhD-positive RBC (volunteer; n = 21), at least one RhD-positive RBC unit (volunteer; n = 39), and at least one RhD-positive RBC unit (patients; n = 66) fit the data well (R2 = 0.99). Individual regressions for each of these three recipient subgroups were also performed, and all subgroup regressions fit the data well (R2 = 0.99; Figure 2). The 95% confidence intervals of the variables in the subgroup regressions overlapped indicating that they were not statistically different. The results from the logistic regression analysis using a non-linear least squares method are shown in Data S1.
3.2 Applying the weighted model to the D-alloimmunization rate at a large regional hospital
At this large regional hospital, 228 RhD-negative patients who received ≥1 unit of RhD-positive RBC in the study period were identified. There were 123 patients who were excluded: 105 did not have an antibody screen performed at least 14 days after the index RhD-positive transfusion, 14 patients developed another RBC-antibody before an anti-D could potentially be detected and future antibody screens were not performed (see discussion below), three formed an anti-D within 14 days of the index RhD-positive transfusion, and one patient's anti-D was due to RhIg administration. Thus, there were 105 RhD-negative patients available for analyses. The characteristics of the patients who were included in the study are presented in Table 2.
| Patient group | Number of recipients | Female proportion (p)a | Median age, years (IQR) | Median age of male recipients, years (IQR) | Median age of female recipients, years (IQR) | Median length of serological follow-up, days (IQR) | Median length of serological follow-up for male recipients, days (IQR) | Median length of serological follow-up for female recipients, days (IQR) | Median number of RhD-positive units transfused (IQR)b |
|---|---|---|---|---|---|---|---|---|---|
| Total number of patients who did not become D-alloimmunized during the study period | 84 | 42% (0.01) | 73 (16) | 72 (16) | 76 (17) | 212 (687) | 264 (789) | 207 (328) | 2 (5) |
| Patients in Group 1 | 45 | 42% (0.02) | 74 (15) | 74 (16) | 74 (18) | 604 (1464) | 801 (1672) | 365 (1392) | 2 (5) |
| Patients in Group 2 | 39 | 41% (0.03) | 72 (18) | 68 (14) | 78 (11) | 37 (51) | 36 (60) | 46 (36) | 2 (6) |
| Patients who became D-alloimmunized | 21 | 10% (1.0) | 69 (9) | 69 (10) | 71 (2) | 276 (700) | 239 (541) | 845 (50) | 4 (5) |
| Aggregate | 105 | 35% (0.04) | 73 (16) | 71 (16) | 76 (15) | 239 (716) | 252 (721) | 216 (662) | 2 (5) |
- Note: Groups 1 and 2 are the two subsets of patients stratified by the timing of the last antibody screen that did not detect anti-D and who did not become D-alloimmunized during the study period.
- a p-value (Chi2 test) compares the non-D-alloimmunized patients against anti-D alloimmunized patients.
- b RhD-positive units transfused within 72 h after index RhD-positive transfusion.
The unweighted rate of anti-D-alloimmunization in this patient population was 21/105 (20.0%; CI 95% 12.3%–27.7%). When the weighted model was applied to the 39 recipients in Group 2 whose last antibody screen did not reveal anti-D between days 14 and 180, the weighted rate of D-alloimmunization rose to 26.3% (CI 95% 17.9%–34.7%).
4 DISCUSSION
In this study, the application of a weighted model for D-alloimmunization increased the uncorrected rate of D-alloimmunization by an absolute value of 6.3% points. It was expected that applying the weighted model would raise the D-alloimmunization rate because it accounts for the potential for D-alloimmunization to occur amongst recipients with short serological follow up lengths. The weighted model is itself probabilistic based on the timing of anti-D detection in recipients who seroconverted. Thus, the actual rate of D-alloimmunization in this cohort likely lies somewhere between 20.0% and 26.3%. Applying this weighted model to account for the likelihood that recipients with relatively short serological follow up lengths might have become D-alloimmunized in the future can improve the accuracy of future studies of the seroconversion rate.
In building the weighted model it was found that the kinetics of anti-D detection between the three subgroups of recipients were quite similar, despite differences in the doses of RhD-positive RBCs administered and the recipients' underlying health status. This is interesting because it is recognized that the rate of D-alloimmunization differs considerably between healthy volunteers and hospitalized patients1 as well as the dose of RhD-positive RBCs administered (increasing rates of alloimmunization up to approximately 30 mL of RhD-positive RBCs, after which the rate becomes constant17). Thus, it seems that the volume of the RhD-positive RBCs administered and the recipient's underlying health status modulate the likelihood of D-alloimmunization but not the kinetics of anti-D formation in a recipient who seroconverts.
The weighted model was not applied to the patients in Group 1 whose last antibody screen that did not show anti-D was performed >180 days after the index RhD-positive transfusion because it was assumed that the absence of anti-D beyond 180 days reflected their true immunohematological status. While it is possible that patients with a negative antibody screen that long after the index RhD-positive transfusion could have been D-alloimmunized with an evanescent antibody, anti-D has a low evanescence rate.18-20 Thus, the assumption that the absence of anti-D on their last antibody screen reflects a truly not D-alloimmunized state is reasonable.21
This study had several other limitations. The retrospective nature of its design prevented the investigators from taking a more proactive role in obtaining serial antibody screens on the patients who were exposed to RhD-positive RBCs, hence the need for the weighted model for those with short serological follow up lengths. As is common for retrospective studies of alloimmunization rates, the date on which the anti-D was detected was taken to be the day on which the patient actually produced the antibody, although these dates might not perfectly coincide. For those who seroconverted, knowing the amount of time between the date of the last antibody screen that did not contain anti-D and the date of the screen that first demonstrated the anti-D allowed for a more accurate determination of the month in which the anti-D was produced. RhD-positive platelets were not considered to be an alloimmunizing stimulus because of the much lower rate of D-alloimmunization following RhD-positive platelet exposure compared to RhD-positive RBC exposure and none of the patients or volunteers in these studies only received RhD-positive platelets.22
The hospitalized patients who were included in the rate of D-alloimmunization part of this study might have received RhD-positive transfusions at hospitals at which the investigators did not have access to transfusion histories, and they could also have been sensitized during pregnancy. However, any patient whose anti-D was detected within 14 days of the index RhD-positive transfusion at this hospital was excluded due to the possibility that the anti-D was due to an anamnestic response thereby reducing the potential confounding effect of antibody evanescence. The clinical diagnoses and treatments of the RhD-negative recipients who received RhD-positive transfusions were not known, however they were drawn from a large, regional hospital over a long period of time thereby likely reflecting a pragmatic sample of the population of hospitalized patients. Also, at this hospital, once a patient has a positive antibody screen, additional antibody screens are not performed before future blood products are issued. Instead, the RBC units are serologically crossmatched with recipient plasma and an incompatibility would be further investigated for the presence of additional antibodies before the unit is issued. Thus, it is possible that some patients with anti-D might not have been detected if RhD-negative RBCs were issued after a non-anti-D antibody had been detected.
Some of the limitations of building the weighted model included the fact that at the single center that contributed data, RhD-negative recipients of RhD-positive RBCs underwent serial antibody screening at months 1, 2, 3, and 6 following the index RhD-positive transfusion but not at months 4 or 5. Thus, the data used to build the model for months 4 and 5 came solely from the patients in the previously published studies and from the sites that participated in the RADAR study. Also, it was assumed that all recipients who were destined to produce anti-D would do so by 6 months following the RhD-positive index transfusion. This assumption might overestimate the weight of a negative antibody screen result >180 days post RhD-positive exposure. However, setting an upper time limit for D-alloimmunization was necessary for building the model. Six months was chosen as the upper limit because the dataset from the single center that featured serially screened recipients contained only one patient who developed an anti-D between months 6 and 12. A published study also found that primary D-alloimmunization after 6 months was rare.16 Therefore, it is unlikely that a recipient would become D-alloimmunized beyond 6 months.
These data suggest that if RhD-negative patients are to have an antibody detection test performed after RhD-positive transfusion to determine if they have become D-alloimmunized, the optimum timing of the test is around 6 months after the exposure. This timing has been adopted for screening RhD-negative recipients of RhD-positive RBCs in Germany because it helps to reduce the collection of samples at times that are less likely to demonstrate seroconversion.23 Furthermore, knowing the optimal time to screen recipients could have implications for screening RhD-negative females of childbearing potential following an RhD-positive transfusion during resuscitation from life threatening bleeding for antenatal counseling about her risk of HDFN and on recipients who might require future transfusions as part of their ongoing therapy to ensure their safety from delayed hemolytic reactions.
The weighted model can be applied to future studies of D-alloimmunization to help correct for the uncertainty that accompanies the immunohematological status of patients with relatively short lengths of serological follow up. While several weeks of serological follow up might be sufficient to detect a new antibody, weighting the results of those recipients who did not produce anti-D within 180 days post exposure offers a novel method of accounting for those with a relatively short length of serological follow up.
ACKNOWLEDGMENTS
KS thanks Kathrin Denker for her efforts to systematically collect the immunohematogical results of the RhD-negative patients after RhD-positive RBC transfusion in the University hospital Greifswald.
CONFLICT OF INTEREST
MHY has given paid lectures/reimbursed travel/consulting fees for: Grifols, Hemanext, Terumo, Legacy Innovations. MHY owns equity in Velico Medical, Inc.




