Tranexamic acid in trauma: After 3 hours from injury, when is it safe and effective to use again?
Christopher D. Barrett first authors.
Abstract
Tranexamic acid (TXA) has proven mortality benefit if used early after traumatic injury, likely related to a combination of bleeding reduction and other non-bleeding effects. If TXA is given more than 3 h after traumatic injury, there is a significant and paradoxical increased risk of death due to bleeding. TXA has level 1 evidence for use as a bleeding reduction agent in isolated orthopedic operations, but in polytrauma patients undergoing orthopedic operations, it is not clear if and when TXA is safe or effective once outside the 3-h window of proven trauma efficacy.
Abbreviations
-
- TXA
-
- tranexamic acid
-
- uPA
-
- urokinase
Tranexamic acid (TXA) is a lysine analogue1 that has been an essential medicine for preventing or treating major bleeding through its antifibrinolytic effects. Multiple randomized trials have shown that TXA is effective and safe as a bleeding control agent in various medical fields including trauma2, 3 and elective orthopedics.4, 5 Traumatic injury is a significant cause of death and disability worldwide,2 with approximately half of polytrauma patients admitted to the hospital having orthopedic injuries that require surgical intervention.6
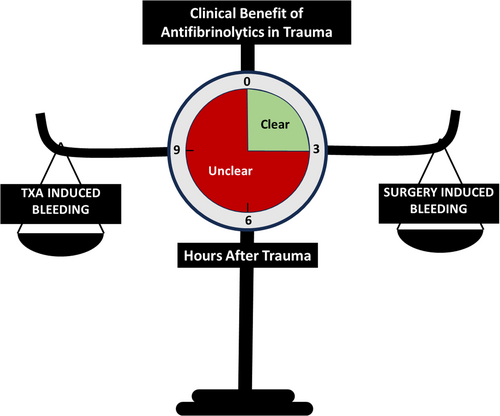
In severe polytrauma, TXA has been shown to save lives through bleeding reduction when dosed within 3 h of injury. Similarly in the orthopedic surgery setting, TXA has been shown to significantly reduce bleeding in certain isolated operative populations, including spine and pelvic/lower extremity surgeries, which are known to cause hyperfibrinolysis. With the isolated (non-polytrauma) orthopedic literature as a basis, it has become common to observe TXA administration in orthopedic trauma operations performed on patients several hours to days after their polytrauma. This raises a significant clinical dilemma in trauma patient care with competing level 1 evidence, as (1) TXA use more than 3 h after injury has demonstrated an increased risk of death due to an apparent increase in bleeding in the trauma literature,7 and (2) their orthopedic operations are known to stimulate fibrinolysis-induced bleeding and, absent their preceding polytrauma, are candidates for intraoperative TXA administration which is known to reduce operative bleeding in these procedures. It is unknown whether the risk of bleeding from TXA delivered more than 3 h after severe injury or from the fibrinolysis instigated by any subsequent orthopedic surgical procedure, if not treated with TXA, is worse. This is particularly concerning in patients with solid organ and brain injuries, where subjecting patients to any increased risks of bleeding and/or hypotension may have catastrophic consequences, whether from delayed TXA-induced bleeding at the sites of injury or from large volume blood loss from orthopedic operations that may benefit from TXA.
The reason underlying the deleterious effect of TXA when given later than 3 h remains elusive, but preclinical studies suggest that this may be related to a delayed surge in urokinase (uPA) expression and changes in its conformation that promote plasmin activity, leading to bleeding.8 In addition, plasmin generation may also have non-bleeding related impacts on inflammation and immune function that can be both protective and deleterious, depending on circumstance and timing, that may further contribute to how TXA impacts outcomes.9-12
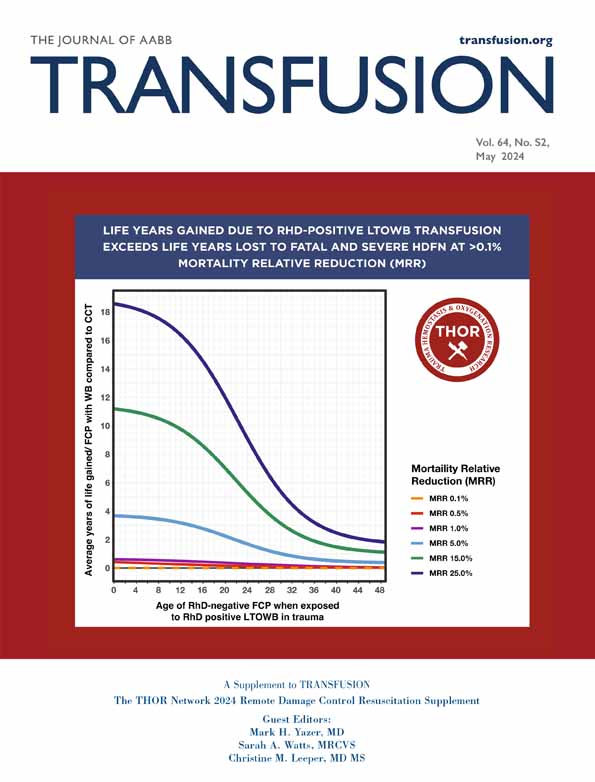
With competing randomized trial evidence between benefit and harm in the relevant fields of medicine, and no available clinical trial to mitigate the knowledge gap, it remains to be determined when TXA is safe and effective to use beyond 3 h from severe polytrauma (Figure 1) where many post-trauma orthopedic operations occur. This question needs to be addressed via a randomized clinical trial. While awaiting a trial to clarify this clinical paradox, the decision to use TXA beyond 3 h from injury in complex polytrauma patients should be made on a case-by-case basis that carefully considers the patient's overall clinical situation.
ACKNOWLEDGMENT
Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
CDB has patents pending related to both coagulation/fibrinolysis diagnostics, previously received grant support from Genentech, Inc. and Werfen, and has received consulting fees from Atheneum Partners. MDN has previously received grant support from Haemonetics, Alexion, and Instrumentation Laboratories/Werfen, has received consulting fees from Haemonetics and Takeda, and serves as the Chief Medical Officer and has equity in Haima Therapeutics. All other authors have nothing to disclose.