Analyzing and modeling massive transfusion strategies and the role of fibrinogen—How much is the patient actually receiving?
Abstract
Background
Hemorrhage is a leading cause of preventable death in trauma, cardiac surgery, liver transplant, and childbirth. While emphasis on protocolization and ratio of blood product transfusion improves ability to treat hemorrhage rapidly, tools to facilitate understanding of the overall content of a specific transfusion strategy are lacking. Medical modeling can provide insights into where deficits in treatment could arise and key areas for clinical study. By using a transfusion model to gain insight into the aggregate content of massive transfusion protocols (MTPs), clinicians can optimize protocols and create opportunities for future studies of precision transfusion medicine in hemorrhage treatment.
Methods
The transfusion model describes the individual round and aggregate content provided by four rounds of MTP, illustrating that the total content of blood elements and coagulation factor changes over time, independent of the patient's condition. The configurable model calculates the aggregate hematocrit, platelet concentration, percent volume plasma, total grams and concentration of citrate, percent volume anticoagulant and additive solution, and concentration of clotting factors: fibrinogen, factor XIII, factor VIII, and von Willebrand factor, provided by the MTP strategy.
Results
Transfusion strategies based on a 1:1:1 or whole blood foundation provide between 13.7 and 17.2 L of blood products over four rounds. Content of strategies varies widely across all measurements based on base strategy and addition of concentrated sources of fibrinogen and other key clotting factors.
Discussion
Differences observed between modeled transfusion strategies provide key insights into potential opportunities to provide patients with precision transfusion strategy.
Abbreviations
-
- COI
-
- Circular of Information
-
- Cryo AHF
-
- cryoprecipitated antihemophilic factor
-
- FVIII
-
- factor VIII
-
- FXIII
-
- factor XIII
-
- IFC
-
- INTERCEPT Fibrinogen Complex
-
- MTP
-
- massive transfusion protocol
-
- PAS
-
- platelet additive solution
-
- TIC
-
- trauma-induced coagulopathy
-
- USA
-
- United States of America
-
- vWF
-
- von Willebrand factor
-
- WB
-
- whole blood
1 INTRODUCTION
Hemorrhage is a leading cause of preventable death in trauma,1, 2 cardiac surgery,3 liver transplant,4, 5 and childbirth.6 Over the past several decades, civilian traumatic hemorrhage treatment has improved by leveraging results from studies of combat hemorrhage treatment7-9 and incorporating these practices into civilian care. Studies of civilian massive transfusion protocols (MTPs) in trauma show that both time and ratio of blood products impact mortality.10, 11 Specifically, every minute from time of activation of MTP to arrival of the first blood products increases the odds of mortality by 5%.10 Balanced transfusion ratios show consistent benefit for major hemorrhage treatment in trauma,11 cardiovascular,12 liver transplant,13, 14 and obstetric cases.15 Despite these improvements, hemorrhage remains a significant cause of mortality in adults and children.11, 16
Studies of hemorrhage during planned surgical procedures show correlation between preoperative and perioperative hypofibrinogenemia-related coagulopathy and increased blood utilization and worse outcomes.17-24 Use of whole blood (WB) or a 1:1:1 transfusion ratio in trauma provides a balanced resuscitation; however, neither provides concentrated fibrinogen that can rapidly address hypofibrinogenemia or fibrinogen dysfunction when it occurs. Identification of the need for a concentrated source, and ability to immediately respond, is critical for those who would benefit from supplementing fibrinogen. Fibrinogen, the precursor for fibrin, a key protein in clot formation, is the first clotting factor to reach critically low levels during hemorrhage.20, 25, 26 Fibrinogen level below 200 mg dL−1 is an independent risk factor for severe hemorrhage in trauma,20 cardiovascular,18 liver transplant,21, 22 and obstetric19 patients. The European Society for Anaesthesiology guidelines indicate that a fibrinogen concentration of less than 150 to 200 mg dL−1 is considered hypofibrinogenemic.27 Treating acquired hypofibrinogenemia earlier shows promise for positive impact in trauma,28, 29 cardiovascular surgery,30, 31 pediatric major surgery,32 and obstetric hemorrhage,33 but the ability to provide earlier treatment, or more rapid correction with fibrinogen supplementation, is limited. Until recently, common concentrated sources have had delayed access due to need to thaw cryoprecipitated antihemophilic factor (cryo-AHF),34 or the need to reconstitute fibrinogen concentrates, both of which require approximately 5–20 minutes to achieve (cryo-AHF, COI; Fibryga, Octapharma, Paramus, NJ; and RiaSTAP, CSL Behring, King of Prussia, PA).34-37 Additionally, fibrinogen concentrates are FDA approved in the United States of America (USA) for the treatment of congenital hypofibrinogenemia only. Outside of the USA, there are fibrinogen concentrate products approved for treatment of acquired hypofibrinogenemia. A newer concentrated source of fibrinogen and other key clotting factor products, Pathogen-Reduced Cryoprecipitated Fibrinogen Complex (INTERCEPT Fibrinogen Complex, IFC, Cerus, Concord, CA), can be stored thawed in the blood bank or operating room (OR) and be ready for immediate transfusion, for 5 days post-thaw. IFC is approved in the USA for the treatment and control of bleeding, including massive hemorrhage, associated with acquired hypofibrinogenemia, but it is not available outside the USA. Indications and approvals for use may change in the future.
While emphasis on the ratio and protocol for administration of blood products improves ability to treat hemorrhage rapidly, understanding the overall content of what specific resuscitation regimens provide allows for the opportunity to optimize protocols based on patient condition and begin to achieve precision transfusion medicine. This can be used to facilitate and improve the MTP design and provide opportunities for future studies of hemorrhage treatment. Medical modeling provides insights into what may be happening based on changing patient conditions. While modeling is not meant to indicate the right clinical course of action, it can provide insights into where deficits in treatment could arise and key areas for clinical study. A new tool is described below to provide insight into the content of multiple currently used treatment strategies for patients with trauma-induced coagulopathy (TIC). The model can be accessed at rdcr.org/mtp-model. Future prospective studies may determine clinical utility of this model; currently, it is intended to provide information comparing transfusion strategies only. The model's products and parameters are based on blood product and component averages, which may be from limited data.
2 METHODS
2.1 Model design
This transfusion model is intended to describe the content within multiple transfusion and factor concentrate resuscitation strategies to allow for their comparison. Up to four rounds of an MTP are utilized to illustrate that the total content of blood elements and coagulation factor changes over time. The configurable model calculates hematocrit, platelet concentration, percent volume plasma, total grams and concentration of citrate, percent volume anticoagulant and additive solution, and concentration of clotting factors: fibrinogen, factor XIII (FXIII), factor VIII (FVIII), and von Willebrand factor (vWF) provided by the MTP strategy. This information may help clinicians choose the best transfusion strategy for their patient by understanding the factors that contribute to dilutional coagulopathy, predicting depleted clotting factors, or recognizing the increased risk of hypocalcemia from high levels of citrate. Endogenous and exogenous causes of hemostatic dysfunction that occur with prolonged bleeding and resuscitation are not accounted for in model calculations. Results are only intended to describe what is being given with different resuscitation strategies. Once the hemostatic system and its interactions are more thoroughly described, future models can incorporate different endotypes of biologic responses to hemorrhagic shock that occur in patients. Likewise, as new products come to market or current products become approved for new indications (e.g., lyophilized plasma, lyophilized cryo, and prothrombin complex concentrates), they can be incorporated in future models.
Blood products have natural variation in volume and concentration based on the donor characteristics and collection technique. Composition values for specific blood components and WB units included in the model reflect average value, by component type, as set by the Circular of Information34 for a representative donor using a common collection device. The representative donor has the following characteristics: 45% hematocrit,38 Clauss fibrinogen of 280 mg dL−1,34 and platelet concentration of 236,000 μL−1.39 The collection parameters are for WB donation, total collected volume of 500 mL34 with the addition of 70 mL of CPD citrate solution40 within WB collection kit34 for a total volume of 570 mL. Total blood product volume, volume of anticoagulant, and volume of additive solution are determined by the collection device manufacturer's package inserts.41-43 WB and WB-derived components are assumed to use CPD citrate solution,40 and apheresis-derived units are assumed to use ACD-A citrate solution.44 Platelet concentration, hematocrit, and clotting factor levels for vWF, factor VIII, and fibrinogen in blood products reflect the 2021 AABB Circular of Information (COI),34 when possible. Values for products with individual package inserts, Pathogen-Reduced Cryoprecipitated Fibrinogen Complex (INTERCEPT Fibrinogen Complex, IFC),45 Fibryga,35 and RiaSTAP,36 reflect the package insert, when possible. Factor XIII levels are calculated based on literature review of antigen and activity assays used in the evaluation of FXIII levels in cryo-AHF,46-48 IFC,46, 47 RiaSTAP,46, 47, 49 and Fibryga.48, 49 Therefore, quantitative comparisons are limited as concentrations might change, or be different, based on different methods of analysis or production of products. A conservative estimate was used that assumes IFC and plasma cryoprecipitated products have the same level of FXIII, as found in cryo-AHF. Default pool size used in this model for cryo-AHF is a pool of five donors, for IFC a four-pool (FC15). Factor XIII and vWF levels are not provided in the package insert for either of the fibrinogen concentrate products. The values in the model are taken from a separate in vitro analysis performed by Octapharma, the manufacturer of Fibryga.48, 49 Default blood product model values are given in Table 1. In the model, these can be modified to reflect individual blood center quality control data.
| Product/component | Unit volume (mL) | Plasma (mL) | Additive solution (mL) | Anticoagulant (mL) | Citrate (g) | Red cells (mL) | Platelet (count) | Fibrinogen (mg) | FXIII activity (U) | vWF antigen (U) | FVIII activity (U) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FFP (WB derived) | 275 | 236.5 | 0 | 38.5 | 1.00 | 0 | 0 | 662 | 495 | 270 | 223 |
| PF24 (WB derived) | 275 | 236.5 | 0 | 38.5 | 1.00 | 0 | 0 | 662 | 495 | 363 | 182 |
| FFP (apheresis) | 500 | 430 | 0 | 70 | 1.54 | 0 | 0 | 1204 | 900 | 490 | 405 |
| Platelets in PAS (apheresis) | 200 | 59.5 | 130 | 10.5 | 0.23 | 0 | 3E+11 | 167 | 107 | 58 | 48 |
| Platelets in plasma (apheresis) | 200 | 169.2 | 0 | 30.8 | 0.68 | 0 | 3E+11 | 474 | 305 | 166 | 137 |
| WB-derived platelets (5 pools) | 275 | 232.7 | 0 | 42.3 | 1.10 | 0 | 2.75E+11 | 652 | 419 | 228 | 188 |
| RBC–apheresis | 320 | 12.8 | 110 | 6.8 | 0.15 | 190.4 | 0 | 36 | 23 | 5 | 10 |
| RBC WBD | 350 | 22.6 | 110 | 7.4 | 0.19 | 210 | 0 | 63 | 41 | 9 | 18 |
| Whole blood | 570 | 275 | 0 | 70 | 1.82 | 225 | 1.18E+11 | 770 | 495 | 150 | 223 |
| Cryoprecipitated AHF (5 pools) | 120 | 103.2 | 0 | 16.8 | 0.37 | 0 | 0 | 2000 | 504 | 1032 | 756 |
| IFC (4 pools) | 120 | 103.2 | 0 | 16.8 | 0.37 | 0 | 0 | 1500 | 504 | 780 | 340 |
| Fibrinogen concentrate (1 vial RiaSTAP) | 50 | 0 | 50 | 0 | 0 | 0 | 0 | 1000 | 55 | 215 | 0 |
| Fibrinogen concentrate (1 vial Fibryga) | 50 | 0 | 50 | 0 | 0 | 0 | 0 | 1000 | 160 | 10 | 0 |
| Circulating blood | 5000 | 2750 | 0 | 0 | 0 | 2250 | 1.18E+12 | 7700 | 4950 | 1482 | 2228 |
2.2 Transfusion strategies
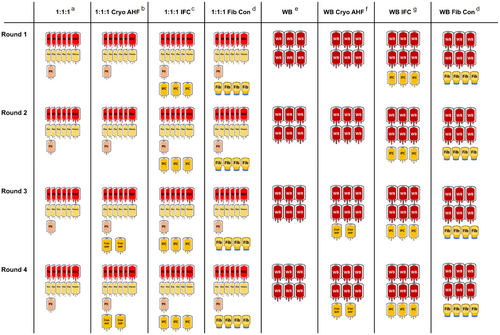
Any combination of blood products can be added to the model to achieve a wide range of potential goals. In this example of the model, the goal is to compare two base transfusion strategies with or without three options for concentrated fibrinogen to effectively treat a patient with severe hemorrhage and TIC. These examples use either a base strategy of six red cell units, six plasma units, and one apheresis platelet unit (1:1:1), or 6 units of WB per strategy round (Figure 1). Platelet units in this model reflect the average platelet content in plasma as this provides some additional coagulation factors in comparison to platelet additive solution (PAS). In the model, either platelets in PAS or plasma can be entered. Approximately 4 grams of fibrinogen per round is the target in this model for the treatment of hemorrhage and TIC. To achieve this, the fibrinogen sources compared are two 5 pools of cryo-AHF, three 4 pools of IFC (FC15), or four 1-gram vials of Fibryga (Figure 1). Fibryga was chosen as a representative fibrinogen concentrate, rather than RiaSTAP, for the model because of its ability to be stored at room temperature prior to reconstitution. Access to these products differs based on availability and preparation time. Cryo-AHF is usually not available until the third round of MTP due to the time needed to thaw it and also to balance wastage vs earlier use during an MTP activation. If not immediately used, cryo-AHF waste risk is high due to a short 4–6-hour post-thaw shelf life. Time to reconstitute fibrinogen concentrate differs depending on which product is used and storage conditions. For the model, we use the best-case scenario of Fibryga stored at room temperature, with room temperature sterile water available, to reconstitute in 5 to 10 minutes.35 In this case, it would be possible to provide Fibryga in round 1 of MTP strategy, assuming it could be returned to the pharmacy or blood bank if not used. Similar to cryo-AHF, Fibryga must be used within 4 hours of reconstitution. IFC can be stored for 5 days post-thaw at room temperature45; therefore, it is available for the first round of an MTP with reduced waste risk as it can be returned if unused. Since the model does not account for the patient's condition, hemoconcentration in vivo, cellular or factor half-life or consumption, the model does not predict clinical outcomes.
3 RESULTS
Table 2 shows the aggregate values for four rounds of the different commonly used transfusion strategies in a patient with severe bleeding. All strategies provide between 13.7 L and 17.2 L of blood products over four rounds, but differ in hematocrit, platelet concentration, fibrinogen and other key clotting factors, grams of citrate and g/L citrate, and percent volume anticoagulant/additive solution, from circulating blood (Table 2).
| Transfused product mix | Circulating blood | 1:1:1 | 1:1:1 + cryo-AHF | 1:1:-1 + IFC | 1:1:1 + fib con | WB | WB + cryo-AHF | WB + IFC | WB + fib con |
|---|---|---|---|---|---|---|---|---|---|
| Fibrinogen (g) | n/a | 18.1 | 27.3 | 37.3 | 35.3 | 18.5 | 26.5 | 36.5 | 34.5 |
| Clauss fibrinogen (mg dL−1) | 280 (200–500) | 168 | 243 | 306 | 305 | 223 | 302 | 375 | 380 |
| Platelets (count μL−1) | 236,000 | 75,949 | 73,710 | 69,606 | 72,289 | 207,018 | 200,000 | 187,302 | 195,580 |
| vWF (IU mL−1) | 1.1 | 0.4 | 0.7 | 1.0 | 0.5 | 0.3 | 0.5 | 0.9 | 0.3 |
| FXIII (U mL−1) | 1 | 0.8 | 1.0 | 1.2 | 1.0 | 0.9 | 1.0 | 1.2 | 1.0 |
| FVIII (IU mL−1) | 1.2 | 0.4 | 0.6 | 0.6 | 0.4 | 0.4 | 0.6 | 0.6 | 0.4 |
| Hematocrit | 36%–54% | 32% | 31% | 29% | 30% | 39% | 38% | 36% | 37% |
| Hemoglobin (g dL−1) | 12–18 | 10.6 | 10.3 | 9.7 | 10.1 | 13.2 | 12.7 | 11.9 | 12.4 |
| Plasma volume (%) | 50%–60% | 41% | 45% | 47% | 42% | 48% | 50% | 52% | 46% |
| Citrate (g) | 0 | 29.6 | 32.8 | 35.8 | 31.4 | 43.7 | 45.2 | 48.1 | 43.7 |
| Citrate (g/L) | 0 | 1.9 | 2.0 | 2.1 | 1.9 | 3.2 | 3.2 | 3.2 | 3.0 |
| Anticoagulant and additive solution (%) | 0 | 27% | 24% | 24% | 28% | 12% | 12% | 12% | 17% |
| Volume (L) | 4.5–5.7 | 15.8 | 16.3 | 17.2 | 16.6 | 13.7 | 14.2 | 15.1 | 14.5 |
Hematocrit: All model strategies using a base of 1:1:1 fall below the lower end of normal, 36%, for hematocrit. All strategies using a base of WB are either at, or above, 36% hematocrit.
Platelet count: All model strategies provide either an apheresis unit of platelets or WB, with associated platelets, in each round. With this approach, all strategies are above 50,000 platelet μL−150; however, the only strategies that provide platelets above the threshold used to define thrombocytopenia in patients (<150,000 platelet μL−1) are WB based (Table 2).
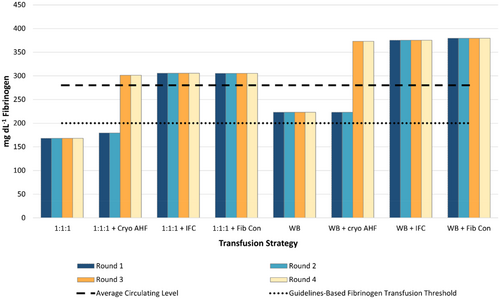
Fibrinogen: In a 1:1:1 strategy, without a concentrated source of fibrinogen, fibrinogen levels remain below the 200 mg dL−1 guideline for transfusion of fibrinogen threshold27 (Figure 2). Addition of two cryo-AHF 5 pools in rounds three and four raises the aggregate amount of fibrinogen to above 200 mg dL−1 (Table 2), but prior to round three, patients receive over 6 L of blood components with low levels of fibrinogen. WB-based strategies surpass 200 mg dL−1 without a concentrated source of fibrinogen; however, they do not surpass 280 mg dL−1, the average circulating level of fibrinogen in a healthy adult according to the COI34 (Figure 2). Addition of three IFC 4 pool, or four 1-gram vials of Fibryga, in round one of either 1:1:1 or WB-based strategies, provides a consistent source of fibrinogen, not only above the guideline threshold of 200 mg dL−1 but also above the average circulating level of 280 mg dL−1, allowing for potential replacement of fibrinogen lost during bleeding or consumed by clot formation (Figure 2).
Anticoagulant and additive solutions: All strategies contain anticoagulant and/or additive solution, predominantly from platelet and plasma units or WB41-43 (Table 1), representing 12%–28% of the total volume (Table 2). 1:1:1-based strategies have the highest percentage of anticoagulant and additive solution, ranging 24%–28%, and WB-based strategies are lower, ranging 12%–17% (Table 2). While lower in percent volume addition of these solutions, WB-based strategies have more citrate in both total grams and overall concentration than 1:1:1-based strategies (Table 2).
Financial impact: Model allows for calculation of the financial impact of each transfusion strategy based on individual costs. This is not discussed in these examples as costs vary significantly between countries, blood providers, and hospitals. Potential financial savings from decreased overall blood utilization, OR time, intensive care unit time, personnel time, and wastage rate are not calculated within this model.
4 DISCUSSION
During massive hemorrhage, there is sufficient evidence to assume that early access to blood is critical10 and that a balanced ratio of blood products may improve outcomes.11 As such, the burning question remains as to how to further optimize transfusion strategies to reduce mortality rates from hemorrhage. Goal-directed transfusion therapy is an evidence-based approach to treating bleeding, but there may be a delay in availability of laboratory testing. In the interim, balanced resuscitation is needed. The modeling of potential transfusion strategies described here provides a tool that clinicians and laboratorians can use to gain a deeper understanding of transfusion protocols and their potential impact on what content they are providing to their patients. For hospitals, modeling explains the value behind the addition of certain blood products to MTP strategy, above and beyond cost. Not every hospital has access to the same blood components, or fibrinogen concentrate, for MTPs. Understanding potential shortcomings of transfusion strategy drives awareness to look for the impact of these issues before they impact patient outcomes. The model is available for use at rdcr.org/mtp-model to allow for alternative strategies not represented in this study to be compared to other resuscitation strategies.
Clinicians performing transfusions are typically poorly informed about blood collection and manufacturing effects on blood product characteristics. Therefore, product content is not immediately factored into treatment strategy, and many are not aware that a portion of every blood product is not blood, but anticoagulant and/or additive solution, even for WB. Addition of anticoagulant and/or additive solutions is necessary during blood product collection to allow storage. The main reason that all strategies fall below normal concentration of fibrinogen, without the addition of a concentrated source, is due to these added non-blood solutions. The 1:1:1 transfusion strategy, prior to the addition of a concentrated source of fibrinogen, contains 27% combined additive and anticoagulant solution, and WB is 12% anticoagulant solution. This has a combined dilutional and inhibitory effect on coagulation; a concentrated source of fibrinogen and other key clotting factors is important in regaining hemostasis.
In this example, transfusion strategies reflect the potential ways cryo-AHF, fibrinogen concentrate, or IFC can be incorporated into base MTP strategies of 1:1:1 or WB (Figure 1). Given the predominance of cryo-AHF as the concentrated source of fibrinogen for the treatment of acquired coagulopathy in the USA and the UK, the vast majority of hospital MTPs currently do not include any concentrated source of fibrinogen in rounds one and two. Many have shifted to independent ordering of cryo-AHF based on results of goal-directed therapy in order to reduce wastage risk. Across etiologies of hemorrhage, there are no randomized control trials, or high-quality data, suggesting superiority of fibrinogen concentrates to cryo-AHF for the treatment of acquired hypofibrinogenemia in blood product use or patient outcomes.37, 51, 52
Given the difficulties accessing cryo-AHF early in hemorrhage treatment, some facilities in the USA have started to use fibrinogen concentrate off label, especially when hypofibrinogenemia is known to significantly impact hemorrhage risk. IFC is a newer product; therefore, general hospital experience is low. Unlike fibrinogen concentrates, IFC is indicated for hemorrhage-related acquired hypofibrinogenemia in the USA. Pre-thawed IFC can be available immediately without the need to thaw or reconstitute on demand since it can be kept thawed at room temperature for 5 days.
Goals for transfusion strategy may differ between etiologies of hemorrhage. Depending on this, different goal-directed therapies may be used. In cases where a resuscitation strategy closest to circulating blood would be most beneficial for the patient, this model shows that WB plus any concentrated source of fibrinogen may provide this more effectively than the use of blood components in a 1:1:1 unit ratio and any source of concentrated fibrinogen.
Modeling has limitations. It does not take into account the patient status, total blood volume, ability to cope with a fluid load and the different etiologies of hemorrhage, comorbidities, or conditions. In the future, additional mechanical techniques and pharmacological treatments may change how blood is used. Future iterations of this model may be developed to include these new techniques and/or treatments (e.g., prothrombin complex concentrates or lyophilized plasma) as their clinical efficacy is clarified. The example model strategies used in this paper are based on the most common approach to MTPs in the USA (1:1:1 or WB), and not all facilities align with these strategies. In addition to the base strategy (1:1:1 or WB), the strategies modeled provide similar doses of fibrinogen. IFC is available as a 2 (FC10), 4 (FC15), 6 (FC20), 8 (FC30), or 10 (FC40) pools of donors and therefore has a range of sizes and fibrinogen quantity.45 Three bags of FC15 (1.5 grams of fibrinogen per bag) were chosen per round to target a goal of 4 grams of fibrinogen per round. FC15 was used rather than two bags of FC20 (2.25 grams of fibrinogen per bag) since FC15 is the most used dose in the USA. It is rare for hospitals to carry FC20. Twelve plasma donors are required to manufacture either two FC20 or three FC15. Institutions preferring a larger quantity of fibrinogen in a single bag have the option of the 6, 8, or 10 pools. The additional fibrinogen provided does not necessarily reflect typical transfusion patterns as fibrinogen is usually missing in the first rounds of MTP strategy in the USA. Many institutions, especially those outside of the USA, have very different approaches. The live version of the model can be configured to each institution's preference.
5 CONCLUSIONS
Models are a common tool in hospitals to show potential areas for improvement in health care protocols, treatment options, and economic implications. This model does not take into account the individual patient condition but provides a basic assessment of the hospital MTP, allowing both improved awareness of shortcomings in design and alerting clinicians to aspects that may require additional attention when minutes impact patient survival.
CONFLICT OF INTEREST STATEMENT
Nadia Keltner is an employee of Cerus Corporation and receives compensation and equity options as part of employment. Melissa Cushing is on the advisory boards for CSL Behring, Cerus Corporation, Haemonetics, and Octapharma. Melissa Cushing received research funding from Cerus Corporation and is a consultant for Octapharma. Thorsten Haas is on the advisory committees for Octapharma and CSL Behring. Philip Spinella is a consultant to Cerus and Hemanext, an advisory board member for Octapharma and Haima, and CMO and Co-Founder for Kalocyte.