Pooling, room temperature, and extended storage time increase the release of adult-specific biologic response modifiers in platelet concentrates: a hidden transfusion risk for neonates?
Abstract
BACKGROUND
Adult donor platelets (PLTs) are frequently transfused to prevent or stop bleeding in neonates with thrombocytopenia. There is evidence for PLT transfusion–related morbidity and mortality, leading to the hypothesis on immunomodulatory effects of transfusing adult PLTs into neonates. Candidate factors are biologic response modifiers (BRMs) that are expressed at higher rates in adult than in neonatal PLTs. This study investigated whether storage conditions or preparation methods impact on the release of those differentially expressed BRMs.
STUDY DESIGN AND METHODS
Pooled PLT concentrates (PCs) and apheresis PCs (APCs) were stored under agitation for up to 7 days at room temperature (RT) or at 2 to 8°C. The BRMs CCL5/RANTES, TGFβ1, TSP1, and DKK1 were measured in PCsʼ supernatant, lysate, and corresponding plasma. PLT function was assessed by light transmission aggregometry.
RESULTS
Concerning the preparation method, higher concentrations of DKK1 were found in pooled PCs compared to APCs. In supernatants, the concentrations of CCL5, TGFβ1, TSP1, and DKK1 significantly increased, both over standard (≤4 days) and over extended storage times (7 days). Each of the four BRMs showed an up to twofold increase in concentration after storage at RT compared to cold storage (CS). There was no difference in the aggregation capacity.
CONCLUSION
This analysis shows that the release of adult-specific BRMs during storage is lowest in short- and CS APCs. Our study points to strategies for reducing the exposure of sick neonates to BRMs that can be specifically associated to PLT transfusion–related morbidity.
ABBREVIATIONS
-
- ADP
-
- adenosine diphosphate
-
- APC(s)
-
- apheresis-derived platelet concentrate(s)
-
- BPD
-
- bronchopulmonary dysplasia
-
- BRM
-
- biologic response modifier(s)
-
- CCL5
-
- chemokine C-C motif 5 alias RANTES
-
- CRP
-
- C-reactive protein
-
- CS
-
- cold storage
-
- DKK1
-
- Dickkopf-related protein 1
-
- IL-6
-
- interleukin-6
-
- LoD
-
- limit of detection
-
- PPC(s)
-
- pooled platelet concentrate(s)
-
- RCT
-
- randomized controlled trial
-
- RT
-
- room temperature
-
- TGFβ1
-
- transforming growth factor β1
-
- TSP1
-
- thrombospondin 1
Thrombocytopenia and hemorrhage are frequent issues in very preterm or sick neonates.1 To prevent or to stop bleeding in neonates with thrombocytopenia, adult donor platelets (PLTs) are commonly transfused.2 However, neither a causal association between PLT count and bleeding risk nor a reduction of bleedings by prophylactic PLT transfusion could be confirmed in a recent systematic review.3 Most recently, the randomized controlled trial (RCT) on PLTs for Neonatal Transfusion (PlaNeT2) showed that among preterm infants with thrombocytopenia those who received a prophylactic PLT transfusion at a threshold of 50 × 109/L had a significantly higher rate of death or major bleeding than those neonates with a restrictive PLT transfusion threshold of 25 × 109/L.4 In another RCT on PLT transfusion for closure of a patent ductus arteriosus in preterm infants, liberal PLT transfusion thresholds were associated with a significantly higher rate of intraventricular hemorrhage.5 Thus, against common beliefs, clinical data indicate that PLT transfusions may indeed harm preterm and sick term neonates.6
The exact cause for the association between PLT transfusion and adverse outcomes is unknown. More generally, transmission of infectious diseases, transfusion-related lung injury, and transfusion-related circulatory overload were repeatedly discussed, especially in adult intensive care. In pediatric patients, PLT transfusion was associated with an increased incidence of allergic transfusion reactions when compared to adults.7 In neonates, PlaNeT2 showed increased rates of bronchopulmonary dysplasia (BPD) in infants randomly assigned to the high-threshold PLT transfusion group.4 The higher risk of hemorrhage and the increased rate of BPD associated with a PLT transfusion in infants suggest that adult donor PLTs lead to organ damage as consequence of disturbances in the developing hemostatic system and/or of reactive proinflammatory properties.8
The accumulation of various molecules released from donor PLTs, such as (pro-) inflammatory mediators, microparticles, membrane lipids, and biologic response modifiers (BRMs) has been theorized as cause for noninfectious adverse events after PLT transfusion. This theory is even more relevant for neonates, since certain BRMs were found at higher concentrations in adult than in neonatal PLTs. Such BRMs include chemokine C-C motif 5 (CCL5 alias Regulates on Activation Normal T cell Expressed and Secreted [RANTES]), Transforming growth factor β1 (TGFβ1), thrombospondin 1 (TSP1), and Dickkopf-related protein 1 (DKK1). These proteins are present in the PLTʼs alpha-granule9-12 and have the capacity to regulate recruitment and/or activation of various immune cells,13-17 inhibiting both megakaryopoiesis and angiogenesis.17, 18 Among these proteins, CCL5 has previously been studied in transfusion reactions,19-21 and both TGFβ1 and TSP1 have been found to be associated with BPD and retinopathy of prematurity.22 This raises the question of whether such BRMs are released from stored adult donor PLTs and if the release is impacted by PLT preparation (apheresis-derived PLT concentrates [APCs] or whole blood–derived pooled PLT concentrates [PPCs]) and storage conditions (time, temperature).
MATERIALS AND METHODS
PCs
Both PPCs, prepared by the buffy coat method, and APCs were leukoreduced and nonirradiated.23 PLTs were stored in clot-preventing additive solutions (Supplement S1, available as supporting information in the online version of this paper). PCs were limited to blood group O, while Rh factor status or sex were not considered. Analysis was started when the PCs would have been available for transfusion. Matched pairs of APCs and nonmatched pairs of PPCs were stored either at 22°C (room temperature [RT]) or at 2 to 8°C (cold storage [CS]), both with constant horizontal agitation. PCs were sampled on Days 1 (only APCs), 2, 3, 4, and 7.
Blood count and immature PLT fraction
Blood count variables, including mean PLT volume (MPV) and immature PLT fraction values, were determined by an automated hematology analyzer (SN-1000, Sysmex).
PC supernatants and lysates, analysis of IL-6 and C-reactive protein
Platelet supernatants were collected by centrifugation at 3000 × g for 10 minutes at RT and stored in aliquots at −80°C until enzyme-linked immunosorbent assays (ELISAs) were performed. Corresponding PLT lysates were prepared by an optimized freeze–thaw method.24 PC lysates were cleared from cellular components by centrifugation at 3000 × g for 5 minutes at RT and stored at −80°C. Interleukin 6 (IL-6) and C-reactive protein (CRP) concentrations were determined using the Elecsys IL-6 (on Cobas e801 analyzer) and CRP Gen.3 assay (on Cobas c701 analyzer, all Roche), respectively.
ELISA
Concentrations of CCL5, TGFβ1, TSP1, and DKK1 were determined in supernatants and lysates, as well as in plasma of the APC donor, using DuoSet ELISAs (R&D Systems). Absorbance was read with an microplate absorbance reader (iMARK, Bio-Rad). Samples were diluted in reagent diluent (Supplement S2, available as supporting information in the online version of this paper).
Light transmission aggregometry
For light transmission aggregometry, a fresh 600-μL aliquot of PC was centrifuged at 939 × g without brake for 7 minutes at 37°C. The pellet was resuspended in 1 mL of suspension buffer (Supplement S3, available as supporting information in the online version of this paper). The PLT count was measured with an analyzer (KX 21 N, Sysmex ), and suspension buffer was added to achieve a final PLT concentration of 250 × 109/L. Before testing, PLTs rested for 60 to 70 minutes at 37°C with manual agitation every 15 minutes. Light transmission aggregometry was performed using an aggregometer (PAP-4 series, Biodata Corporation) with collagen (final concentration 10 μg/L) and thrombin receptor-activating peptide 6 (TRAP6; final concentration 0.1 mmol/L, both from HART Biologica).
Swirling, pH, and sterility
To verify the quality of the PC, the presence of swirling was assessed and the pH was determined by soaking pH paper. Sterility of the PCs was evaluated after 7 days in an automated blood culture system (BacT/ALERT, bioMérieux).
Statistical analysis
Data were analyzed with computer software (SPSS Statistics 22, IBM Corp.). Two-way analysis of variance (ANOVA) was used to examine differences over time between the four groups and each condition and for comparing standard versus extended storage time. Longitudinal changes were examined by Friedmanʼs test (K-linked sample analysis). The t test was used to investigate differences in PLT count and MPV between conditions at each time point.
Ethical approval
Approval for the study was given by the institutional review board (EA2/152/17).
RESULTS
Swirling, sterility, and pH
At the initiation of the experiments, swirling was assessed in each PC. PCs stored at RT retained their swirling throughout the whole storage time, unlike CS PCs where no swirling could be observed from 24 hours of CS onward. A 7-day incubation in a blood culture system confirmed sterility in each PC on Day 7 of storage. In all PCs, pH values did not change from initiation with a mean (range) of 7.0 (6.5-7.3) to 6.9 (6.6-7.4) on the final trial day.
Blood count, MPV, and immature PLT fraction
To evaluate degradation or changes in the shape of PLTs during the entire storage time, PLT count and MPV were determined comparing both the methods of preparation and the different types and times of storage. Generally, the measured PLT counts and MPV were higher in PPCs than in APCs, that is, at both RT and CS and at all times (Table 1). Comparing time points of storage, significantly higher PLT counts and MPV were measured 24 hours after initiation of CS compared to baseline values in both CS PPCs and APCs, but not at RT. Both variables remained stable during the later course of storage. For CS PPCs and APCs, PLT counts and MPV also did not change after return to RT (data not shown). Baseline values varied 1) between different PPCs (2.5 ± 1.1%) and 2) between the APCs of the individual donors (1.5 ± 1.0%), but showed no significant changes over storage time (data not shown).
| Baseline (at initiation of storage) | 24 hours (after initiation of storage) | Day 4 | Day 7 | |
|---|---|---|---|---|
| A. PLT count | ||||
| RT APC | 821 ± 154 | 789 ± 105 | 777 ± 110 | 807 ± 109 |
| p value | NS | |||
| RT PPC | 1055 ± 263 | 1074 ± 300 | 1046 ± 282 | 1043 ± 252 |
| p value | NS | |||
| CS APC | 858 ± 118 | 989 ± 79 | 961 ± 137 | 962 ± 114 |
| p value | 0.004 | |||
| CS PPC | 1044 ± 138 | 1290 ± 121 | 1307 ± 187 | 1290 ± 130 |
| p value | <0.001 | |||
| B. MPV | ||||
| RT APC | 9.2 ± 0.5 | 9.1 ± 0.5 | 9.0 ± 0.4 | 9.1 ± 0.5 |
| p value | NS | |||
| RT PPC | 9.6 ± 0.4 | 9.8 ± 0.3 | 9.8 ± 0.3 | 10.1 ± 1.1 |
| p value | NS | |||
| CS APC | 9.2 ± 0.5 | 9.9 ± 0.6 | 10.0 ± 0.5 | 10.1 ± 0.7 |
| p value | 0.003 | |||
| CS PPC | 9.4 ± 0.2 | 10.3 ± 0.3 | 10.5 ± 0.4 | 10.9 ± 0.3 |
| p value | <0.001 | |||
- * Absolute values (A, ×109 /L; B, fL) are presented as mean ± SD, n = 6 in each group. Significance (p < 0.05) was determined by paired t test.
- NS = not significant.
IL-6 and CRP
IL-6 and CRP concentrations were measured to evaluate whether the amount of standard inflammatory markers varied depending on PC preparation method, storage time, or storage temperature. In 50% of specimens, the concentration of IL-6 (as early inflammatory marker protein) was found slightly above the limit of detection (LoD) of the assay (LoD 1.5 ng/L, range of measured values <1.5-3.6 ng/L). This finding was equally distributed between PPCs versus APCs and RT versus CS (Supplement S4, available as supporting information in the online version of this paper). In 96% of supernatants from PPCs, CRP (as unspecific late inflammatory marker protein) was present at concentrations above LoD (LoD, 0.3 mg/L; maximal value measured, 1.2 mg/L), while detectable CRP was found only in 20% of APC supernatants (Supplement S5, available as supporting information in the online version of this paper). This difference was significant (p < 0.001). However, the concentrations of both IL-6 and CRP were extremely low in all specimens (data not shown), suggesting lack of any clinical significance.
CCL5, TGFβ1, TSP1, and DKK1
CCL5, TGFβ1, TSP1, and DKK1 concentrations were determined to examine whether the putative release of BRMs from PLTʼs alpha-granules varies depending on the preparation method, storage time, or storage temperature. To reduce the influence of varying PLT counts, the protein concentrations were normalized to the initial PLT count of each PC. All four BRMs were measured at concentrations far above the LoD in both lysates and supernatants (Figs. 1-4), but below (TGFβ1, DKK1) or just above (CCL5, TSP1) the LoD in the donor's plasma (data not shown).

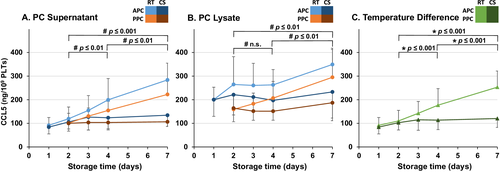
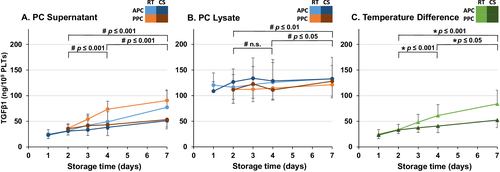
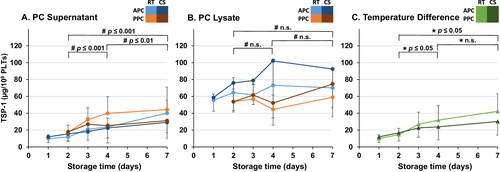
At the initiation (Day 2), only the concentration of DKK1 differed significantly with higher baseline concentration in PPCs than in APCs (Fig. 1A). Depending on the preparation method and the storage conditions, both in PC supernatants and in PC lysates, specific patterns of changes in CCL5, TGFβ1, TSP1, and DKK1 concentrations were observed. In PC supernatants, a significant increase of CCL5, TGFβ1, TSP1, and DKK1 was observed over both standard (Days 2-4) and extended (Days 2-7) storage times (Figs. 1-4).
At all time points, the mean concentration of all four BRMs was higher in lysate than in supernatant. In contrast to PC supernatant, the concentrations of CCL5 and TGFβ1 in PC lysates showed a significant increase only over extended storage time, but not during standard storage time (Figs. 2B and 3B). There was no significant change in the concentration of TSP1 in lysates of PCs (Fig. 4B). The concentration of all four alpha-granule proteins with higher expression in adult PLTs increased significantly more in RT PCs than in CS PCs over both standard and extended storage times (Figs. 1C-4C). Notably, the direct comparison between undetectable/very low BRM concentrations in the donorʼs plasma and those from PC supernatant and PLT lysate indicates that the BRMs were indeed released from the stored PLTs.
PLT function test
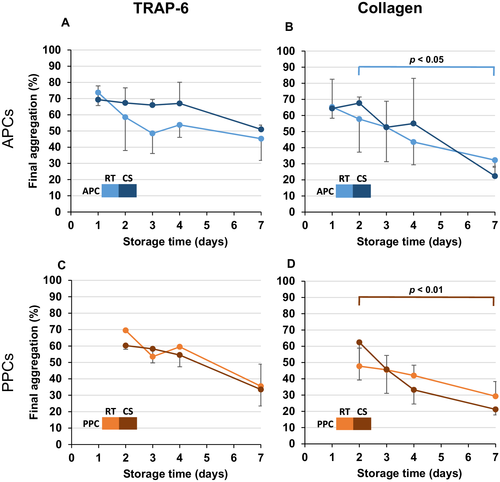
Light transmission aggregometry was used for monitoring PLT function. In CS, PCs PLT counts increased after at least 24 hours of CS (Table 1). During storage time, both APCs and PPCs showed a decrease of the final aggregation with both agonists tested (TRAP6 and collagen; Fig. 5), which was generally pronounced, but significant only if activation was performed with collagen. However, this phenomenon was obvious only in APCs stored at RT and in PPCs stored at 4°C (Figs. 5B and 5D). Since the type of PLT preparation had no effect on final aggregation (data not shown), the question of the impact of temperature on PLT function was addressed by comparing PLT function in RT versus CT APCs (Figs. 5A and 5B) as well as RT versus CT PPCs (Figs. 5C and 5D). However, there was also no significant difference in the aggregation depending on the storage at RT or at 2 to 8°C (Figs. 5E and 5F). In addition, adenosine diphoshate (ADP) was tested at different concentrations (3-20 μmol/L), but no aggregation was observed (data not shown).
DISCUSSION
Herein, we present the first systematic analysis on the release of BRMs during storage of PCs known to be expressed at higher levels in adult than in neonatal PLTs (CCL5/RANTES, TGFβ1, TSP1, and DKK1). Notably, the release of each of these BRMs changed specifically depending on the pretransfusion variables: 1) preparation method, 2) storage time, and 3) temperature. Concerning the preparation method, a significant difference in the initial concentration of the four BRMs was found only for DKK1. Particularly, APCs showed a significantly lower release of DKK1 than PPCs (Fig. 1A). The most robust findings for all four factors were 1) the increased release of the BRMs with storage time and 2) the higher concentrations in the supernatants of PCs stored at RT versus CS (Figs. 1-4). These experimental data argue for using rather fresh PLTs in transfusing preterm and term neonates. However, there is a continuous debate on whether transfusing fresh versus standard-issue blood products into critically ill pediatric patients reduces the incidence of new or progressive multiple organ dysfunction. Concerning red blood cell (RBC) transfusion, the recently published RCT “Age of Blood in Children in Pediatric Intensive Care Unit” (ABC-PICU) did not find a significant correlation between the storage length of the blood product and the clinical outcome variables.25 However, such RCT is currently not available for stored PCs.
Quantitatively, the most prominent impact of storage temperature was found when determining the concentration of CCL5 in supernatants (Fig. 2A). More generally, a previous study has also shown CS PCs to release less BRMs than RT PCs.26 However, each BRM analyzed in our panel deserved further attention regarding its biologic function and putative consequence for PLT transfusion in clinical neonatology.
The concentration of CCL5 increased by 10% during standard (Days 2-4) and by 17% during extended storage (Days 2-7) in CS, while the corresponding values for RT were 60 and 130%, respectively (Fig. 2A). The accumulation of CCL5 over storage time and the decreased release in CS found in this study are concordant to findings of previous studies.26-29 CCL5 is a proinflammatory chemoattractant released by a variety of cells, including PLTs.15 It attracts lymphocytes, neutrophils, and basophils among others and has also been shown to induce histamine release in basophils and degranulation of eosinophils.15, 30, 31 Due to these allergic properties, CCL5 has been investigated in association with allergic response(s) after PLT transfusion.19-21 Assuming that CCL5 was limited to the intravascular space and had a molecular weight of approximately 8 kDa,20 an adult 70-kg patient would be exposed to a final concentration of 1.6 or 1.1 nmol/L after being transfused with 250 mL (approximately 3 mL/kg body weight) of a 4-day-old RT or CS PC, respectively. In neonates, donor PLTs are commonly transfused with a volume of 15 mL/kg body weight,32 which would result in a fivefold higher CCL5 exposure of this patient group if PLTs were stored as RT and CS PC, respectively, resulting in 8.1 or 5.4 nmol/L in neonatal plasma.
Cold-stored PCs and APCs showed a significantly lower release of DKK1 than RT PCs and PPCs, both over standard and over extended storage (Fig. 1). DKK1 is a proinflammatory protein, a major suppressor of the Wnt/β-catenin signaling pathway and exerts important regulatory functions in white blood cell (WBC) infiltration and T-cell cytokine production.13, 33 DKK1 has also been shown to increase PLT-mediated release of inflammatory cytokines from endothelium.34 Such mechanism could also contribute to transfusion-associated morbidity in neonates.
Transforming growth factor β1 was also released less in CS PCs than in RT PCs (Fig. 3). The concentration of TGFβ1 increased 23% for standard and 58% for extended storage in the CS PCs, while the corresponding values for RT PCs were 81 and 148%, respectively. TGFβ1 is a predominantly anti-inflammatory protein involved in fibrosis, immune response suppression, angiogenesis, and inflammation.16 TGFβ1 is stored in its latent form and released from various immune cells, including PLTs upon activation, and can then be activated by different molecules. The half-life of activated TGFβ1 is only a couple of minutes. TGFβ1 is a potent inhibitor of megakaryopoiesis,18 and thus TGFβ1 could have counterproductive effects when being transfused to a thrombocytopenic neonate. Former studies investigating the concentrations of BRM in PCs have performed screenings with preloaded cytokine antibody arrays to determine which BRMs to further examine.26, 29 Considering the samples in these studies were not activated before measurement, the concentrations of TGFβ1 were very low or undetectable and TGFβ1 was not studied further.26, 29 However, the activation of TGFβ1 is required—as we did in our experiments—to reflect it endogenous biologic response that it would exert in the human body.
TSP1 is one of the molecules identified as an activator of TGFβ135 and was also released less in CS PCs compared to RT PCs (Fig. 4). TSP1 is a BRM with functions in both pro- and anti-inflammatory pathways, including regulation of migration, proliferation, cell adhesion, hemostasis, and growth factor activity.17 TGFβ1 and TSP1, as regulators of angiogenesis, have been suggested as potential protective mediators in BPD and retinopathy of prematurity.22 Thus, increased concentrations of TGFβ1 and TSP1 could contribute to the higher rate of BPD associated with liberal PLT transfusion thresholds in the PlaNeT2 trial.4, 8
More generally, we observed an early increase of PLT count in CS, which is not in line with the results of other authors who found a decrease over storage time.36-38 The observed increase in MPV in CS PCs was expected and reflects the CS-induced conformational change from discoid to spherocytic shape of the PLTs.36 There were also no changes in the very low numbers of RBCs and WBCs counted. The early increase of PLT counts in CS PCs is difficult to explain and is likely an artifact of counting in the fully automated blood analyzer. Moreover, the decrease in final aggregation by light transmission aggregometry reflects the known storage lesion dependent on glycolytic metabolism of stored PLTs.38, 39 CS may reduce this glycolytic metabolism leading to higher maximal aggregation.39 Multiple studies showed that PCs and whole blood stored at 4°C are hemostatically more effective than those stored at RT.40, 41 Such effect was not obvious in our analysis (Fig. 5). Interestingly, the releasate from TRAP6-activated PLTs was able to increase megakaryocyte proPLT formation, probably via CCL5.42
In neonatal intensive care, it should be considered, however, that CS PCs contain more microparticles than those stored at RT,26 which might be of concern since microthromboembolisms are accused of causing transfusion-associated necrotizing enterocolitis. Furthermore, it should be taken into account that in a humanized animal model CS, PLTs display an accelerated clearance from the circulation,43 predisposing them rather for treatment of acute bleeding than for prophylactic PLT transfusion in the thrombocytopenic neonate. In pediatric trauma patients with hemorrhagic shock, however, a recent clinical trial on the transfusion of CS versus RT whole blood PLTs showed no differences in posttransfusion PLT numbers and function.44
Development-specific differences between neonates and adults are evident both for PLT biology as well as plasmatic coagulation and hemostasis.45, 46 Transfusion of either adult PLTs into the neonatal plasmatic coagulation system or of adult plasma that reacts with neonatal PLTs seem to disturb the unique hemostatic balance in the neonate, particularly in the very preterm infant, resulting in a higher risk of (secondary) major hemorrhage. Our data strongly suggest that besides this development-specific mismatch on PLT function, the accumulation of BRMs with higher adult than neonatal expression could be causal or contribute to noninfectious adverse events after PLT transfusion.
There is currently no experimental evidence that a single BRM (at a certain concentration) can be responsible for PLT transfusion–associated morbidity (e.g. BPD), but this hypothesis needs to be tested in appropriate animal models. However, our study points to an easy strategy for reducing the exposure of sick neonates to BRMs with high adult expression levels by using the freshest PC available, potentially even stored in the cold.
ACKNOWLEDGMENTS
The authors appreciate the discussion of preliminary data on BRMs in adult donor platelets with the investigators of the Program Project Grant (2 P01 HL046925-16A1) “Neonatal Anemia and Thrombocytopenia: Pathophysiology and Treatment.” We thank Mirelle Möckel and Patricia Greiser (both Institute of Transfusion Medicine, Berlin) for their technical assistance.
AUTHOR CONTRIBUTIONS
LKS, OM, ZJL, CAB, RT, AP, MSV, and CD designed the research; MWdP, LKS, OM, HS, and RT performed the research; MWdP and LKS analyzed the data; MWdP, LKS, OM, and CD interpreted data; MWdP, LKS, OM, and CD wrote the manuscript; and all authors approved of the final version of the manuscript.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.