Comparative effectiveness of plasma prepared with amotosalen-UVA pathogen inactivation and conventional plasma for support of liver transplantation
Supported in part by funding from Cerus Corporation. ER received salary support provided through Association ARMESA.
Abstract
BACKGROUND
Liver transplant may require large-volume plasma transfusion with increased risk of transfusion-transmitted infection (TTI). Pathogen inactivation of plasma with amotosalen-UVA offers the potential to mitigate TTI risk.
STUDY DESIGN AND METHODS
A retrospective cohort design was used to compare the therapeutic efficacy and key safety outcomes for liver transplants supported with quarantine plasma (Q-FFP [reference]) or amotosalen-UVA plasma (IBS plasma [test]). The outcomes evaluated were volume of plasma, the numbers of red blood cell (RBC) components, and the total dose of platelets (PLTs) transfused during and 7 days after transplant. The safety outcomes were acute hepatic artery thrombosis (HAT) and mortality.
RESULTS
Transplantation and transfusion records for 212 Q-FFP transplants and 215 IBS plasma transplants were reviewed. Not all transplants required plasma; 161 received Q-FFP and 174 received IBS plasma. Among the transplants that required plasma, there were significant differences in median values between cohorts for delay to transplantation (p = 0.002), model end-stage liver disease score (p < 0.001), pretransplant hematocrit (p = 0.006), and graft cold perfusion time (p = 0.033). The median volumes of plasma transfused were not different for test and reference (2.160 L vs. 1.969 L, p = 0.292). Transplants in the test cohort required a mean of 3.7% more RBC components (p = 0.767) and on average a 16.5% increase in total PLT dose (p = 0.518). No significant differences were observed for the frequency of acute HAT or mortality.
CONCLUSION
In this retrospective study, IBS plasma provided therapeutic support of liver transplant not different from Q-FFP.
ABBREVIATIONS
-
- ANCOVA
-
- analysis of covariance
-
- EFS
-
- Établissement Français du Sang (French National Blood Transfusion Service)
-
- GLM
-
- general linear model
-
- HAT
-
- hepatic artery thrombosis
-
- IBS plasma
-
- pathogen inactivated fresh frozen plasma prepared by using the INTERCEPT Blood System for plasma referred to as test plasma
-
- LS mean(s)
-
- least-squares mean(s)
-
- MELD score
-
- model end-stage liver disease score
-
- PI
-
- pathogen inactivation
-
- Q-FFP
-
- quarantine fresh-frozen plasma referred to as reference plasma or conventional plasma
-
- TTI(s)
-
- transfusion-transmitted infection(s)
Improvements in surgical methods and perioperative patient blood transfusion management have reduced the use of blood components during liver transplantation.1 However, for many patients, plasma transfusion continues to be an important supportive therapy during liver transplantation. Some transplant patients may require transfusion with large volumes of plasma, resulting in multiple donor exposures with an increased risk of transfusion-transmitted infection (TTI). These risks may arise from pathogens that escape detection during the window period,2 undetectable pathogens due to low copy number,3 or emerging pathogens for which no tests are available.4-6 Treatment of plasma with amotosalen and UVA light inactivates a broad spectrum of blood-borne pathogens providing a means to mitigate the risk of TTI.7, 8
In some countries, plasma prepared with pathogen inactivation (PI) has been implemented to reduce the risk of TTI.9, 10 As different PI methods are introduced into routine clinical practice, it is relevant to assess the impact of these interventions on therapeutic efficacy and safety.11 Randomized clinical trials are inherently limited in size and the scope of the patient population included9 and may not fully characterize the experience in routine use for broad patient populations.12 Studies of comparative effectiveness during routine use can be informative to define the efficacy and safety of new types of blood components because they offer the opportunity to examine a larger experience.13
PI treatment of plasma with amotosalen and UVA light, IBS plasma (INTERCEPT Blood Systems, Cerus Corporation, Concord, CA) was introduced into routine practice in some countries in Europe for therapeutic support of patients with congenital and acquired coagulopathies and for therapeutic plasma exchange.10 A randomized clinical trial to evaluate the therapeutic efficacy and safety of IBS plasma for patients with the acquired coagulopathy of liver disease was conducted and included 51 patients undergoing liver transplant.14 Due to the limited number of liver transplant patients enrolled, the study had limited power to compare the effectiveness of conventional and PI plasma for this important subset of patients. Universal adoption of IBS plasma prepared with amotosalen and UVA light by the Établissement Français du Sang (French National Blood Transfusion Service [EFS], Alsace, France) in 2007 provided an opportunity to conduct a more extensive evaluation of the effectiveness of this novel plasma in comparison to conventional plasma in a regional liver transplant center. A multiyear retrospective review of plasma transfusion during liver transplantation was conducted to compare therapeutic efficacy and safety of pathogen inactivated and conventional plasma. Therapeutic efficacy was defined by the volume of plasma transfused, the number of red blood cell (RBC) components transfused, and the total dose of platelets (PLTs) transfused during and 7 days after transplant surgery. Safety outcomes were defined by the frequency of hepatic artery thrombosis (HAT) and mortality.
MATERIALS AND METHODS
Study design
This study was designed as a retrospective two-period cohort analysis to compare the effectiveness of IBS plasma (test) prepared with amotosalen and UVA light (INTERCEPT Blood System for Plasma, Cerus Corporation)7, 15 with conventional plasma prepared as quarantine fresh-frozen plasma (Q-FFP, reference). During both periods, all plasma components were prepared by EFS Alsace using approved standard operating procedures. Clinical data related to patient characteristics and clinical laboratory data were obtained by review of the transplant center clinical database. Data for the amounts of plasma, RBC components, and total PLT dose transfused were obtained from the EFS Alsace blood center database. This retrospective review was conducted under the auspices of the EFS Alsace hemovigilance program without patient informed consent in accordance with national procedures. Patient confidentiality was protected by use of coded study numbers using date of birth and date of transplant as unique identifiers for each transplant.
Transplants that entailed multiple organ transplants at the same time as liver transplant were excluded from analysis because they involve a greater level of surgical complexity than liver transplant alone. Transplants utilizing both types of plasma during the transitional period from Q-FFP to IBS-FFP were excluded. Repeat liver transplants performed more than 9 days after a prior transplant were analyzed as separate transplant events due to the elapsed period from prior exposure to study plasma. Repeat transplants within 9 days of the prior transplant were not analyzed for outcomes due to the proximity to the prior exposure to study plasma (test or reference) and the increased level of clinical confounding factors associated with early repeat transplantation. Contiguous review periods for the two cohorts were selected to minimize differences with respect to surgical team procedures and clinical transfusion practices. All liver transplant procedures that met the defined inclusion criteria and for which plasma transfusion was administered at surgery and during 7 days after the transplant procedure were included in the analyses.
The efficacy endpoints of the study were the volume of plasma, the number of RBC components, and the total dose of PLTs transfused during liver transplant and the 7 days after surgery. The volume of plasma transfused provides an integrated clinical endpoint indicative of hemostasis reflecting the clinical decision to transfuse plasma either for active bleeding or for coagulopathy associated with risk of bleeding.9 In the setting of the coagulopathy of liver disease, and especially during transplant, correction of abnormal clinical laboratory tests of hemostasis (prothrombin time or activated partial thromboplastin time) are not highly correlated with clinical hemostasis and frequently do not correct substantially after plasma transfusion.16-19
Outcome measures included transfusion use of RBC and PLT components during the same period of observation. Transfusion use of RBC components was selected as an outcome measure because it is partially indicative of blood loss due to bleeding and hemostatic efficacy during the intraoperative and perioperative period, although some bleeding may be the result of technical surgical complications that do not respond to plasma transfusion. The total dose of PLTs transfused during the observation period also was analyzed, but is of limited sensitivity due to declining use in liver transplant procedures.
The primary safety outcome measure was HAT within 9 days of transplantation. Mortality within 7 days of liver transplant was a secondary safety outcome measure. The observation period for mortality was limited to the exposure period to plasma, whereas the observation period for HAT was extended to 9 days to allow for follow-up for 2 days after the last plasma exposure during which time HAT could continue to evolve in the presence of transfused plasma. HAT is a relevant endpoint that is partly dependent on the balance between the procoagulant and antithrombotic function of plasma in patients with chronic liver disease.20, 21 Mortality was selected as a relevant secondary outcome measure because increased use of blood products has been associated with increased mortality.22-24
Statistical analysis
The distributions of all continuous baseline variables and endpoints were initially assessed for normality visually by histograms and QQ-plots. Additionally, the Shapiro-Wilk normality test was also used as a confirmation tool. In all cases, the results from the three assessments were consistent with each other, and the only variables resembling a normal distribution were the baseline hematocrit (Hct) and hemoglobin (Hb) levels. Due to the overwhelming skewness, tabulations for the baseline covariates were presented based on the median statistic, and furthermore, endpoints were log transformed and/or analyzed using a skewed distribution to ensure the validity of the subsequent modeling process. The differences between the cohorts with respect to the characteristics before liver transplant were presented by description of the treatment differences and the associated 95% confidence intervals (CIs). A bootstrap distribution was used to determine the 95% CI for the treatment difference of medians. The Wilcoxon rank-sum test was used to assess location shifts in distributions of data or for differences in medians if identically shaped distributions were assumed. Fisher's exact test was used for differences in categorical data.
For the modeling process of blood product utilization, treatment differences in plasma volume transfused, RBC components transfused, and total PLT dose transfused were analyzed using an analysis of covariance (ANCOVA) and/or a negative binomial general linear model (GLM). In both modeling procedures, potential confounding variables that could impact clinical hemostasis and the requirement for blood components were included as covariates. No variable selection procedures were adopted and each potential covariate was controlled for regardless of its significance.
Due to a highly skewed distribution, all endpoints were log-transformed and then analyzed using ANCOVA. Additionally, the use of RBC components and total PLT dose were also analyzed using a negative binomial GLM, which could accommodate for the skewness of the data along with the transplants that did not require transfusion of RBCs or PLTs. The covariates included in both models were selected on the basis of clinical relevance to coagulopathy or risk of bleeding in the setting of liver disease: patient demographics (age and sex), days of delay from decision to transplant to time of transplant, model end-stage liver disease (MELD) score,20 pretransplant Hb and Hct levels, pretransplant PLT count, pretransplant white blood cell (WBC) count, pretransplant hepatic function enzymes, and graft cold perfusion time. The MELD score (10 × [0.957 × ln serum creatinine + 0.378 × ln total bilirubin + 1.12 × ln INR + 0.643]) is informative about the severity of liver disease before transplant and was selected as opposed to the prothrombin time and INR alone because it correlates more highly with the overall severity of liver disease and risk of bleeding.25 For plasma, RBC, and PLT use, the treatment difference in least-squares (LS) means on the log scale (test – control) was estimated from the ANCOVA model adjusted for the aforementioned covariates. For RBC and PLT use, the log ratios of the treatment means (test/control) were also determined from the negative binomial GLM and were subsequently exponentiated to obtain the ratios in the raw scale.
To explore changes in the level and trend of plasma usage over time before and after the adoption of IBS, a graphical and segmented regression analysis based on an interrupted time series technique described by Wagner and colleagues26 was conducted. For the graphical analysis, the entire study period between January 2005 and December 2010 was partitioned into nonoverlapping time intervals that contained only transplants using the same type of study plasma. Once the intervals were constructed, the mean, median, and 25th and 75th percentiles were plotted to evaluate the volume of plasma transfused on a per-transplant level. For visualization purposes, the means and medians across the time intervals were connected to highlight potential temporal changes. The formal segmented regression analysis was then conducted as recommended by the authors. Note that across both applications, the analysis was constructed using time intervals of 6 months. However, due to the fact that the entire study period cannot be partitioned into evenly spaced, nonoverlapping time intervals that only summarize transplants using the same type of study product, inconsistencies in the duration of the first and last time intervals exist. The primary and secondary safety outcome measures, the incidence of HAT and mortality, were assessed via Fisher's exact test.
RESULTS
By review of the transplant center database, 441 liver transplants were identified at the University Hospital of Strasbourg during the period from January 1, 2005, through December 31, 2010, in the respective periods in which either Q-FFP or IBS plasma were used exclusively. Thirteen liver transplants (nine with Q-FFP and four with IBS plasma) were excluded from review due to multiple organs transplanted within the same operative procedure. One transplant was excluded due to exposure to both types of plasma during the transitional period from Q-FFP to IBS-FFP.
Pretransplant demographic and clinical characteristics
In total, 215 liver transplants in 211 patients were included in the IBS plasma (test) cohort over 40 months (September 1, 2007, through December 31, 2010) and 212 liver transplants in 207 patients were included in the conventional Q-FFP (reference) cohort in the 32-month period (January 1, 2005, through August 31, 2007). Nine patients (test, four; reference, five) received more than one liver transplant and were included in the analyses.
In the reference cohort, 51 of 212 (24.1%) transplant procedures did not require use of plasma during and within 7 days of the procedure. In the test cohort, 41 of 215 (19.1%) did not require use of plasma during and within 7 days of the procedure. The clinical indications for liver transplant were similar between test and reference cohorts for patients receiving plasma and those not receiving plasma (Table 1). Among transplants supported with plasma, there were more males in the reference cohort, but the proportions were similar between cohorts for the transplants not requiring plasma (Tables 2 and 3). Analysis of median pretransplant characteristics between the cohorts receiving plasma demonstrated potentially relevant differences in the following characteristics: delay to transplant from decision to transplant (p = 0.002), MELD score (p < 0.001), Hct (p = 0.006), and graft cold perfusion time (p = 0.033). For transplants not requiring plasma transfusion similar differences were observed between cohorts except for Hct (p = 0.488), which was not significantly different (Table 3).
| Patients requiring plasma | ||
| Indication | Test (n = 174)b | Reference (n = 161)b |
| Cirrhosis | 94 (54.0) | 68 (42.2) |
| Carcinoma | 43 (24.7) | 49 (30.4) |
| Hepatic failure | 15 (8.6) | 9 (5.6) |
| Retransplantation | 11 (6.3) | 12 (7.5) |
| Hepatitis | 7 (4.0) | 12 (7.5) |
| Other | 4 (2.3) | 9 (5.6) |
| Multiorgan failure | 0 (0.0) | 2 (1.2) |
| Patients not receiving plasma transfusion | ||
| Indication | Test (n = 41)c | Reference (n = 51)c |
| Carcinoma | 22 (53.7) | 25 (49.0) |
| Cirrhosis | 10 (24.4) | 12 (23.5) |
| Hepatic failure | 4 (9.8) | 1 (2.0) |
| Retransplantation | 3 (7.3) | 5 (9.8) |
| Other | 2 (4.9) | 7 (13.7) |
| Multiorgan failure | 0 (0.0) | 1 (2.0) |
- a Data are reported as number (%).
- b p value for differences using Fisher's exact test = 0.093.
- c p value for differences using Fisher's exact test = 0.389.
| Characteristics | Test | Reference | CIb | p valuec |
|---|---|---|---|---|
| Proportion male (%) | ||||
| Number | 63.8 | 72.0 | 0.5-1.0 | 0.128 |
| Age (years) | ||||
| Number | 174 | 161 | ||
| Median | 53 | 51 | −1.0 to 5.0 | 0.244 |
| 25th-75th percentile | 46-59 | 44-59 | ||
| Delay to transplant (days) | ||||
| Number | 174 | 161 | ||
| Median | 89.5 | 45 | 16.0-65.0 | 0.002 |
| 25th-75th percentile | 14-176 | 19-93 | ||
| MELD score | ||||
| Number | 164 | 147 | ||
| Median | 19 | 14 | 3.0-7.5 | < 0.001 |
| 25th-75th percentile | 14-27 | 10-21 | ||
| Hb (g/dL) | ||||
| Number | 138 | 154 | ||
| Median | 10.5 | 11 | −1.3 to 0.2 | 0.092 |
| 25th-75th percentile | 9.2-11.9 | 9.4-13.0 | ||
| Hct (%) | ||||
| Number | 128 | 153 | ||
| Median | 30.6 | 33 | −4.1 to −0.6 | 0.006 |
| 25th-75th percentile | 27.3-34.6 | 28.0-38.3 | ||
| WBC count (×109/L) | ||||
| Number | 138 | 154 | ||
| Median | 6.1 | 5.9 | −0.6 to 1.0 | 0.390 |
| 25th-75th percentile | 4.6-7.9 | 4.2-8.2 | ||
| PLT count (×109/L) | ||||
| Number | 138 | 154 | ||
| Median | 95.0 | 103.5 | −23.0 to 3.5 | 0.096 |
| 25th-75th percentile | 65.0-134.0 | 72.0-151.0 | ||
| SGOT (IU/L) | ||||
| Number | 138 | 153 | ||
| Median | 68.5 | 50 | 4.0-38.0 | 0.042 |
| 25th-75th percentile | 42.0-175.0 | 35.0-144.0 | ||
| SGPT (IU/L) | ||||
| Number | 138 | 153 | ||
| Median | 40.5 | 38 | −7.5 to 17.0 | 0.457 |
| 25th-75th percentile | 24.0-166.0 | 23.0-103.0 | ||
| Cold perfusion time (min) | ||||
| Number | 174 | 161 | ||
| Median | 567 | 590 | −62.5 to 2.0 | 0.033 |
| 25th-75th percentile | 515-660 | 516-729 | ||
- a Since the CIs and the p values were computed using different statistical techniques, inconsistencies between the results may exist.
- b 95% CIs for the treatment difference were computed using a bootstrap distribution.
- c p values for the treatment differences were computed using a Wilcoxon rank-sum test.
| Characteristics | Test | Reference | CIb | p valuec | |
|---|---|---|---|---|---|
| Proportion male (%) | 82.9 | 84.3 | 0.4-2.3 | 1.00 | |
| Age (years) | |||||
| Number | 41 | 51 | |||
| Median | 55 | 52 | −2.0 to 7.0 | 0.316 | |
| 25th-75th percentile | 46-61 | 48-57 | |||
| Delay to transplant (days) | |||||
| Number | 41 | 51 | |||
| Median | 187 | 86 | 23.0-147.0 | < 0.001 | |
| 25th-75th percentile | 89-260 | 44-129 | |||
| MELD score | |||||
| Number | 41 | 46 | |||
| Median | 11 | 9 | 0.0-4.0 | 0.006 | |
| 25th-75th percentile | 8-14 | 6-11 | |||
| Hb (g/dL) | |||||
| Number | 39 | 48 | |||
| Median | 12.9 | 13 | −1.7 to 1.0 | 0.901 | |
| 25th-75th percentile | 10.8-14.1 | 11.2-14.0 | |||
| Hct (%) | |||||
| Number | 38 | 47 | |||
| Median | 38.1 | 37.9 | −6.1 to 2.7 | 0.488 | |
| 25th-75th percentile | 30.6-41.4 | 34.0-41.9 | |||
| WBC count (×109/L) | |||||
| Number | 39 | 48 | |||
| Median | 5.9 | 6.1 | −1.1 to 1.1 | 0.871 | |
| 25th-75th percentile | 4.3-7.5 | 4.4-7.5 | |||
| PLT count (×109/L) | |||||
| Number | 39 | 48 | |||
| Median | 126 | 121.5 | −53.0 to 41.0 | 0.878 | |
| 25th-75th percentile | 88.0-197.0 | 79.0-191.0 | |||
| SGOT (IU/L) | |||||
| Number | 39 | 48 | |||
| Median | 54 | 52 | −24.0 to 51.0 | 0.413 | |
| 25th-75th percentile | 34-199 | 33-106 | |||
| SGPT (IU/L) | |||||
| Number | 39 | 48 | |||
| Median | 53 | 47 | −15.0 to 29.0 | 0.403 | |
| 25th-75th percentile | 27-233 | 27-95 | |||
| Cold perfusion time (min) | |||||
| Number | 41 | 51 | |||
| Median | 590 | 533 | 5.0-129.0 | 0.017 | |
| 25th-75th percentile | 535-675 | 465-603 | |||
- a Since the CIs and the p values were computed using different statistical techniques, inconsistencies between the results may exist.
- b 95% CIs for the treatment difference were computed using a bootstrap distribution.
- c p values for the treatment differences were computed using a Wilcoxon rank-sum test.
Efficacy outcomes for transplants receiving plasma
Plasma volume transfused
The raw distributions of the volumes of plasma transfused were skewed with a long tail toward high values (Fig. 1). Logarithmic transformation of the data exhibited good approximation to a normal distribution. Without any covariate adjustment, the median volume of plasma transfused over the 7-day period was 2160 mL for the test (IBS plasma) cohort and 1969 mL in the reference (Q-FFP) cohort (Table 4). With ANCOVA using log-transformed data including all covariates, the significant covariates were cold ischemia time (p < 0.001) and MELD score (p = 0.002). ANCOVA using log-transformed data demonstrated modest treatment differences without significance between treatment cohorts (p = 0.772) for the volumes of plasma transfused (Table 4).

Distribution of the volumes (mL) of test and reference plasma transfused to support liver transplants is shown. The median (thick black line), mean (Δ), interquartile ranges, and extreme values are indicated for test and reference cohorts.
| Variable | Test (n = 174) | Reference (n = 161) | Treatment difference (95% CI)b | p valuec |
|---|---|---|---|---|
| Median, 25-75th percentile | 2160, 1187-3666 | 1969, 1067-3298 | 191 (−124 to 757) | 0.292 |
| LS mean (log scale) | 3.295§ | 3.309d | −0.014 (−0.110 to 0.082) | 0.772 |
- a Treatment difference = test – control.
- b 95% CIs for the treatment difference were computed using a bootstrap distribution (for medians) and ANCOVA (for LS means).
- c p values for the treatment differences were computed using a Wilcoxon rank-sum test (for medians) and ANCOVA (for LS means).
- d Antilog values for test = 1972 mL, control = 2037 mL.
A segmented regression analysis using 6-month intervals (Fig. 2) demonstrated that for the first cohort (reference plasma), the mean plasma volume per 6-month segment increased slightly but not significantly (+179 mL per 6-month segment, p = 0.294). Immediately after adoption of IBS plasma, mean plasma volume transfused decreased (−606 mL, p = 0.385), and overall within the second cohort (test plasma), there was a nonsignificant increase with time (+6 mL per 6-month segment, p = 0.963). In summary, there was no significant linear effect of plasma dose transfused over time in either cohort and the difference between the mean volumes transfused also appeared nonsignificant between the reference (Q-FFP) and test (IBS plasma) groups (Fig. 2).
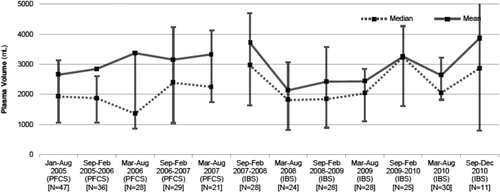
Segmental interrupted time series in 6-month intervals for the test and reference plasma transplant cohorts. Mean and median values for each segmented period are presented. Note that the initial period for the reference cohort (PFCS/Q-FFP) cohort and the final period for the test cohort (IBS) are respectively more and less than 6 months.
RBC components transfused
The distributions for the number of RBC components transfused were skewed to the right (Fig. 3). Among transplants supported with plasma, slightly more transplants (12/161, 7.5%) in the reference group than in the test group (5/174, 2.9%) did not require RBC transfusions (p = 0.080). Without any covariate adjustment, the median number of RBC components transfused was significantly different between the cohorts (p = 0.004; Table 5). With ANCOVA using the log-transformed numbers of RBC components transfused for all transplants receiving plasma, none of the pretransplant covariates were significant at the α = 0.05 level, and the estimated mean treatment difference (test – control) on the log scale was also not significant among transplants that required RBC components (p = 0.255; Table 5). Additional analysis using the negative binomial GLM including all transplants receiving plasma, with or without RBC component transfusion, demonstrated a log ratio of the treatment means (test/control) of 0.0363. Thus test transplants required 3.7% more RBC components, although this difference was also not significant, (p = 0.767; Table 5).
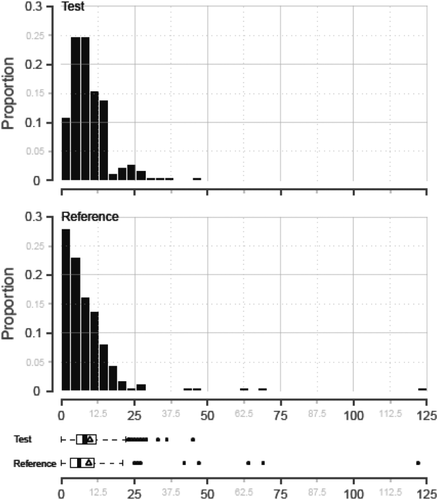
Distribution of the number of RBC components transfused to support liver transplants using test and reference plasma is shown. The median (thick black line), mean (Δ), interquartile ranges, and extreme values are indicated for test and reference cohorts.
| Variable | Test (n = 174) | Reference (n = 161) | Treatment difference/ratio (95% CI)b | p valuec |
|---|---|---|---|---|
| Median, 25-75th percentile | 8.0, 5-12 | 6.0, 3-11 | 2.0 (0.0-3.0) | 0.004 |
| LS mean (ANCOVA) (log scale) | 0.900§ | 0.846d | 0.053d (−0.039 to 0.146) | 0.255 |
| Log treatment ratio (GLM) (raw scale) | NA | NA | 0.0363e (−0.2040 to 0.2767) | 0.767 |
- a Treatment difference = test – control. Treatment ratio = test/control. Subjects without RBC transfusions were excluded from the ANCOVA analysis but included in the negative binomial GLM.
- b 95% CIs for the treatment difference and log treatment ratio were computed using a bootstrap distribution (for medians), ANCOVA (for LS means), and a negative binomial GLM (for the treatment ratio).
- c p values for the treatment difference and log treatment ratio were computed using a Wilcoxon rank-sum test (for medians), ANCOVA (for LS means), and a negative binomial GLM (for the treatment ratio).
- d Antilog values for test = 7.9, control = 7.0, and 1.1 for the treatment difference.
- e Treatment ratio (test/control) = exponentiated (0.0363) = 1.037.
Total PLT dose transfused
Among transplants supported with plasma, 63 of 161 (39.1%) in the reference cohort and 58 of 174 (33.3%) in the test cohort did not require PLT transfusions during transplant and through Day 7. The distributions for total dose of PLTs transfused were highly skewed (Fig. 4). For all transplants receiving plasma, the median total doses of PLTs transfused during the 7-day observation period were relatively small, but greater for test than reference (Table 6). The total dose of PLTs transfused was evaluated with the ANCOVA model using a log transformation while adjusting for all the pretransplant covariates in the model. With adjustment for the covariates, the LS mean of total PLT dose transfused remained significantly different between the cohorts (Table 6). The antilog of the mean treatment difference (test – control) in total PLT dose was 1.35 × 1011 PLTs, which is less than half a PLT component over the 7-day observation period. Because a substantial proportion of liver transplants required no PLT support, the analysis was repeated for all transplants, including those who required no PLTs, using the negative binomial GLM and with all covariates in the model. PLT count pretransplant was a significant covariate in the model (p < 0.001). The adjusted log ratio of the treatment means (test/control) was 0.1524 indicating that test transplants required a 16.5% greater total PLT dose than reference transplants, but this was not significant (p = 0.518).
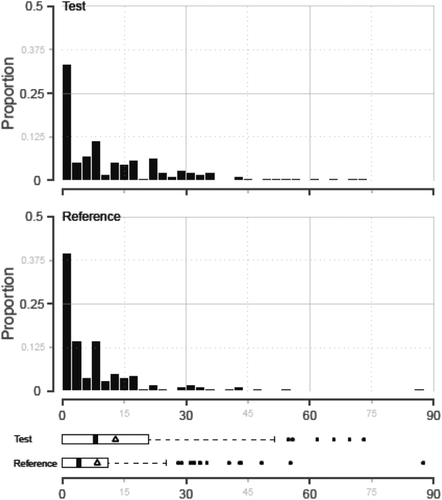
Distribution of the total PLT doses (×1011) transfused to support liver transplants using test and reference plasma is shown. The median (thick black line), mean (Δ), interquartile ranges, and extreme values are indicated for test and reference cohorts.
| Variable | Test (n = 174) | Reference (n = 161) | Treatment difference/ratio (95% CI)b | p valuec |
|---|---|---|---|---|
| Median, 25-75th percentile | 8.0, 0.0-21.0 | 4.0, 0.0-11.1 | 4.0 (0.8-5.7) | 0.005 |
| LS mean (ANCOVA) (log scale) | 1.144d | 1.015d | 0.129§ (0.013-0.245) | 0.030 |
| Log treatment ratio (GLM) (raw scale) | NA | NA | 0.1524e (−0.3099 to 0.6148) | 0.518 |
- a Treatment difference = test – control. Treatment ratio = test/control. Subjects without RBC transfusions were excluded from the ANCOVA analysis but included in the negative binomial GLM.
- b 95% CIs for the treatment difference and log treatment ratio were computed using a bootstrap distribution (for medians), ANCOVA (for LS means), and a negative binomial GLM (for the treatment ratio).
- c p values for the treatment difference and log treatment ratio were computed using a Wilcoxon rank-sum test (for medians), ANCOVA (for LS means), and a negative binomial GLM (for the treatment ratio).
- d The anti-log for test = 13.9 × 1011, control = 10.5 × 1011, and a treatment difference of 1.3 × 1011 PLTs.
- e Treatment ratio (test/control) = exponentiated (0.1524) = 1.165.
Use of RBC and total PLT dose transfused for transplants not requiring plasma
As noted, 41 transplants in the test cohort period and 51 transplants in the reference cohort period did not require plasma transfusion (Table 3). For transplants not requiring plasma support, the test cohort received a median of 2.0 RBC components (0.0-4.0, 25th-75th percentile) and the reference cohort received a median of 0.0 RBC components (0.0-3.0, 25th-75th percentile). The median total dose of PLTs transfused was 0.0 across both cohorts and the 25th and 75th percentiles were also 0.0 to 0.0.
Safety outcomes
As previously noted, both cohorts included a number of transplants for which no plasma was required. To avoid “diluting” potential safety signals by including patients who received no exposure to plasma, these transplants were excluded from the safety analyses. There was increased pretransplant morbidity in the test cohort compared to the reference cohort, as indicated by treatment difference in median values for delay to transplant (p = 0.002), MELD score (p < 0.001), and Hct (p = 0.006). Due to the retrospective design of this study, safety assessments were limited to two key outcomes: HAT within 9 days of transplant resulting in repeat transplantation and mortality within 7 days of initial exposure to plasma. Of note, exclusion of patients who did not receive plasma transfusions did not exclude any of the key safety events that were of interest (HAT and mortality), as all patients with these events received plasma.
HAT and mortality data were analyzed on a per-transplant basis and a per-patient basis for transplants and patients exposed to plasma. The incidence of HAT was not significantly different between the cohorts on a per transplant basis (4/174, 2.3% for test plasma vs. 8/161, 5.0% for reference FFP; p = 0.244) or on a per-patient basis (4/171, 2.3% test plasma vs. reference plasma 8/157, 5.1%; p = 0.242). Mortality within 7 days of transplantation was not different between the two cohorts (8/174, 4.6%) for test plasma versus reference FFP (6/161, 3.7%, p = 0.788) on a per-transplant basis or on a per-patient basis (8/171, 4.7% test plasma vs. reference FFP 6/157, 3.8%; p = 0.789).
DISCUSSION
This study was designed to compare the impact of PI treatment of plasma on the utilization of plasma and other blood components during and for 7 days after liver transplant. Potential limitations of the study design were the use of retrospective analysis, performance bias in ordering plasma, observer bias in reporting safety outcomes, possible variation in patient populations during the two observation periods, and changes in surgical practice. To minimize some of these potential limitations, we utilized two consecutive, contiguous observation periods within the same surgical center. Importantly, the treating surgeons did not control the decision to implement PI plasma; thus they had no opportunity for ordering bias in terms of competing types of plasma, and only one type of plasma was available during each cohort period.
Another potential limitation was the use of a surrogate measure, the volume of plasma transfused, rather than a direct measure of clinical hemostasis. However, the volume of plasma transfused provides an integrated measure of response to plasma transfusion therapy and is more precise than estimated blood loss, which can be affected by technical complications during a complex surgical procedure, and the clinical assessment of hemostasis may be subjective.9 Thus, we believe that the volume of plasma transfused provides a rational efficacy endpoint indicative of hemostasis. Relevant clinical covariates were used in the analyses to adjust for potential impact on the outcomes. Only a small number of transplants were excluded from the analyses due to multiple concurrent organ transplants as these patients have different risk factors than patients undergoing only liver transplantation. We believe that this was justifiable to make the model applicable to the majority of patients without inclusion of these infrequent highly complex surgical conditions. Although we excluded patients from the primary analysis who were not exposed to plasma transfusion, a substantial number of transplants remained in each cohort.
We observed significant differences between the two cohorts for some pretransplant characteristics: delay to transplant, MELD score, pretransplant Hct, and graft cold perfusion time. These differences were likely due to increased national demand for organ grafts between the two study periods resulting in increased morbidity due to longer delay until transplant. Notably, patients in the second cohort had significantly worse MELD scores. This increased morbidity in the later cohort also may be reflected in a slightly higher proportion of patients who required plasma support in the later cohort. Although pretransplant Hct levels were lower in the test cohort, this covariate did not exert a significant effect in the ANCOVA model for plasma volume transfused, and only the MELD score and cold perfusion time remained significant in the model. A recent study reported pretransplant Hb level as an important determinant for RBC transfusion requirements for liver transplant, but we did not observe this effect in our study.1
The period of observation in this study was different from other liver transplant studies because we examined usage during a 7-day period starting with surgery. We believe this is important as hemostasis in the immediate postsurgical period is partly dependent on plasma transfusion until the graft becomes functional. Of note, the volume of plasma transfused in this study was similar to that reported in a recent randomized controlled study in France that examined plasma use only during surgery.9 The prior study identified different significant pretransplant covariates than our study, but similar to our study included only those patients who received plasma. Interestingly, the numbers of RBC and PLT components transfused over 7 days in this study were similar to those transfused only during the surgical procedure in the prior study reported by Bartelmaos and colleagues.9
This study utilized two key safety outcomes: HAT and mortality. We extended the observation period for diagnosis of HAT to 9 days after transplant, 2 days after the last exposure to plasma, to include any delayed HAT events after exposure to transfused plasma. No significant differences in the frequencies of HAT were detected between the treatment groups despite worse MELD scores seen in the test group. Some types of PI plasma have been associated with increased risk of transplant-associated thrombosis, and the study by Bartelmaos and coworkers observed an increased number of thrombotic events with solvent/detergent plasma, which has been reported to contain lower protein S levels in some studies.9, 27 Recent data suggest that in chronic liver disease there is an increased risk of thrombosis, rather than hemorrhage, due to decreased resistance to thrombin generation mediated by altered thrombomodulin regulation.20, 21, 28
In summary, this retrospective study utilized two large cohorts undergoing liver transplant in routine practice and did not detect a difference in the use of plasma treated with PI technology (INTERCEPT Blood System) required to support liver transplantation compared to the volume of conventional plasma. In addition, use of RBC components was not substantially different between the cohorts. The unadjusted median total dose of PLTs in the IBS cohort was significantly greater than in the conventional plasma cohort. However, in both cohorts, a substantial portion of transplants did not require PLT transfusion support, and the total doses required during the 7-day observation period were small. When the negative binomial GLM was used, transplants without PLT transfusion were included in the analysis, and no significant difference between plasma cohorts in total PLT dose transfused was detected.
In this multiyear experience, IBS plasma prepared with a broad-spectrum PI process provided hemostatic support without a substantial increase in blood component utilization. The experience obtained in this review of routine practice liver transplant is consistent with the prior reported experience from the randomized controlled clinical trial.14 The cumulative experience with IBS plasma supports the therapeutic efficacy and safety of IBS plasma for transfusion of patients with acquired complex coagulopathy in a major surgical setting.
ACKNOWLEDGMENTS
JC directed the surgical teams that performed the transplant surgery, directed the collection of clinical data used to compile the database, and reviewed the manuscript and received research support to cover costs of data review and validation. DK contributed to the study design, directed the preparation and issue of blood components used in the study, directed the collection of data for blood components used in the study, and contributed to writing the manuscript. ER extracted clinical data from the transplant surgery database and entered the data into the database used for conduct of the data analyses. NH conducted the statistical analyses, and contributed to writing the manuscript. LC contributed to the study design, interpretation of the data analyses, and contributed to writing the manuscript. JPC contributed to the study design and interpretation of the data analyses and to the writing the manuscript and served on the Cerus speaker bureau and received honoraria for presentations unrelated to this manuscript.
CONFLICT OF INTEREST
NH is employed by Cerus Corporation. LC is employed by Cerus Corporation and has beneficial ownership of stock in Cerus. JC, DK, ER, and JPC have disclosed no conflicts of interest.