Time-course transcriptome and proteomic dynamics during the de novo shoot organogenesis in Chinese fir (Cunninghamia lanceolata)
SUMMARY
De novo shoot organogenesis (DNSO) enables plants to regenerate shoots from various explants, offering valuable opportunities for research and plant biotechnology applications. While significant progress has been made in understanding regeneration in angiosperms, the regulatory mechanisms in gymnosperms, particularly Chinese fir (Cunninghamia lanceolata), remain poorly understood, despite its importance as a key timber species in China. This study successfully established an efficient DNSO protocol for Chinese fir, identifying six distinct stages in the process through cellular-level analysis. Time-course transcriptome and proteomics analyses revealed dynamic changes in mRNA and protein levels during regeneration. Notably, proteins showed more significant alterations across a broad range of biological processes, often independent of corresponding mRNA changes. Key pathways associated with ethylene metabolism and abiotic stress responses were enriched, highlighting their critical roles in regeneration. Further experiments confirmed that moderate osmotic stress treatments (150 mm mannitol) and ethylene treatment (100 μm ACC and 5 μm AgNO3) substantially enhanced DNSO efficiency. In summary, this study uncovers the molecular mechanisms underlying Chinese fir DNSO, providing valuable insights into improving plant regeneration efficiency in this economically important species. These findings contribute to advancements in plant biotechnology and sustainable forestry practices.
INTRODUCTION
Plants exhibit a remarkable regenerative capacity, driven by the totipotency or pluripotency properties of their cells, allowing them to reconstruct tissues or organs and adapt to fluctuating environmental conditions (Ikeuchi et al., 2016; Ikeuchi et al., 2019; Sang et al., 2018; Shin et al., 2020). Three primary modes of regeneration have been identified in higher plants, namely tissue repair, DNSO, and somatic embryogenesis (Ikeuchi et al., 2019; Sun & Irvine, 2014). Among these, DNSO stands out as particularly significant in in vitro plant regeneration due to its simplicity and robustness (Duclercq et al., 2011; Shin et al., 2020). DNSO involves the in vitro formation of new shoots from cultured explants, making it a widely utilized technique in plant biotechnology (Shin et al., 2020). Its relative simplicity and efficiency have rendered it a preferred method for not only fundamental research in plant biology but also various biotechnological breeding approaches, such as in vitro micropropagation, haploid production, and genetic engineering (Duclercq et al., 2011; Sang et al., 2018).
In recent years, DNSO has been extensively studied in higher plants, with a particular focus on the model plants and crops. Considerable focus has been directed toward understanding the impact of phytohormones on DNSO processes, with particular emphasis on the opposing effects of auxins and cytokinins (CKs) on root and shoot regeneration (Ikeuchi et al., 2013). It is well established that a relatively low auxin/CK ratio is anticipated to induce shoot regeneration by provoking a series of coordinated steps for cell fate transition, including pluripotency acquisition, shoot pro-meristem formation, establishment of the confined shoot progenitor, and shoot outgrowth (Su & Zhang, 2014; Xu et al., 2006). The pluripotent property of callus is crucial for DNSO, and this process resembles the process of lateral root initiation in Arabidopsis (Ikeuchi et al., 2013). Multiple genes that are mainly expressed in the root primordium or root apical meristem, such as WUSCHEL RELATED HOMEOBOX 5 (WOX5), SHORT-ROOT (SHR), SCARECROW (SCR), PLETHORA 1 (PLT1), PLT2, and PIN-FORMED1 (PIN1) (Sena et al., 2009; Xu et al., 2006), and genes related to maintaining quiescent center properties, including QUIESCENT CENTER 25 (QC25), ROOT-CLAVATA HOMOLOG 1 (RCH1) and GLABRA 2 (GL2) (Ikeuchi et al., 2016; Mendez-Hernandez et al., 2019), are vital for callus pluripotency acquisition. Additionally, their upstream regulators, such as the histone acetyltransferase protein HISTONE ACETYLTRANSFERASE OF THE GNAT/MYST SUPERFAMILY 1 (HAG1)/GENERAL CONTROL NONREPRESSED 5 (GCN5) and the WOX11–LATERAL ORGAN BOUNDARIES DOMAIN 16 (LBD16) module, contribute to the pluripotency acquisition of developing callus by modulating the expressions of these genes (Kim et al., 2018; Liu et al., 2018; Servet et al., 2010). During the shoot pro-meristem formation stage, the spatial expression of No Apical Meristem/Arabidopsis thaliana activating factor/Cup-shaped cotyledon 2 (NAC) transcription factor genes, CUP-SHAPED COTYLEDON 1 (CUC1) and CUC2, induces polar localization of PIN1, determining the generation of shoot progenitors (Gordon et al., 2007; Pulianmackal et al., 2014). ENHANCER OF SHOOT REGENERATION 1 (ESR1)/DORNRÖSCHEN (DRN), which belongs to the AP2/ERF family, is involved in shoot pro-meristem formation by binding to the promoter of the CUC1 gene and then activating its expression (Banno et al., 2001; Gordon et al., 2007; Ikeda et al., 2006; Kirch et al., 2003). In addition, ESR2/DRN-LIKE (DRNL) which was the closest homolog of ESR1 and was transiently induced during the later stages of DNSO, could directly activate the expressions of CYCLIN D1;1, ARABIDOPSIS PHOSPHOTRANSMITTER 6 (AHP6), and CUC1 (Ikeda et al., 2006; Matsuo et al., 2011). The plant regeneration process is closely linked to the wounding response (Ikeuchi et al., 2013). The wound-responsive transcription factor WOUND-INDUCED DEDIFFERENTIATION 1 (WIND1) directly activates the expressions of ESR genes, connecting wound signaling to shoot regeneration (Iwase et al., 2011; Iwase et al., 2017). During the establishment of the shoot progenitor stage, the expression of WUSCHEL (WUS) plays a critical role in directing the shoot meristem program by suppressing cell division and elongation to maintain stem cell fate, thereby contributing to shoot progenitor formation (Wang et al., 2017; Zhang et al., 2017). The expressions of WUS are tightly regulated by multiple intricate signaling pathways, including DNA methylation (Ishihara et al., 2019; Li et al., 2011), histone modification (Li et al., 2011), type-B ARR that functions in the cytokinin signaling pathway (Dai et al., 2017; Leibfried et al., 2005), and the miRNA156-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) module (Fouracre & Poethig, 2019). Once the shoot progenitor is formed, it continues to grow, and genes involved in the shoot outgrowth, such as ARABIDOPSIS THALIANA HOMEOBOX GENE1 (ATH1), SAWTOOTH 1 (SAW1), SAW2, and TCP FAMILY TRANSCRIPTION FACTOR 10 (TCP10), are activated at this stage, contributing to the following shoot outgrowth (Caggiano et al., 2017; Koyama et al., 2007; Li et al., 2011).
Despite significant progress in elucidating the molecular network of DNSO in angiosperms, particularly in Arabidopsis, research focusing on gymnosperms—organisms that diverged from a common ancestor roughly 300 million years ago (Bowe et al., 2000)—is notably limited. This deep evolutionary separation has likely led to both shared and lineage-specific mechanisms governing regeneration. Compared to many angiosperms, gymnosperms—including conifers—are generally more recalcitrant to regeneration, with success rates highly dependent on species, genotype, and explant type. This recalcitrance is further complicated by their large and complex genomes (e.g., Pinus tabuliformis, ~24 Gb), which contain high proportions of repetitive sequences and pose challenges to gene annotation and functional genomics (Niu et al., 2022).
Although some embryogenesis-related genes in gymnosperms exhibit conservation with their angiosperm counterparts, studies suggest the existence of conifer-specific regulatory elements (Cairney & Pullman, 2007). In recent years, limited progress has been made in understanding the DNSO process in gymnosperms. For example, endogenous hormonal levels in Pinus pinea cotyledons have been shown to influence organogenic competence (Alvarez et al., 2020; Cortizo et al., 2009; Cuesta et al., 2009; Moncaleán et al., 2005; Valdés et al., 2001). Specific genes, such as PipiRR1, a type-A ARR gene, function in cytokinin signaling (Alvarez et al., 2012; Cortizo et al., 2010), and PipiCLV1L, a CLV gene implicated in shoot apical meristem (SAM) homeostasis, is upregulated during the DNSO in P. pinea (Alvarez et al., 2013). Additional meristem-related genes have also been characterized. For instance, orthologs of WUS and WOX5 in Pinus pinaster are expressed in the SAM and root apical meristem (RAM), respectively (Alvarez et al., 2018), and four class I KNOX genes (PpKN1-PpKN4) have been identified (Belmonte et al., 2007; Bueno et al., 2020; Guillet-Claude et al., 2004; Hjortswang et al., 2002; Sundås-Larsson et al., 1998). These genes are induced by cytokinin and are tightly coordinated with the endogenous balance of cytokinin and auxin to regulate SAM formation during regeneration (Alvarez et al., 2020). Despite these insights, the global transcriptional and translational dynamics underpinning regeneration in gymnosperms, especially in coniferous species, remain largely unknown. Comprehensive multi-omics studies are thus essential to unravel the unique regulatory networks governing shoot organogenesis in this evolutionarily distinct group of plants.
Chinese fir is an evergreen conifer belonging to the family Taxodiaceae and has become one of the most widely planted and economically important tree species in southern China (Xue et al., 2017). While recent progress has been made in establishing somatic embryogenesis protocols using immature zygotic embryos as explants (Hao et al., 2023), limited advances have been achieved to understand the molecular mechanisms governing regeneration in this species. With the development of modern transcriptomic and proteomic approaches—focusing on the identification and quantification of key pathways and proteins— these approaches offer a direct window into the functional executors of cellular processes (Campos et al., 2017). Such approaches are particularly valuable in uncovering mechanisms underlying morphogenetic competence during organogenesis (Heringer et al., 2018). Therefore, integrative transcriptomic and proteomic analyses represent a powerful strategy to dissect the regulatory landscape of DNSO in Chinese fir and advance our understanding of its developmental and physiological foundations.
In this study, we developed an efficient DNSO protocol for Chinese fir regeneration using cotyledons as explants. Detailed cellular analysis allowed us to divide the DNSO process into six distinct stages. We also performed transcriptomic and proteomic profiling to thoroughly investigate the transcriptional and translational dynamics during Chinese fir DNSO, revealing stage-specific regulation of numerous functional genes. Additionally, we examined the correlation between mRNA expression and protein levels, observing how this relationship evolves throughout the regeneration process. These findings not only deepen our understanding of plant regeneration but also offer practical tools for rapid clonal propagation and genetic improvement of Chinese fir, while providing a valuable resource for future research in gymnosperm regeneration and plant developmental biology.
RESULTS
Establishment of the DNSO protocol for Chinese fir
The development of the DNSO procedure for Chinese fir holds paramount significance in the fields of genetic engineering and the exploration of regeneration mechanisms. Thus, our initial focus was on establishing the conditions for Chinese fir DNSO. Previous reports showed that the DCR (De Fossard Conifer Regeneration) base medium stands out as the most effective medium for both direct and indirect organogenesis in conifer tissue culture (Fraga et al., 2023; Klimaszewska et al., 2005). In line with this, DCR medium was used in this study. Based on our preliminary investigations, cotyledons derived from the germinated Chinese fir seeds were used as explants. The phytohormone cytokinins, specifically 6-Benzylaminopurine (6-BA) and Thidiazuron (TDZ), demonstrated efficacy in facilitating Chinese fir regeneration. Therefore, we optimized the concentration of TDZ and 6-BA and tested their effects on inducing cotyledon regeneration. Our findings showed that TDZ substantially affected the frequency of shoot induction and the mean number of shoots (Table 1). Notably, an increase in TDZ concentration corresponded with a substantial enhancement in shoot induction efficiency. Particularly noteworthy is the observation that a concentration of 0.005 mg L−1 TDZ resulted in a remarkable 81.1% DNSO efficiency, yielding an average of 6.28 shoots per explant (Table 1). Additionally, we conducted experiments to assess the combined effects of TDZ and 6-BA. The results indicated that under lower TDZ concentration conditions (0.0005 mg L−1), the addition of 6-BA led to an augmentation in Chinese fir shoot induction. Conversely, in the context of elevated TDZ concentrations (0.005 mg L−1), the efficiency of DNSO displayed a reduction in tandem with an increase in 6-BA concentration (Table 2).
| Concentration of TDZ (mg L−1) | Frequency of shoot induction (%) | Mean number of shoots per explant |
|---|---|---|
| 0.0005 | 72.11 ± 0.94a | 3.89 ± 0.17a |
| 0.002 | 77.50 ± 1.77ab | 4.25 ± 0.17a |
| 0.005 | 81.10 ± 1.31b | 6.28 ± 0.11b |
- Note: Explants were cultured on DCR media supplemented with 6-BA (1 mg L−1) and NAA (0.2 mg L−1). Data are presented as the mean LSD and recorded after 8 weeks. n = 150–160. Letters indicate that the values are significantly different at P < 0.05 according to Duncan's multiple range test.
| Concentration of BA (mg L−1) | Concentration of TDZ (mg L−1) | Frequency of shoot induction (%) | Mean number of shoots per explant |
|---|---|---|---|
| 1.0 | 0.0005 | 66.85 ± 0.75e | 2.39 ± 0.09bc |
| 2.0 | 0.0005 | 62.96 ± 0.44cd | 2.95 ± 0.09bc |
| 3.0 | 0.0005 | 33.27 ± 0.91a | 1.68 ± 0.24ab |
| 1.0 | 0.002 | 62.77 ± 0.95c | 3.98 ± 0.25e |
| 2.0 | 0.002 | 66.72 ± 0.73de | 3.44 ± 0.13de |
| 3.0 | 0.002 | 35.52 ± 1.17a | 2.33 ± 0.10abc |
| 1.0 | 0.005 | 82.64 ± 0.96g | 7.28 ± 0.21g |
| 2.0 | 0.005 | 73.75 ± 0.34f | 4.93 ± 0.05f |
| 3.0 | 0.005 | 43.67 ± 0.32b | 1.61 ± 0.04a |
- Note: Explants were cultured on DCR media supplemented with NAA (0.2 mg L−1). Data are presented as the mean LSD and recorded after 8 weeks. n = 150–160. Letters indicate that the values are significantly different at P < 0.05 according to Duncan's multiple range test.
The pivotal role of auxin in stimulating DNSO within in vitro plant tissue cultures is widely acknowledged. Remarkably, an impressive average DNSO efficiency of over 80% was achieved, yielding an average of 6.84 shoots per explant using 0.1 mg L−1 1-Naphthaleneacetic acid (NAA) (Figure S1). Noteworthy is the discernible trend that, as the concentration of NAA increased, both the efficiency of shoot induction and the average shoot number exhibited a decline. For instance, at a NAA concentration of 0.3 mg L−1, the induction efficiency and the mean shoot number diminished to 66.14% and 3.08, respectively (Figure S1). Furthermore, a noteworthy outcome emerged at an NAA concentration of 0.5 mg L−1, where the induction of adventitious shoots was suppressed, concurrently resulting in the observation of adventitious roots (Figure S1). These results suggested that the introduction of exogenous auxin was not an essential requirement for shoot induction efficiency in Chinese fir. Conversely, the utilization of lower NAA concentrations contributed to the attainment of more robustly regenerated seedlings.
In summary, the most optimal shoot induction medium (SIM) for Chinese fir DNSO consisted of the DCR medium supplied with 3% sucrose, 0.42% phytagel, 0.1 mg L−1 NAA, 1 mg L−1 6-BA, and 0.005 mg L−1 TDZ. This formulation facilitated the induction of shoots from the explants (Figure 1a-i–vii). Following the shoot incubation (Figure 1a-viii) and rooting stages (Figure 1a-ix), the plants were successfully transplanted into soil and displayed vigorous growth (Figure 1a-x).

De novo shoot organogenesis in Chinese fir.
(a) Overview of the DNSO process in vitro. Panels (i–vii) show shoot formation from explants, (viii) shoot development, (ix) rooting, and (x) planting. Scale bars, 1 cm.
(b) Histological analysis of the shoot organogenesis process, with stages (i–vi) corresponding to six documented developmental phases. Red arrows indicate shoot meristems and shoot primordia. Scale bars, 1 mm.
In-depth cellular analysis of the Chinese fir shoots organogenesis process
To determine the developmental phases of Chinese fir organogenesis at the cellular level, we assayed the tissue structure using sample slices. Upon a comprehensive cellular-level analysis, the Chinese fir DNSO process was unfolded through six distinct stages (S1–S6, Figure 1b). We initiated the process with cotyledons obtained from germinated seeds, wherein the initial composition of these structures consisted of well-organized cells prior to their transfer to the SIM (S1, Figure 1b-i). After incubating on SIM for approximately 7 days, the cell structures underwent deformation. Mesophyll cells exhibited dynamic de-condensation, resulting in a shift toward rounded cell shapes with augmented cytoplasm volume and amplified nuclear dimensions, resembling characteristics of undifferentiated cells (S2, Figure 1b-ii). Following an additional week's growth, a significant portion of cells exhibited division activity. Characterized by compactly packed cytoplasm, these cells lacked distinctive cell types or evident tissue organization, displaying a more uniform morphology compared to differentiated cells (S3, Figure 1b-iii). Subsequently, cells underwent a reduction in size while maintaining dense packing, and a prevalent proliferation of cells was observed. This indicated the emergence of organized arrangements akin to early-stage shoot meristems, signifying the initiation of shoot pro-primordia approximately 7 days after S3 (S4, Figure 1b-iv). Following another week, the shoot pro-primordia continued to divide and differentiate. Cells exhibited elongation and organized configurations of dividing cells, leading to the development of shoot primordia (S5, Figure 1b-v). As the process advanced, protruding shoot structures emerged from the callus tissue, eventually evolving into leaf primordia. The progression continued to encompass various shoot components, including stems, leaves, and buds, each characterized by specialized cell types tailored for distinct functions (S6, Figure 1b-vi).
SMRT sequencing of full-length transcriptome of Chinese fir DNSO process
To generate a representative full-length transcriptome for Chinese fir DNSO process, we sequenced the mixed sample of all six developmental stages mentioned above with the PacBio HiFi sequencing platform. A total of 4,354,449 HiFi reads were obtained, and the average length of HiFi reads was 2093 bp. Among the total reads, 3,895,942 (89.47%) FLNC sequences were identified. After clustering, 237,509 consensus isoforms were obtained, while 237,445 were HQ consensus isoforms. Then, 137,369 non-redundant isoforms were gained after removing redundant isoforms and error correction from high-quality consensus isoforms. The length of these full-length transcripts mainly ranged from 1000 to 3000 bp, and the mean size was 1966 bp (Figure S2a). These isoforms were used for subsequent analysis.
CDSs were predicted using TransDecoder, resulting in 127,011 CDSs with an average length of 929 bp (Figure S2b). The average protein length was 310 amino acids (Figure S2c). Pathway classification and functional annotation of the isoforms were performed based on several databases, including NCBI non-redundant protein sequences (NR), Gene Ontology (GO), EuKaryotic Orthologous Groups (KOG), Kyoto Encyclopedia of Genes and Genomes (KEGG), SwissProt, and InterPro. More than 87% of the transcripts were annotated in SwissProt, with 111,290 transcripts (87.6%) in KOG, 104049 transcripts (81.9%) in GO, and 105,161 transcripts (82.8%) in InterPro (Figure S3). The species most closely related to Chinese fir were Cryptomeria japonica (30%), Picea sitchensis (15%), and Picea glauca (14%) (Figure S2d). A total of 115,483 isoforms were aligned to the KOG database. Among the 24 KOG categories, the largest group was ‘General function prediction only’ (14 379; 12.45%), followed by ‘Post-translational modification, protein turnover, chaperones’ (9133; 7.91%), ‘Signal transduction mechanisms’ (6466; 5.6%), and ‘Carbohydrate transport and metabolism’ (5560; 4.81%) (Figure S2e). Additionally, GO annotation was conducted across categories such as biological process, cellular component, and molecular function, encompassing 55 subcategories. In the biological process category, genes associated with cellular and metabolic processes were notably prevalent (Figure S2f). The full-length exome of Chinese fir generated here will be a comprehensive genetic resource for upcoming transcriptome and genetic analyses.
Transcriptional dynamics during the Chinese fir DNSO process
To unravel the dynamic changes at the transcriptome during DNSO of Chinese fir, RNA-seq analysis was conducted on samples across the six distinct developmental stages as outlined above. Pearson correlation coefficients (PCCs) were calculated using R software to evaluate the possible relationships among the 18 samples representing these six developmental stages (Figure S4). In total, short-read Illumina sequencing yielded an average of 49.54 million reads per sample. A substantial number of isoforms were generated across S1 to S6, with 42,510, 42,172, 44,483, 44,718, 44,475, and 43,968 transcripts were identified at each respective stage (Table S1). A core set of 35,554 transcripts was found to be consistently expressed across all stages, representing a common transcriptional landscape of the regeneration process (Figure 2a). Additionally, distinct transcripts were specifically expressed at individual developmental stages, with 502, 279, 444, 510, 451, and 453 transcripts unique to S1 to S6, respectively, suggesting their potential stage-specific functions at the corresponding stage (Figure 2a). The reliability of the RNA-seq findings was validated using qRT-PCR, targeting 12 DEGs associated with phytohormone signaling, stress response, and energy metabolism, and this validation underscores the accuracy of the RNA-seq results (Figure S5).
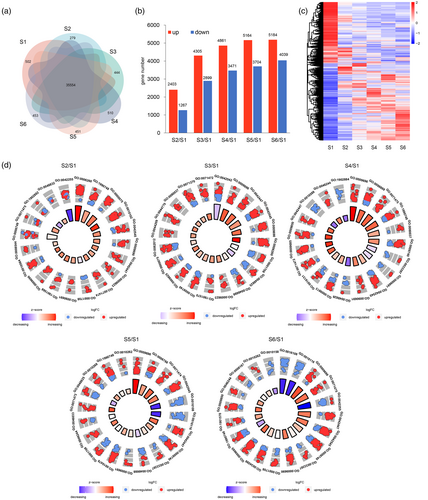
Transcriptomic profiles during Chinese fir DNSO.
(a) Venn diagram showing transcripts expressed across different developmental stages.
(b) Number of upregulated and downregulated transcripts relative to S1.
(c) Heatmap and hierarchical clustering of differentially expressed transcripts during Chinese fir DNSO.
(d) GO enrichment analysis of differentially expressed transcripts at each stage, .
A total of 14 017 transcripts are substantially activated or repressed during different developmental stages of Chinese fir DNSO (Table S2). Of these, 2403, 4305, 4861, 5164, and 5184 transcripts were upregulated, while 1267, 2899, 3471, 3704, and 4039 transcripts were downregulated at S2-S6, respectively, compared to S1 (Figure 2b,c). GO enrichment analysis indicated that key processes, including the salicylic acid metabolic process, hormone catabolic process, cell wall modification, cytokinin metabolic process, and cellular detoxification, were consistently enriched throughout the entire Chinese fir regeneration process (Figure 2d, Table S3). Specific insights were gleaned from individual stages. S2 was characterized by enriched genes related to epidermis development, lateral root morphogenesis, regulation of the cell cycle process, and DNA replication, suggesting active cell division at this stage (Figure 2d). At S3, in addition to cell cycle-related genes, there was a notable increase in genes associated with the cellular response to gibberellin stimulus, AMP salvage, and multidimensional cell growth. This suggests that gibberellin stimulus may have induced cell cycle-related processes involved in cell development (Figure 2d). S4 exhibited enhanced expression of genes involved in plant organ boundary formation and metabolic processes for salicylic acid (SA), zeatin (ZT), brassinosteroids (BRs), gibberellin (GA), and auxins. This suggests enhanced multidimensional cell growth and organ formation, influenced by various phytohormones during this stage (Figure 2d). S5, associated with promodia formation, exhibited an enrichment of genes related to somatic embryogenesis, brassinosteroid homeostasis, AMP metabolic process, and brassinosteroid metabolic process (Figure 2d), suggesting a shift from meristematic tissues to nutrient meristems and corroborating with the cytological phenotypes of bud formation at this stage, probably regulated by BR and AMP pathways. In S6, which indicates continued shoot development, there was an enrichment of genes linked to cotyledon morphogenesis, somatic embryogenesis, and organ boundary specification between lateral organs and the meristem (Figure 2d). These findings highlight the capacity of Chinese fir to swiftly reprogram its transcriptome, facilitating new shoot regeneration.
Phytohormones play a pivotal role in plant regeneration, and here we identified 144 auxin-related genes (Figure S6, Table S4); key genes related to auxin biosynthesis, such as YUCCA (YUC5 and YUC9) were consistently upregulated, and the genes involved in the auxin signaling pathway were also identified, such as receptor TIR1, which exhibited stage-specific induction in S2 and S6, suggesting the signals are enhanced in proliferated callus and shoot development. Signaling genes AUX/IAA, 8, 10, and 27 showed strong upregulation in the early stage (S2-S3), coinciding with shoot meristem establishment, and response genes ARF6, 17, and 18 showed substantial upregulation. Moreover, auxin transporters, such as transporter-like protein 1 (LAX1), LAX4, and PIN-Likes 7, displayed stage-specific induction, particularly in S4–S6, marking the SAM development in these stages (Table S4). In addition, the regeneration process involves a complex interplay of various regulatory factors, such as the WUS-related genes (WOX1, WOX4, WOX6, WOX8); core regulators of stem cell maintenance (SCR, SCL6, SCL32, PAT1, CUC2, TCP3, TCP4, and TCP8); and the transcription factors (SPL8, LBD4, LBD 15, LBD 29, LBD 6, LBD 11), and CYCD 3–3, DOF1.7, DOF 3.5, DOF 5.3, DOF 5.6 were also identified during Chinese fir shoot organogenesis (Table S5), highlighting the intricate regulatory network involved in this developmental process. Cytokinin regulation was highlighted by 94 genes (Table S6). Among these, cytokinin dehydrogenase (CKX) genes, including CKX1, CKX3, CKX7, CKX9, and CKX11, along with 2 cytokinin hydroxylase (CYP735A1 and CYP735A2) genes, exhibited upregulation. Moreover, genes related to cytokinin signaling, such as histidine kinase 4 (AHK4), were rapidly induced after transfer onto SIM, and two-component response regulators (ARR6, ARR9, RR4, RR9, RR22, RR23) showed gradual downregulation during Chinese fir DNSO, indicating the repression of cytokinin signals was substantially decreasing. Jasmonic acid (JA) influence was evidenced by 85 genes, including key genes involved in JA synthesis, such as 3 allene oxide synthase genes (AOS) and 3 OPDA reductase genes (OPR11, OPR3 and OPR7) (Table S7). Additionally, 43 BR-, 44 GA-, and 54 SA-related genes exhibited differential expression (Tables S8–S10), suggesting the involvement of these phytohormones in the complex regulation of Chinese fir DNSO.
To deepen understanding of the functional implications during Chinese fir DNSO, DEGs were grouped into 15 clusters using K-means clustering analysis, and GO enrichment and transcription factor analysis for each cluster were conducted (Figure 3a). C12, C14, C11, C8, C3, and C2 were substantially upregulated from S1 to S6, respectively (Figure 3a). C12, enriched in C2H2 and ERF transcription factors, were associated with processes like DNA recombination, osmotic stress response, and vascular pattern formation (Figure 3a,b). C14, peaking at S2, consists of ERF, C2H2, and WRKY transcription factors, involved in ABA metabolism, lateral root formation, and copper ion response (Figure 3a,b). C11, enriched in bHLH and ERF transcription factors, was linked to ethylene biosynthesis and cell morphogenesis regulation (Figure 3a,b). C8, active at S4, includes bHLH, WRKY, and C3H transcription factors, and was associated with meristem development, cell regulation, and flavonoid biosynthesis (Figure 3a,b). C3, enriched in NAC and ERF transcription factors, was involved in ovule morphogenesis and wound healing (Figure 3a,b). C2, enriched in C2H2, ERF, and C3H TFs, regulated organ growth, stem cell differentiation, and hormone transport (Figure 3a,b, Table S11).
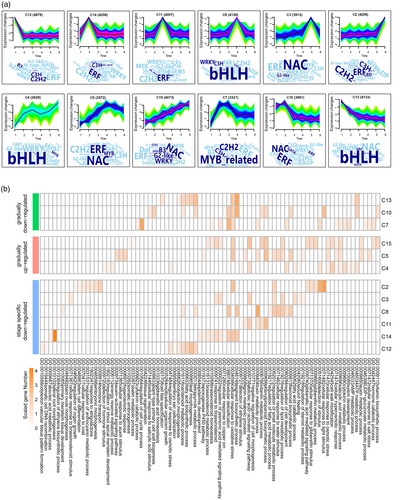
K-means clustering and enrichment analysis of transcript expression.
(a) Selected K-means clusters representing transcript expression patterns across six developmental stages, with transcription factors identified for each cluster.
(b) GO enrichment analysis for gene clusters of stage-specific, continuous upregulation, or continuous downregulation patterns during Chinese fir DNSO.
Clusters 4, 5, and 15 exhibited consistent upregulation during Chinese fir DNSO and were enriched with bHLH, ERF, and NAC transcription factors (Figure 3a). These clusters were linked to hormone stimulus detection, shoot apical meristem development, auxin signaling, hyperosmotic response, salicylic acid metabolism, and somatic embryogenesis (Figure 3b, Table S11), highlighting their roles in DNSO. Conversely, clusters 7, 10, and 13 exhibited consistent downregulation, involving genes related to cytokinin catabolism, cell cycle regulation, tissue regeneration, salicylic acid response, cell wall biogenesis, gibberellic acid homeostasis, and cell fate specification (Figure 3a,b, Table S11). Overall, these results emphasize the complex regulatory network underlying Chinese fir DNSO.
Proteomics dynamics during the DNSO of Chinese fir
To detect protein dynamics during Chinese fir regeneration, we employed Data-Independent Acquisition (DIA) technology, identifying a total of 137,835 peptides corresponding to 14 558 proteins. Across S1 to S6, the numbers of identified proteins were 10 021, 10 015, 10 013, 10 025, 10 014, and 13 784, respectively (Table S12). As indicated by the PCC analysis, the reproducibility of the proteomic analysis across most biological replicates was high (Figure S7). The Venn diagram showed that a substantial number of proteins were uniquely expressed at each developmental stage. Specifically, 657, 24, 16, 18, 14, and 32 proteins were exclusively expressed at S1 through S6, respectively (Figure 4a). Furthermore, compared with S1, a total of 4905 differentially expressed proteins (DEPs) at S2, 5641 DEPs at S3, 6123 DEPs at S4, 6505 DEPs at S5, and 6417 DEPs at S6 were identified (Figure 4b,c, Table S13). Interestingly, there were more downregulated proteins than upregulated proteins among these DEPs (Table S13). The GO enrichment analysis results revealed substantial involvement of proteins in ATP metabolism, tetraterpenoid metabolism, auxin biosynthesis, detoxification, fatty acid biosynthesis, growth regulation, the tricarboxylic acid cycle, and vitamin metabolism during regeneration (Figure 4d, Table S14). Notably, detoxification and AMP metabolic processes were upregulated from S2 to S6, while ATP metabolism showed increased enrichment from S3 to S6 (Figure 4d, Table S14), highlighting the energy demands of regeneration. Additionally, proteins related to embryonic body morphogenesis and multicellular growth were consistently upregulated throughout the regeneration process.
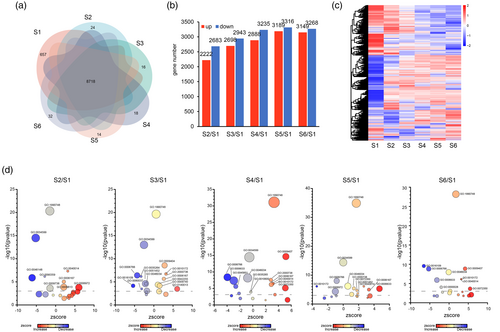
Proteomic data analysis during Chinese fir DNSO.
(a) Venn diagram illustrating protein expression across stages.
(b) Number of upregulated and downregulated proteins compared to S1.
(c) Heatmap and hierarchical clustering of differentially expressed proteins during Chinese fir DNSO.
(d) GO enrichment analysis of the different expressed proteins at each stage, .
Rapid plant growth and organogenesis are energy-intensive processes, necessitating robust cellular energy metabolism. In this study, genes and proteins involved in the ATP metabolic and AMP metabolic processes were substantially enriched during Chinese fir DNSO. Notably, proteins such as ATP Synthase (ATPb, ATP6), ATP-dependent 6-phosphofructokinase (PFK2, PFK3 and PFK5), and isocitrate dehydrogenase (IDH) were markedly upregulated, suggesting enhanced ATP biosynthesis and flux through the TCA and glycolytic pathways to meet the energy demands of morphogenesis. In contrast, key enzymes involved in AMP catabolism, including Aspartokinase 1 (AK1) and AMP deaminase (AMPD), were substantially downregulated. This pattern likely reflects a metabolic shift toward the accumulation and conservation of energy-rich intermediates, supporting the energy requirements for subsequent stages of shoot development.
To determine the protein accumulation status at a specific stage of Chinese fir DNSO, we performed K-means clustering analysis. Enrichment analysis of transcription factors and GO was performed to understand the biological processes underlying each cluster (C1 to C6) (Figure 5a–c, Table S15). C1 exhibited the highest expression at S1 (Figure 5a), enriched with ERF, C3H, C2H2, and FAR1 transcription factors, associated with auxin metabolism, stem cell differentiation, root development, and DNA demethylation (Figure 5b,c, Table S15). C2 exhibits upregulation at S2 and S3 (Figure 5a), and is characterized by ERF, C3H, and bZIP transcription factors, involved in cellular hyperosmotic response, multicellular organism growth, mitotic cell cycle phase transition, and ethylene-activated signaling pathways (Figure 5b,c, Table S15), indicating that ethylene may potentially regulate the cell cycle and growth during these stages. C3 exhibited gradual upregulation (Figure 5a), enriched with ERF, MYB, and LBD transcription factors, linked to embryonic pattern specification, stem vascular tissue formation, and organ growth (Figure 5b,c, Table S15), suggesting that enhancing stress response contributes to embryonic and vascular tissue development. In contrast, C4 showed a decreasing trend from S1 onwards (Figure 5a). This cluster is enriched with C2H2 and bZIP transcription factors (Figure 5b) and is involved in regulating secondary cell wall biogenesis, brassinosteroid-mediated signaling pathways, somatic cell DNA recombination, salt response, auxin import, cytokinin metabolic processes, and shoot apical meristem development (Figure 5c, Table S15), suggesting that cytokinin, brassinosteroid, and auxin pathways are crucial at the initial stages and may contribute to the following shoot apical meristem development. C5 showed low expressions at S2 and S3 (Figure 5a), enriched in C2H2, ERF, and C3H transcription factors (Figure 5b). These proteins are linked to cytokinin transport, hormone metabolism and signaling, histone H3-K4 demethylation, tissue regeneration, and negative regulation of the mitotic cell cycle (Figure 5c, Table S15), indicating that negative regulation of the cell cycle is less active in early stages, while hormone signaling becomes prominent later. C6 showed low expression at S1 (Figure 5a) and included NAC, ERF, and WRKY transcription factors (Figure 5b). These proteins are primarily involved in DNA methylation, brassinosteroid homeostasis, cellulose biosynthesis, histone H3-K9 demethylation, and nitrogen utilization regulation (Figure 5c, Table S15). This suggests that brassinosteroid metabolism remains active throughout the entire DNSO process in Chinese fir. Additionally, pathways like fatty acid biosynthetic process, ATP transport, regulation of response to osmotic stress, cellular response to oxidative stress, salicylic acid-mediated signaling pathway, and root meristem growth were also enriched in all expression profiles (Figure 5, Table S15), highlighting their critical roles in regeneration. These findings reveal the dynamic proteomic landscape during Chinese fir DNSO.
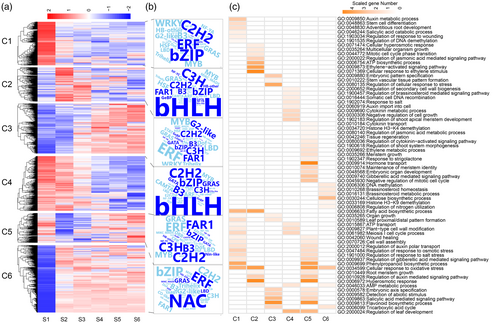
K-means clustering and enrichment analysis of protein expression.
(a) K-means clustering analysis of differentially expressed proteins.
(b) Transcription factor enrichment for each cluster.
(c) GO enrichment for clusters of transcription factors across different developmental stages.
Proteins involved in auxin and cytokinin signaling were also identified during the Chinese fir DNSO. Consistent with transcriptomic data, key auxin-related components such as TIR1, Gretchen Hagen 3 (GH3.5, GH3.6, GH3.17), and the auxin transporter PIN1 were substantially upregulated during the early stage (S2-S3) (Table S23), suggesting their involvement in initiating the shoot apical meristem development. In parallel, the cytokinin biosynthesis genes, such as LONELY GUY (LOG3 and LOG7) were upregulated, along with cytokinin signaling genes, including AHK4 and RR9, which also showed a consistent expression pattern at the transcriptional level (Table S23). These results underscore the critical regulatory roles of auxin and cytokinin signaling during the initiation and progression of shoot regeneration in Chinese fir.
Integrated transcriptome and proteome analysis
The relative contribution of transcriptional versus translational regulation in controlling gene expression has been the subject of intense debate, and few studies were performed in gymnosperm species. To address the question in a dynamic Chinese fir DNSO system, we performed a combined analysis of DEGs and DEPs over the six stages, with a total of 18,815 genes commonly detected at both the transcript and protein levels (Table S16). Nine-quadrant scatterplot association analysis at each stage was performed, and categories showing the correlation status across the Chinese fir DNSO process were generated, and then the following functional enrichment analyses were performed.
The first category represents genes exhibiting concordant increases (Quadrant 3) or decreases (Quadrant 7) in both transcript and protein levels (Figure 6, Table S16), suggesting that these changes in mRNA levels strongly determine protein dynamics during the Chinese fir DNSO process. Compared to S1, there are 1349, 1689, 1964, 2122, and 2186 genes that showed a concordant increase (Figure 6a). In this quadrant, we observed a strong enrichment of genes involved in key biological pathways, such as plant phytohormone responses, including auxin, cytokinin, gibberellin (GA), salicylic acid (SA), and brassinosteroids (BR), with genes involved in abiotic stress responses being highly expressed at both the transcriptional and translational levels during early stages (S1 to S3) (Figure 6b, Table S17). At later stages (S3 to S6), genes related to plant development, such as embryonic morphogenesis and stem vascular tissue pattern formation, became substantially enriched (Figure 6b, Table S17). Moreover, key genes involved in cell reprogramming and meristem formation, such as LBD40 and DOF5.7, as well as the ethylene biosynthesis gene ACS3, might be a core regulatory gene set during Chinese fir DNSO. Conversely, in Quadrants 7, 741, 1126, 1250, 1438, and 1896 genes displayed concordant decreases in mRNA and protein from S2 to S6, respectively (Figure 6a), which might be influenced by epigenetic silencing. In addition, these genes are predominantly linked to metabolic processes, including xanthophyll, tetrapyrrole, porphyrin-containing compounds, pigment, and chlorophyll metabolism (Figure 6b, Table S17), indicating that the dynamics of these chemical compounds play a crucial role in the Chinese fir DNSO process. Additionally, we observed that genes involved in abiotic stress, such as wound healing, defense response, and oxidative stress, were substantially enriched at various stages of the DNSO process (Figure 6b, Table S17). The genes that negatively regulate regeneration, such as RR9 and methyltransferase 1 (MET1) were distributed in this quadrant, suggesting that the regeneration inhibitors were silenced during Chinese fir DNSO.
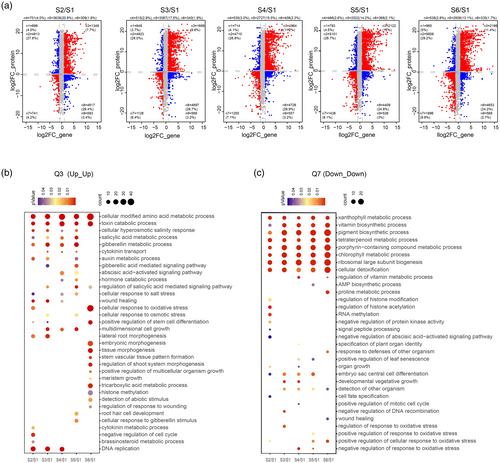
Integrated analysis of proteomic and transcriptomic data.
(a) Nine-quadrant plots illustrating the relationship between mRNA and protein expression across different stages compared to Stage 1. The ratios of DEPs and DEGs were classified into nine quadrants, grouped into four main categories based on the direction and correlation of mRNA and protein expression changes. Category I includes gene–protein pairs with concordant expression trends, where both mRNA and protein levels were either upregulated (quadrant 3) or downregulated (quadrant 7). Category II represents discordant expression, where mRNA and protein levels changed in opposite directions—either mRNA was downregulated while protein was upregulated (quadrant 1) or mRNA was upregulated while protein was downregulated (quadrant 9). Category III consists of cases where significant changes occurred at the protein level, but mRNA levels remained stable (quadrants 2 and 8). Category IV includes gene–protein pairs in which the mRNA levels were significantly altered while protein levels showed no corresponding change (quadrants 4 and 6).
(b) GO enrichment for Q3 (mRNA and protein upregulated).
(c) GO enrichment for Q7 (mRNA and protein downregulated).
The second category comprises genes displaying opposing trends: mRNA levels decrease while protein levels increase (Quadrant 1), or mRNA levels increase while protein abundance decreases (Quadrant 9). This category contains a relatively smaller number of genes. A total of 698, 648, 714, 793, and 965 genes showed an increase in mRNA levels while their corresponding proteins were downregulated from S2 to S6 compared to S1 (Figure 6a, Table S16). Quadrant 1 is strongly enriched for genes involved in translation-related processes, such as the snRNA metabolic process, cytoplasmic translation, translation initiation, and ribosome assembly (Figure S8a, Table S17). Notably, these translation-related genes are present throughout the DNSO process (Figure S8a, Table S17). Since cell growth and proliferation are closely tied to ribosome biogenesis, we hypothesize that the translational upregulation of these genes likely reflects enhanced cell growth during Chinese fir DNSO. Additionally, we observed that certain development-related genes, including those involved in meristem growth, embryonic organ morphogenesis, and adaxial/abaxial pattern specification, were specifically enriched at S3 (Figure S8a, Table S17), potentially indicating functional specialization during this stage. Despite the increase in mRNA levels, a total of 309, 340, 408, 366, and 335 genes exhibited decreases in protein levels (Figure 6a, Table S16), and these genes related to mRNA transcription, phospholipid transfer to the membrane, and formation of the plant organ boundary were strongly enriched from S2 to S6 (Figure S8b, Table S17). The development-related genes were also observed, including genes related to embryo sac central cell differentiation and cellular processes involved in reproduction in multicellular organisms, which were enriched at S5 and S6 (Figure S8b, Table S17). Genes in this category might initiate translation, such as translation initiation factors (eIF-2B1, eIF-2B2) and eukaryotic translation initiation factor 4B1 (EIF4B1), suggesting that the epigenetic regulation of transcription, post-transcriptional, and post-translational regulation may influence the expression levels of mRNAs and proteins involved in the Chinese fir DNSO process.
The third category represents genes where mRNA levels remain steady while protein levels either increase (Quadrant 2) or decrease (Quadrant 8) (Figure 6a, Table S16). Interestingly, this category contains the largest proportion of identified genes. Despite no changes in mRNA expression, a total of 4812, 4923, 4710, 5101, and 5606 genes exhibited increases in protein levels (Figure 6a, Table S16), while 4617, 4697, 4728, 4409, and 4653 genes showed decreases in protein levels at S2 to S6, respectively (Figure 6a, Table S16).
The fourth category represents genes where mRNA levels decrease (Quadrant 4) or increase (Quadrant 6) while protein levels remain unchanged. As shown in Quadrant 4, a total of 701, 515, 530, 466, and 538 genes exhibited a decrease in mRNA levels while their protein levels remained unchanged at S2 to S6, respectively (Figure 6a, Table S16). Despite the rise in mRNA levels, a total of 593, 568, 557, 526, and 568 genes remained unchanged in protein levels at S2 to S6, respectively (Figure 6a, Table S16). This suggests that post-transcriptional or post-translation mechanisms, such as mRNA modification, translation inhibition, or protein modification, may play a significant role in regulating protein abundance, even when mRNA levels changed during Chinese fir DNSO.
In summary, the temporal relationship between transcripts and protein changes is not always linear, but is instead governed by a more complex regulatory mechanism. This dynamic reflects the involvement of multiple layers of gene expression control, including post-transcriptional, translational, and post-translational processes, which together contribute to the intricate coordination of mRNA and protein levels during the DNSO process.
Abiotic response pathways were involved in the DNSO of Chinese fir
During the process of Chinese fir DNSO, the expression of transcripts and proteins related to abiotic stress responses was observed (Figure 3b, Figure 6, Table S11, and Table S15). GO enrichment analysis of differentially expressed transcripts and proteins revealed the involvement of various pathways related to abiotic stress, including abscisic acid (ABA) signaling, ethylene response, oxidative stress, salt stress, and osmotic conditions (Figure 7a,b, Tables S18– S22).
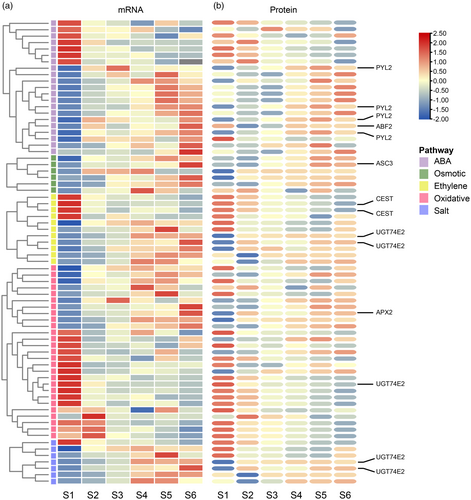
Heatmap of abiotic stress-related transcript and protein expression.
(a) Overview of differentially expressed transcripts related to ABA, ethylene, osmotic, oxidative, and salt stress.
(b) Corresponding protein expression profiles under the same stress conditions.
Ethylene normally accumulated during plant tissue culture and may have either a positive or negative effect on plant shoot regeneration (Chatfield, et al. 2008.). During Chinese fir DNSO, we identified 124 DEGs and 80 DEPs related to ethylene (Table S18). This set included genes like ACS genes and the ACO gene; among them, ACS3 was differentially expressed at both the mRNA and protein levels (Figure 7a,b), indicating the fluctuations in ethylene levels. Furthermore, ethylene receptors ETR1 and ETR2, as well as ethylene-responsive factors like ERFs and WRKYs, exhibited differential expression, signifying the role of the ethylene pathway in Chinese fir DNSO.
ABA, a pivotal player in plant responses to abiotic stress signals, was found to impact plant embryogenesis. As for Chinese fir DNSO, 92 DEGs and 193 DAPs related to ABA were identified; 19 were shared in transcriptome and proteome (Figure 7a,b), including ABA receptor PYL2 and ABSCISIC ACID-INSENSITIVE 5-like protein 5 (ABF2), indicating the activation of ABA signaling during Chinese fir DNSO (Table S19), suggesting that ABA may influence Chinese fir DNSO.
Reactive oxygen species (ROS) metabolism is prevalent during plant tissue culture. Here we identified 79 DEGs and 216 DEPs in response to oxidative stress. Notably, members of the L-ascorbate peroxidase gene (APX) family, including APX2, APX6, and APX7, along with respiratory burst oxidase homolog protein C (RBOHC) (Figure 7a,b, Table S20), were significantly regulated at both mRNA and protein levels. Among them, APX2 showed sustained upregulation from stages S2 to S6, indicating a transcriptional and translational reprogramming of H2O2-scavenging mechanisms. These results highlight the importance of ROS homeostasis during the regeneration process and suggest that oxidative stress signaling contributes to the cellular reprogramming required for DNSO in Chinese fir. 71 DEGs and 176 DEPs related to osmotic stress (7 shared by transcriptome and proteome) and 43 DEGs and 81 DEPs related to salt stress (17 shared) (Figure 7a,b, Tables S20– S22) were also involved in the DNSO of Chinese fir. These findings collectively indicate that abiotic stress-related pathways are intricately connected to the complex process of Chinese fir DNSO. The differential expression of genes associated with these pathways underscores their potential roles and contributions to the regulatory network governing DNSO in Chinese fir.
Exogenous application of stress factors affects the DNOS efficiency of Chinese fir
Based on the results from the above omics data, we hypothesized that abiotic stress may influence the DNSO efficiency of Chinese fir. To test this hypothesis, explants were cultured on the medium supplemented with varying concentrations of (ABA, 0.5–10 μm), 1-aminocyclopropane-1-carboxylic acid (ACC, 10–150 μm), silver nitrate (AgNO3, 5–20 μm), sodium chloride (NaCl, 25–100 mm), and mannitol (50–200 mm); the regeneration ratio and condition of the explants were systematically monitored and quantified over 8 weeks (Figure 8). Compared to the control group, NaCl and ABA treatments suppressed DNSO in Chinese fir, whereas the application of 5 μm AgNO3, 50 μm or 100 μm ACC, and 150 mm mannitol substantially promoted regeneration efficiency (Figure 8a,b and Figure S9). At high concentrations of NaCl, explants displayed browning, leading to a substantial decrease in both shoot induction efficiency and the mean number of shoots. Similarly, as the concentration of ABA increased, browning appeared in the distal regions of the explants, which was accompanied by a gradual reduction in shoot induction efficiency and the mean number of shoots (Figure 8b). Specifically, at 5 μm ABA, the shoot induction efficiency was 5%, and the mean number of shoots was 0.07. However, supplementation of the medium with 5 μm AgNO3 substantially enhanced DNSO efficiency in Chinese fir after 8 weeks of culture. Under this condition, the shoot induction efficiency reached 72%, with an average of 2.01 shoots per explant, compared to the control, which exhibited an efficiency of 66% and an average of 1.85 shoots per explant (Figure 8b). Furthermore, the exogenous application of 50 and 100 μm ACC substantially enhanced DNSO efficiency, achieving shoot induction efficiencies of 72% and 70%, respectively, with corresponding mean shoot numbers of 3.64 and 3.72 per explant (Figure 8b). The efficiency of shoot induction was 0.66, and the mean number of shoots was 2.67 with 150 mm mannitol (Figure 8b). These results suggest that ethylene plays a significant role in the DNSO process of Chinese fir and that the exogenous application of ethylene or high osmotic stress substantially enhances DNSO efficiency in Chinese fir.
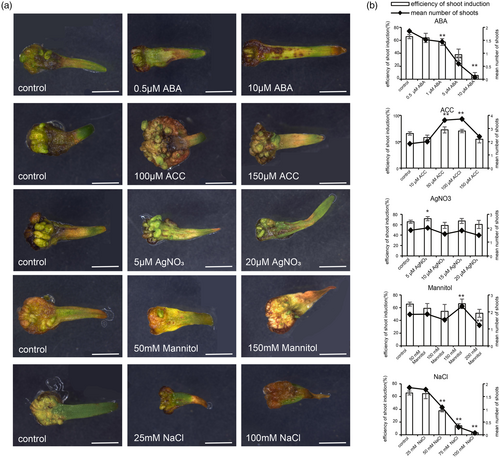
Effects of abiotic stresses on Chinese fir DNSO.
(a) Morphology of explants cultured on SIM medium with varying concentrations of NaCl, ABA, ACC, AgNO3, and mannitol. Scale bars, 5 mm.
(b) Statistical analysis of shoot induction efficiency and shoot numbers after treatment. Data represent mean ± SE from three independent experiments. Efficiency of shoot induction = number of explants forming shoots/total explants, mean shoot number = total shoots/total explants. n = 120 ~ 130, data represent the average of three independent experiments ±SE, * and ** indicate significant differences in comparison to control at P < 0.05 and P < 0.01 (t-test), respectively.
To examine the impact of abiotic stress on the expression of genes related to DNSO in Chinese fir, five candidate transcription factors were chosen: WOX6, WOX8, LBD15, LBD40, and PSK3 (Joshi et al., 2023; Li, Qian, et al., 2024; Sun et al., 2013), all of which potentially play a role in this process. The expression profiles of these genes under normal and abiotic stress conditions were assessed using qRT-PCR. The findings indicated that the expression levels of these genes were higher in S2 to S4 compared to S1 under normal conditions (Figure S10). Additionally, the expression patterns of these genes varied under abiotic stress, with the stress treatments promoting DNSO in Chinese fir (Figure 8a,b). These results further suggest that abiotic stress influences the development of DNSO in Chinese fir. While these data partially confirm the effects of abiotic stress on the efficiency of DNSO, the underlying molecular mechanisms remain unclear.
DISCUSSION
Role of phytohormones
Although various DNSO protocols have been successfully established in numerous angiosperm species, research in gymnosperms, particularly conifer trees, remains limited (Egertsdotter et al., 2019). Chinese fir is the most important conifer species in south subtropical areas of China. Although several studies demonstrated the successful somatic embryogenesis using immature zygotic embryos (Hao et al., 2023; Hu et al., 2017), achieving stable and highly efficient plant regeneration, especially through DNSO, is still a challenge for these important species. In this study, we successfully established a DNSO protocol using cotyledons as explants and optimized the hormone combination, yielding several advantages, including the generation of shoots with high sprouting efficiency, a high rate of reproduction, and minimal variation.
The DNSO process is a highly coordinated developmental process regulated by intricate interactions among multiple phytohormones, with auxins and CKs serving as the primary regulators (Ikeuchi et al., 2019; Kareem et al., 2016; Sang et al., 2018; Tian et al., 2018). Numerous studies have demonstrated that genes involved in auxin and cytokinin biosynthesis, transport, and signaling play pivotal roles in controlling regenerative competence and organ formation (Li et al., 2025; Liu et al., 2023; Yu et al., 2024). In this study, we observed that the auxin and CK biosynthesis and signaling-related genes were differentially expressed at both transcriptional and proteomic levels during the Chinese fir DNSO (Table S4 and Table S23, Figure S6), supporting their critical involvement in regulating this process. Moreover, unlike the two-step regeneration system typically used in Arabidopsis—where callus formation and shoot induction occur sequentially (Zhang et al., 2017), DNSO in Chinese fir and P. pinea is initiated via a single-step induction method using cotyledon explants (Cuesta et al., 2009). This difference may reflect fundamental divergence in regulatory dynamics. In Chinese fir, early expression of auxin-responsive genes such as ARF6, ARF17, ARF18, and PIN1 was observed during shoot initiation. Similarly, in P. pinea, the endogenous IAA concentration increased following BA treatment, reinforcing the role of auxin-cytokinin interaction in promoting shoot regeneration under one-step conditions (Cuesta et al., 2009; Yin et al., 2024). These observations highlight both the conserved and distinct features of hormonal control between gymnosperm and angiosperm regeneration systems.
Ethylene and stress response
Prior research has indicated the significant role of abiotic stress in plant regeneration processes (Ikeuchi et al., 2019; Sugimoto et al., 2019). However, direct connections between plant regeneration and stress responses have been reported infrequently. Our findings reveal substantial alterations in the expression of genes associated with abiotic stress responses at both the transcriptional and translational levels, suggesting the involvement of these pathways in the DNSO process of Chinese fir (Figure 3b, Figure 6). Additional experiments demonstrated that the exogenous application of various stress factors affects the efficiency of Chinese fir DNSO (Figure 8), implying that abiotic stress may activate intrinsic regulators of the cell cycle, thereby initiating subsequent cell proliferation and the DNSO process. Further investigations are necessary to validate this hypothesis.
In the present study, we observed significant impacts of ethylene on the DNSO process in Chinese fir, and the application of either ACC or AgNO3 enhanced shoot regeneration efficiency (Figure 8). Importantly, we also observed that explants treated with ACC exhibited more extensive callus proliferation than those treated with AgNO3 (Figure 8 and Figure S9). The role of ethylene in plant regeneration has historically received limited attention, and to date, few studies have specifically investigated its function in gymnosperm regeneration. The influence of ethylene on regeneration is known to vary significantly across species, genotypes, and explant types (Chatfield & Raizada, 2008; Neves et al., 2021; Trujillo-Moya & Gisbert, 2012). Increasing evidence suggests the effect of ethylene on regeneration with several mechanisms: (1) Ethylene crosstalk with auxin and cytokinin: The increasing evidence suggests that ethylene acts at the intersection of biotic and abiotic stress responses and is intricately linked to hormonal crosstalk, particularly with auxin and cytokinin, which are central to reprogramming and organogenesis (Neves et al., 2021). It is possible that AgNO3 treatment indirectly stabilizes the auxin–cytokinin balance, favoring regeneration in a distinct hormonal environment compared to ACC (Neves et al., 2021). (2) Tissue and species-specific roles of ethylene: Depending on the species and tissue, ethylene may function either as a promoter or an inhibitor of regeneration (Biddington, 1992). For instance, the inhibition of ethylene signaling has been shown to enhance callus formation in Zea mays, Oryza sativa, and Pinus taeda (Adkins et al., 1993; Pullman et al., 2003; Vain et al., 1990), whereas the application of its precursor ACC stimulates callus formation in Citrus and promotes shoot organogenesis in Arabidopsis (Shin et al., 2022; Su & Zhang, 2014; Tadeo et al., 1995). (3) Dose-dependent effect stage-specific roles of ethylene: During early stages, low to moderate ethylene levels (induced by ACC) may promote cell proliferation and dedifferentiation, enhancing callus formation and competence acquisition. However, excessive or prolonged ethylene signaling may inhibit subsequent organogenic differentiation. In this context, AgNO3 could attenuate excessive ethylene responses at later stages, thus maintaining a favorable hormonal balance for shoot formation (Ishizaki et al., 2000). In addition, the effect of ethylene on regeneration within the same species can affect the regeneration process differently regarding each specific stage, and it seems to be related to stress mediated by ethylene in response to auxin and cytokinins (Ewa & Sylwia, 2011; Ishizaki et al., 2000; Kępczyńska et al., 2009; Ptak et al., 2010). (4) Potential involvement of other signaling pathways: Both ACC and AgNO3 may have off-target effects or influence additional signaling pathways beyond ethylene (Li et al., 2022; Polko & Kieber, 2019). A recent study showed ACC inhibited root development by modulating the expression of WOX5 when ethylene signaling is blocked (Mou et al., 2025). Our data further indicate that ethylene may modulate the expression of key intrinsic regeneration-related regulators, such as WOXs, LBD, and PSK, potentially activating intrinsic developmental pathways involved in shoot meristem formation. Nevertheless, the precise molecular mechanisms through which ethylene exerts these effects require further investigation.
In parallel, ABA is a well-established regulator of abiotic stress signaling (Finkelstein & Gibson, 2002; Lee & Luan, 2012) and plays a critical role in plant regeneration, particularly somatic embryogenesis (Fazeli-nasab et al., 2012; Jin et al., 2014). Exogenous ABA has been reported to promote somatic embryogenesis in several conifer species, including Pinus taeda and Larix occidentalis (Pullman et al., 2003; Pullman & Bucalo, 2011; Thompson & von Aderkas, 1992). However, studies addressing its effects on shoot organogenesis in conifers are scarce (Chang et al., 1991; Sen et al., 1989). The effects of ABA on regeneration appear to be concentration-dependent: while moderate levels can enhance organogenic or embryogenic responses, excessive accumulation of ABA—often induced by stress—has been shown to suppress regeneration (Wan et al., 2025). Moreover, hormonal interactions play a critical role in modulating regeneration outcomes. For example, ABA has been found to inhibit shoot regeneration in the presence of both auxin and cytokinin, whereas it has minimal effects in their absence (Ella & Zapata, 1991; Fernando & Gamage, 2000; Maggon & Singh, 1995; Xing et al., 1995). In our study, we found that exogenous ABA significantly inhibited Chinese fir DNSO, potentially due to the excessive concentration of ABA or an imbalanced ABA-to-auxin/cytokinin ratio in the culture medium. These findings highlight the complex regulatory role of ABA in regeneration and suggest that optimization of hormone balance is critical for successful DNSO in gymnosperms. Future studies will be required to systematically test this hypothesis and to explore the underlying hormonal and molecular regulatory networks.
Transcriptome-proteome correlation
The Chinese fir DNSO protocol developed in this study provides a robust platform to investigate the molecular dynamics of gymnosperms, particularly the relationship between mRNA expression and protein abundance. While mRNA levels are traditionally considered reflective of gene function and protein levels (Woodgate & Zenkin, 2023), reports also suggest that mRNA expression is not always a reliable predictor of protein abundance (Greenbaum et al., 2003; Webster & Weixlbaumer, 2021). In this study, we provide further insights into the complexity of molecular changes in the Chinese fir DNSO, revealing that gene expression does not always translate directly into corresponding protein expression (Figure 6a), demonstrating substantial modification of the set of genes during the Chinese fir DNSO process. Cluster analysis of DEGs and DEPs clearly separated the different expression patterns by time and functions. By analyzing these patterns, we found that mRNA and protein levels can vary in both concordant and discordant directions, depending on specific pathways and stages (Figure 3 and Figure 5).
Several mechanisms could explain this disconnect between mRNA and protein expression. One possibility is gene-specific variations in translation rates, which may lead to differences between transcription and protein synthesis. Future comparisons between RNA-seq and Ribo-seq data could help clarify potential changes between transcriptional and translational processes. The regulation of a balanced functional proteome is essential for DNSO and is affected by cellular processes such as protein aggregation, misfolding, oxidative damage, post-translational modifications, and altered protein turnover rates (Ikeuchi et al., 2019; Millar et al., 2019). It is plausible that certain factors drive the global disconnect between gene expression and protein accumulation, and identifying these factors will be key to understanding the mechanisms underlying the Chinese fir DNSO process. Since proteostasis is finely tuned to the specific proteomic requirements of different cell types and the callus is composed of multiple cell types, unraveling these dynamics is particularly challenging in this study. Moreover, our measurements of protein abundance cannot capture the functional state, modifications, or potential damage of these proteins. It is also important to recognize that protein abundance does not necessarily equate to protein function.
Potential implications in forestry
The DNSO protocol developed for Chinese fir provides a promising approach for rapid clonal propagation and genetic improvement of this economically valuable species, and the molecular framework established here lays a critical foundation for the future development and application of functional genomics tools in Chinese fir. The future functional validation—such as targeted gene editing or transgenic overexpression of key regulatory genes identified in this study—will be essential for elucidating the precise mechanisms underlying shoot organogenesis in Chinese fir. Additionally, the comprehensive transcriptomic and proteomic data generated offer a valuable resource for identifying critical genes and regulatory networks involved in Chinese fir DNSO. Moreover, the multi-omics analysis method provides a foundation for an in-depth study of the molecular mechanism of plant regeneration, facilitating future research on gymnosperm regeneration and deepening our knowledge of plant developmental biology.
MATERIALS AND METHODS
Plant materials, growth conditions, and treatment
Chinese fir seeds were collected in Fujian Province, China, and stored in plastic bags at 4°C. The seeds were soaked in warm water (~25°C) for 24 h before being sown in a mixture of organic nutrient soil and vermiculite (3:1). Seedlings were collected after 14 days of germination and then rinsed under running water for 1 h after removing the seed coat and root. The seedlings were surface sterilized in 70% (v/v) ethanol for 1 min, followed by treatment with 0.1% (w/v) HgCl2 for 7 min, and then rinsed five times with sterile distilled water.
Cotyledons were excised from the sterilized seedlings and used as explants for further experiments. These cotyledons were incubated on DCR basic medium supplemented with various concentrations of 6-BA, TDZ, and NAA as indicated. For rooting, the explants were placed on a root induction medium consisting of MS basic medium supplemented with 3% sucrose, 0.42% phytagel, 1 mg L−1 NAA, and 1 mg L−1 4-(3-Indolyl) butanoic acid (IBA). The incubations were conducted at 25 ± 2°C under a 14-h photoperiod using cool white fluorescent lights (80 μmol m−2 s−1), with sub-culturing every 4 weeks. Data were collected after 8 weeks. To investigate the effects of abiotic stress factors on the DNSO efficiency of Chinese fir, cotyledons were cultured on shoot SIM supplemented with various concentrations of NaCl (25, 50, 75, and 100 mm), ABA (0.5, 1, 5, and 10 μm), mannitol (50, 100, 150, and 200 mm), AgNO3 (5, 10, 15, and 20 μm), and ACC (10, 50, 100, and 150 μm). The number of regenerated buds was recorded and statistically analyzed. Each experiment was conducted independently three times, with consistent results obtained, and the data are presented as mean ± standard error (SE). Statistical analysis was performed using SPSS software (version 26.0), employing one-way ANOVA or t-test, with P < 0.05 considered statistically significant.
PacBio cDNA library construction and sequencing
Samples from the Chinese fir DNSO were collected for RNA isolation using the E.Z.N.A. Plant RNA Kit (Omega, Guangzhou, China). RNA samples were combined in equal quantities for subsequent analysis, with a total of 2 μg RNA used as input material for library preparation. Library preparation was conducted according to the manufacturer's instructions using the Iso-Seq Express 2.0 Kit (103–071-500) and the Kinnex Full-Length RNA Kit (103–072-000). Following purification with 1× cleanup beads, the library was sequenced for a duration of 24 h on the Revio system (Pacific Biosciences, Menlo Park, CA, USA).
PacBio data analysis
Sequence data were processed using SMRT Analysis (ISOseq v3.0) and SMRTLink 10.0 to generate High Fidelity (HiFi) reads. Full-length non-chimeric (FLNC) reads were refined, clustered into high-quality (HQ) and polished low-quality (LQ) isoforms, and redundancies (>90% similarity) were removed with CD-HIT (Fu et al., 2012). Non-redundant reads were polished using LoRDEC (Salmela & Rivals, 2014), yielding high-quality full-length isoforms. Coding sequences (CDS) were predicted using TransDecoder (https://github.com/TransDecoder/TransDecoder), and functional annotations were performed against NCBI non-redundant protein sequences (NR), Gene Ontology (GO), EuKaryotic Orthologous Groups (KOG), Kyoto Encyclopedia of Genes and Genomes (KEGG), SwissProt, and InterPro databases using Diamond (Buchfink et al., 2014), InterProScan5, and eggNOG-mapper (Cantalapiedra et al., 2021), ensuring comprehensive characterization of the isoforms.
RNA isolation, cDNA library construction, and data analysis
Chinese fir cotyledons were cultivated and independently pooled from three distinct batches, all maintained under uniform growth conditions. A total of 18 samples representing six developmental stages were collected, and total RNA extraction and RNA-seq library construction were performed as described previously (Wang et al., 2023). Sequencing was conducted on the HiSeq 4000 platform (Illumina, San Diego, CA, USA).
RNA-Seq data analysis was performed as described previously (Wang et al., 2023). Genes exhibiting a fold change >2 and a P-value <0.05 were identified as differentially expressed genes (DEGs). Gene Ontology (GO) enrichment analysis and K-means clustering analysis were performed as described previously (Wang et al., 2023).
Samples preparation for proteomics and data analysis
A total of 18 samples, representing six developmental stages of Chinese fir DNSO, were analyzed using DIA proteomics, with three biological replicates per stage. The protein extraction was performed as described previously (Li, Lu, et al., 2024). Then, the protein digest was performed as previously reported (Wang et al., 2024). The digested peptides were desalted using a C18 desalting column and prepared for analysis. For library construction, equal amounts of peptides (100 μg per sample) were pooled, dried using a refrigerated centrifugal concentrator, and fractionated by high-pH reversed-phase HPLC to obtain eight fractions. These fractions were used for DDA library construction with iRT peptides.
Raw DIA data were processed using Spectronaut 13 (Biognosys AG, Schlieren, Switzerland) with default parameters and dynamic iRT-based retention time prediction. Spectronaut performed data extraction through extensive mass calibration, dynamically adjusting the ideal extraction window based on iRT calibration and gradient stability. An FDR threshold of 1% was applied at both precursor and protein levels. Decoy generation involved mutating amino acid sequences, and selected precursors passing the filters were quantified using the three least interfering fragment ions. Quantification was performed using the average of the top three peptides meeting the 1% FDR cutoff. The initial quantitative data table was filtered to ensure missing values did not exceed 33.3%. Imputation and normalization were performed based on the median. Differential protein analysis was conducted using the T-test, with fold changes >1.2 and P-values <0.05 defining differentially expressed proteins.
Integrated analysis of transcriptomics and proteomics
The integrated analysis (nine-quadrant scatterplot) of the proteome and transcriptome was conducted using the ggplot2 package, and a single protein can correspond to multiple transcripts. Shared transcripts were used to analyze the correlation of transcriptomics and proteomics. The ratios of DEPs and DEGs were classified into nine quadrants, grouped into four main categories based on the direction and correlation of mRNA and protein expression changes. Category I includes gene–protein pairs with concordant expression trends, where both mRNA and protein levels were either upregulated (quadrant 3) or downregulated (quadrant 7). Category II represents discordant expression, where mRNA and protein levels changed in opposite directions; either mRNA was downregulated while protein was upregulated (quadrant 1), or mRNA was upregulated while protein was downregulated (quadrant 9). Category III consists of cases where significant changes occurred at the protein level, but mRNA levels remained stable (quadrants 2 and 8). Category IV includes gene–protein pairs in which the mRNA levels were significantly altered, while protein levels showed no corresponding change (quadrants 4 and 6), and the biological interpretation of each quadrant was described in Table S24. Functional insights were gained through GO enrichment analyses of the paired DEPs and DEGs, visualized via ggplot2, highlighting the complex transcriptional and translational dynamics across the developmental stages of Chinese fir DNSO.
Quantitative real-time PCR analysis
Quantitative real-time PCR (qRT-PCR) was conducted following previously established protocols (Hu et al., 2023). Briefly, Actin was utilized as an internal control, and calculations were performed using the 2-ΔΔCT method. The primers used for PCR are detailed in Table S25. Each quantitative real-time PCR assay included three technical replicates.
AUTHOR CONTRIBUTIONS
ZQ conceived and designed the experiments; DWS performed experiments and analyzed the data; YSW, WDK, WWP, WWJ, and CCY contributed to performing the experiments and collecting the data. ZQ was responsible for project administration and funding acquisition; ZQ wrote the article with contributions from all authors. MXQ, XJJ, and LCT provided critical revisions to the manuscript. All authors have read and approved the final version of this manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (No. 2023YFD2200203). The funding bodies were not involved in the design of the study or in any aspect of the data collection, analysis, interpretation of data, and in paper writing. We sincerely thank the Fujian Agriculture and Forestry University, Instrumental Analysis Center for their help in the analyses of proteomics.
Open Research
DATA AVAILABILITY STATEMENT
All data supporting the findings of this study are available in the Supplementary Data. RNA-seq and SMRT data generated in this study have been submitted to the SRA under BioProject number PRJNA1182841.