Diverse pathways of phosphatidylcholine biosynthesis in algae as estimated by labeling studies and genomic sequence analysis
Summary
Phosphatidylcholine (PC) is an almost ubiquitous phospholipid in eukaryotic algae and plants but is not found in a few species, for example Chlamydomonas reinhardtii. We recently found that some species of the genus Chlamydomonas possess PC. In the universal pathway, PC is synthesized de novo by methylation of phosphatidylethanolamine (PE) or transfer of phosphocholine from cytidine diphosphate (CDP)-choline to diacylglycerol. Phosphocholine, the direct precursor to CDP-choline, is synthesized either by methylation of phosphoethanolamine or phosphorylation of choline. Here we analyzed the mechanism of PC biosynthesis in two species of Chlamydomonas (asymmetrica and sphaeroides) as well as in a red alga, Cyanidioschyzon merolae. Comparative genomic analysis of enzymes involved in PC biosynthesis indicated that C. merolae possesses only the PE methylation pathway. Radioactive tracer experiments using [32P]phosphate showed delayed labeling of PC with respect to PE, which was consistent with the PE methylation pathway. In Chlamydomonas asymmetrica, labeling of PC was detected from the early time of incubation with [32P]phosphate, suggesting the operation of phosphoethanolamine methylation pathway. Genomic analysis indeed detected the genes for the phosphoethanolamine methylation pathway. In contrast, the labeling of PC in C. sphaeroides was slow, suggesting that the PE methylation pathway was at work. These results as well as biochemical and computational results uncover an unexpected diversity of the mechanisms for PC biosynthesis in algae. Based on these results, we will discuss plausible mechanisms for the scattered distribution of the ability to biosynthesize PC in the genus Chlamydomonas.
Introduction
Phosphatidylcholine (PC) is a very common phospholipid in eukaryotes. It is, however, absent in various algae, including Chlamydomonas reinhardtii among others (Sato and Furuya, 1985; Giroud et al., 1988). In these organisms, another zwitterionic lipid, diacylglyceryl-N,N,N-trimethylhomoserine (DGTS), is believed to function in place of PC (Sato and Furuya, 1985; Giroud et al., 1988). The lack of PC-synthesizing enzymes in C. reinhardtii was inferred by genomic analysis (Merchant et al., 2007). The biosynthetic pathways of various lipids, including triacylglycerol, in C. reinhardtii are summarized in Riekhof et al. (2005), Merchant et al. (2012) and Li-Beisson et al. (2015). Various algae possess both DGTS and PC, but DGTS is not present in red algae (Sato, 1992; Sato and Moriyama, 2007). It is thought that DGTS is advantageous for algae or microorganisms in phosphate-limited environments (Riekhof et al., 2014; Senik et al., 2015).
We recently detected PC in four species of the genus Chlamydomonas (Sakurai et al., 2014b). Examination of the phylogenetic relationship of these algae suggested that the loss of PC biosynthesis might have occurred independently in many branches of the genus Chlamydomonas. Alternatively, the ability to biosynthesize PC could have been acquired independently in different species after the initial loss of the ability at the root of the genus Chlamydomonas. To answer these questions, we attempted to analyze the mechanism(s) by which PC is synthesized in these species.
Various pathways are known for the synthesis of PC (Figure 1a) (Cole et al., 2012; Guschina et al., 2014). First, PC is made by methylation of phosphatidylethanolamine (PE). The enzyme is called PE methyltransferase (PEMT) in general, but two different activities are distinguished: the initial methylation is catalyzed by PEMT in the narrow sense, whereas the second and third methylations are catalyzed by phospholipid methyltransferase (PLMT). Second, choline is phosphorylated by choline kinase (CKI) to give phosphocholine, which is then converted to CDP-choline by choline-phosphate cytidylyltransferase (CCT). The choline moiety is transferred from CDP-choline to diacylglycerol (DAG). Third, phosphocholine is synthesized by methylation of phosphoethanolamine via the action of phosphoethanolamine methyltransferase (PEAMT). The remaining part of pathway is identical to the second pathway. In bacteria, PC is also synthesized from CDP-DAG and choline (Geiger et al., 2013). The pathway consisting of methylation of phosphoethanolamine has been known in plants (Nuccio et al., 2000; BeGora et al., 2010), but is now known as an important pathway of PC biosynthesis in the nematode Caenorhabditis elegans (Palavalli et al., 2006) and the malarial parasite Plasmodium falciparum (Bobenchik et al., 2011). In plants (Figure 1b), the initial methylation product of phosphoethanolamine, monomethyl phosphoethanolamine, is considered to be conjugated with CDP and then transferred to DAG to give monomethyl PE, which is further methylated by PLMT (Keogh et al., 2009), because plant PLMT does not catalyze the methylation of PE but rather the methylation of mono- or di-methylated PE (Figure 1b). In vertebrates, however, a PEMT enzyme similar to PLMT catalyzes all three methylations from PE to PC. Ethanolamine is provided by decarboxylation of serine in general, but this enzyme is missing in Cyanidioschyzon merolae. Alternatively, phosphoethanolamine is produced by degradation of sphingosine 1-phosphate (S1P), but this is not normally considered to be a major pathway. Figure 1(a) shows the pathway of synthesis of DGTS in bacteria, non-flowering plants and algae (Riekhof et al., 2005).
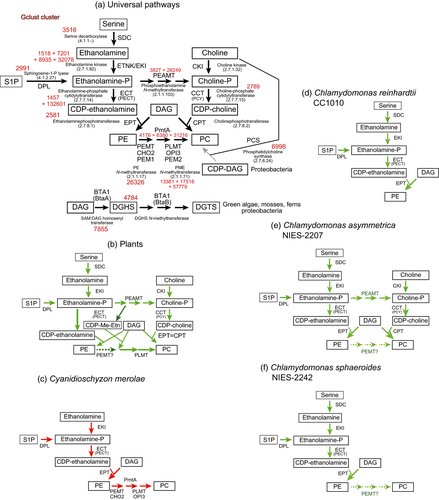
Pathways of synthesis of phosphatidylcholine (PC) in various plants and algae.
(a) Universal pathways. (b) Plants (represented by Arabidopsis thaliana). (c) Cyanidioschyzon merolae (red alga). (d) Chlamydomonas reinhardtii (green alga). (e), Chlamydomonas asymmetrica (green alga). (f), Chlamydomonas sphaeroides (green alga). In diagram (a), enzyme names and corresponding EC numbers are shown, along with the cluster numbers of homolog groups in the Gclust database version 2012_42 (numbers in red). Biosynthetic pathway of diacylglyceryltrimethylhomoserine (DGTS) are also shown in the diagram (a). In plants (b) phospholipid methyltransferase (PLMT) has been known to lack phosphatidylethanolamine methyltransferase (PEMT) activity (Keogh et al., 2009).
The present study was planned to elucidate the pathway(s) of synthesis of PC in two strains of Chlamydomonas by tracer experiments. As a control, Cyanidioschyzon merolae was also analyzed in parallel; this is a small unicellular red alga with a very small genome (16.5 Mbp, encoding 4775 proteins) that we analyzed previously by radiocarbon labeling (Sato and Moriyama, 2007). Background knowledge on the probable pathways of PC synthesis was obtained by comparative genomic analysis using Gclust software (Sato, 2009). Genomic data were also obtained for the Chlamydomonas strains. Cyanidioschyzon merolae was confirmed to synthesize PC by the PE methylation pathway, as in yeasts. Experimental data in conjunction with genome information suggested that Chlamydomonas asymmetrica has the phosphoethanolamine methylation pathway, whereas PE methylation could account for the synthesis of PC in C. sphaeroides, and probably also in C. asymmetrica.
Results
Comparative genomic analysis
The Gclust database was searched for putative enzymes involved in the synthesis of PC (Sato, 2009). The universal pathways are summarized in Figure 1(a). The results suggested that the PE methylation pathway is the sole mechanism of PC synthesis in Cyanidioschyzon merolae (Figure 1c and Figure S1). CMF090C is a candidate for PEMT, and CMP111C and CMA134C are candidates for PLMT or monomethyl PE methyltransferase. In contrast, Chlamydomonas reinhardtii only possesses the pathway for PE synthesis (Figure 1d). Based on these results, we first tried to confirm the PE methylation pathway in intact cells of Cyanidioschyzon merolae, and then analyzed the mechanism of PC biosynthesis in the two species of Chlamydomonas.
Labeling of phospholipids in Cyanidioschyzon merolae
Cyanidioschyzon merolae cells were labeled with [32P]phosphate to follow the labeling of phospholipids with time (Figure 2). Fifteen minutes after the labeling phosphatidylinositol (PI) + phosphatidic acid (PA) was predominant (Figure 2a). In the two-dimensional thin-layer chromatography (2D-TLC) system used in the radioactive experiments, these two lipids were not clearly separated. We estimated that PA was the major labeled lipid at this time. After 2 h of chase, phosphatidylglycerol (PG) and PE were also clearly labeled (Figure 2b), while the label in PC remained very low. After chasing for 21 h, PC became densely labeled, whereas the label in PI+PA decreased (Figure 2c). At this time, the label in this fraction was supposed to reside mostly in PI, because PA is a metabolic intermediate. The time course (Figure 2d) suggests that the total labeling in phospholipids increased during the chase period, possibly due to continued flow of phosphate from some intracellular reservoirs such as lysosomes. Nevertheless, it is clear that PE and PG were fully labeled after 2 h of chasing (Figure 2e).
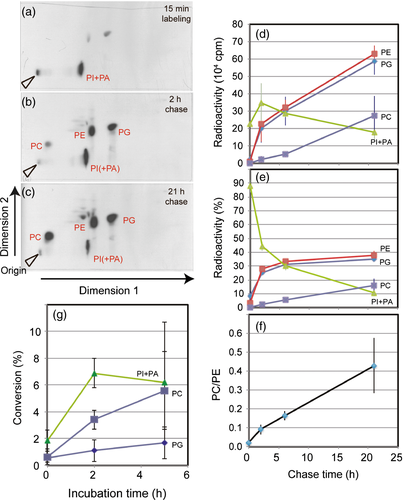
Phosphatidylcholine synthesis in Cyanidioschyzon merolae.
In (a)–(f) the cells were labeled with [32P]phosphate for 15 min and then chased for 21 h. Cells were removed at various time intervals and total lipids were extracted. Lipids were analyzed by 2D-TLC, and then autoradiographed. The radioactive spots were scraped for liquid scintillation counting. An average of three independent experiments is shown. (a)–(c) Representative autoradiograms of 2D-TLC after the labeling and 2 h and 21 h of chasing. (d) Time-course of radioactivity in phospholipids. Error bars indicate the standard deviation of three independent experiments. (e) Time-course of relative radioactivity (percentage). (f) Time-course of the radioactivity ratio PC/PE. In (g), radioactive PE was added to the cells and incubated for 5 h. Results of four independent experiments are shown. The percentages of conversion into phosphatidylinositol (PI), phosphatidylcholine (PC) and phosphatidylglycerol (PG) are plotted (PA, phosphatidic acid). At 5 h, the deviation of PI labeling was large, for reasons that are not known.
Our focus is on the synthesis of PC. The labeling of PC was markedly retarded as indicated by the PC/PE radioactivity ratio (Figure 2f). This is consistent with the supposed PE methylation pathway as inferred from comparative genomics.
Conversion of PE to PC was tested by incubating the cells of C. merolae with radioactive PE (Figure 2g). During the incubation, radioactivity in PC increased linearly. This is an indication that exogenously added PE was converted to PC. The marked labeling of PI+PA was unexpected, but this could be a result of degradation of added PE and incorporation of released phosphate, as in the labeling experiments with radioactive phosphate.
These results suggest that the synthesis of PC in C. merolae can be accounted for by the methylation of PE.
Lipid analysis in the two species of Chlamydomonas
Chlamydomonas asymmetrica and C. sphaeroides are both spherical photosynthetic cells (Figure S2). Chlamydomonas asymmetrica cells tended to stick together after the cell division, engendering four daughter cells. Chlamydomonas sphaeroides cells were flagellated and active in swimming. Except for these differences, no other marked differences were apparent between these two strains or between these and C. reinhardtii.
Lipids and fatty acids were thoroughly analyzed by our standard procedures (Sato and Moriyama, 2007; Sakurai et al., 2014a) in C. asymmetrica and C. sphaeroides. Table 1 shows that these strains contained low but significant levels of PC. The DGTS content was higher than the sum of PE, PI and PC in both strains, suggesting that DGTS is a predominant polar lipid in the extrachloroplast compartment, such as the endoplasmic reticulum (ER) and plasma membrane. This is clearly different from the situation in Cyanidioschyzon merolae, in which PC is a predominant polar lipid in the extraplastidic compartment (Sato and Moriyama, 2007).
| Lipid class | Short name | Composition (%) | |
|---|---|---|---|
| C. asymmetrica | C. sphaeroides | ||
| Monogalactosyl diacylglycerol | MGDG | 39.0 ± 10.4 | 42.0 ± 12.9 |
| Digalactosyl diacylglycerol | DGDG | 23.7 ± 1.5 | 22.1 ± 5.3 |
| Sulfoquinovosyl diacylglycerol | SQDG | 3.5 ± 2.6 | 4.8 ± 0.4 |
| Phosphatidylglycerol | PG | 5.1 ± 0.6 | 7.4 ± 0.9 |
| Diacylglyceryltrimethylhomoserine | DGTS | 16.2 ± 6.2 | 12.9 ± 2.0 |
| Phosphatidylethanolamine | PE | 3.9 ± 1.7 | 4.1 ± 1.3 |
| Phosphatidylinositol | PI | 1.3 ± 0.4 | 1.4 ± 0.4 |
| Phosphatidylcholine | PC | 2.1 ± 0.6 | 1.0 ± 0.2 |
| Triacylglycerol | TAG | 4.0 ± 2.5 | 4.0 ± 4.2 |
| Fatty acids | FA | 1.1 ± 0.7 | 0.3 ± 0.1 |
| Total | 100 | 100 | |
- Each value is the average ± standard deviation of four independent experiments. The total lipid contents in C. asymmetrica and C. sphaeroides cells were 0.0251 ± 0.0085 pmol FA per cell, and 0.0198 ± 0.0073 pmol FA per cell, respectively. The ratio of PC/PE is calculated as 0.54 and 0.24 for C. asymmetrica and C. sphaeroides, respectively.
Fatty acid compositions of individual lipid classes in Chlamydomonas asymmetrica and C. sphaeroides are shown in Tables 2 and S1, respectively. Delta 5 unsaturated fatty acids, such as 18:3(5,9,12) and 18:4(5,9,12,15), were detected in C. sphaeroides (Table S1) as in C. reinhardtii (Sato and Furuya, 1985; Giroud et al., 1988; Sakurai et al., 2014a; Li-Beisson et al., 2015), whereas the delta 6-series of fatty acids, 18:3(6,12,15) and 18:4(6,9,12,15), were found in C. asymmetrica (Table 2). The identity of these fatty acids was confirmed by gas chromatography/mass spectrometry (GC/MS) analysis of pyrrolidide derivatives (Figure S3). The results of selected ion monitoring (SIM) analysis undoubtedly excluded the occurrence of delta 6 fatty acids in C. sphaeroides and delta 5 fatty acids in C. asymmetrica (Figure S4b,d). The presence of 18:4(6,9,12,15) along with 18:4(5,9,12,15) in C. reinhardtii CC-1010, as reported in a previous study (Sakurai et al., 2014a), was not supported by this SIM analysis (Figure S4f). In this respect, C. sphaeroides is similar to C. reinhardtii, whereas C. asymmetrica is distinct from them. These are consistent with the results of phylogenetic analysis (Yumoto et al., 2013), in which C. sphaeroides is placed at a position close to C. reinhardtii.
| Fatty acid | Contenta (FA, mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PC | DGTS | PE | PI | PG | DGDG | SQDG | MGDG | TAG | |
| 14:0 | 0.9 ± 0.8 | 0.7 ± 0.5 | 0.6 ± 0.4 | 7.7 ± 0.6 | 18.3 ± 2.3 | 2.9 ± 0.9 | 0.9 ± 0.2 | 0.2 ± 0.0 | 5.5 ± 0.9 |
| 14:1(9) | 0.5 ± 0.1 | 0.2 ± 0.2 | 0.5 ± 0.3 | 1.1 ± 0.7 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.3 | 0.1 ± 0.1 | 3.2 ± 5.6 |
| 16:0 | 46.7 ± 5.2 | 47.4 ± 5.3 | 57.1 ± 7.9 | 57.5 ± 4.2 | 41.1 ± 4.9 | 50.4 ± 3.1 | 75.2 ± 11.6 | 1.5 ± 0.4 | 33.8 ± 6.3 |
| 16:1(7) | 2.2 ± 0.6 | 1.1 ± 0.2 | 1.3 ± 0.3 | 1.1 ± 1.0 | 0.0 ± 0.0 | 1.2 ± 0.3 | 1.3 ± 1.6 | 1.2 ± 0.4 | 3.0 ± 1.7 |
| 16:1 (3t) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 16.1 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 16:1 (9) | 0.2 ± 0.5 | 0.5 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.9 ± 0.3 | 0.0 ± 0.1 | 0.1 ± 0.2 | 0.0 ± 0.0 | 1.1 ± 2.5 |
| 16:1(11) | 0.5 ± 0.7 | 0.5 ± 0.8 | 0.7 ± 0.9 | 0.7 ± 1.1 | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.5 ± 0.5 | 0.1 ± 0.1 | 0.8 ± 1.6 |
| 16:2(7,10) | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.0 ± 0.9 | 0.3 ± 0.4 | 0.1 ± 0.1 | 4.9 ± 2.0 | 1.3 ± 0.5 | 2.2 ± 0.4 | 1.6 ± 0.4 |
| 16:3(4,7,10) | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.6 ± 0.3 | 0.7 ± 0.5 | 9.5 ± 2.2 | 2.1 ± 1.1 |
| 16:3(7,10,13) | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.1 | 2.1 ± 0.9 | 0.7 ± 0.8 | 0.6 ± 0.4 | 0.6 ± 0.2 |
| 16:4(4,7,10,13) | 2.0 ± 0.1 | 3.7 ± 0.2 | 0.4 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.5 | 33.2 ± 0.8 | 6.2 ± 3.0 |
| 17:0 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.4 ± 0.6 | 0.6 ± 0.3 | 0.3 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.2 | 0.1 ± 0.1 | 2.1 ± 4.7 |
| 17:1(9) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.1 |
| 18:0 | 0.7 ± 0.3 | 0.5 ± 0.2 | 1.6 ± 0.1 | 1.5 ± 0.6 | 0.9 ± 0.7 | 1.6 ± 1.6 | 1.1 ± 0.5 | 0.0 ± 0.0 | 1.7 ± 0.5 |
| 18:1(9) | 2.5 ± 0.7 | 0.8 ± 0.3 | 2.1 ± 1.2 | 1.6 ± 1.0 | 5.3 ± 0.3 | 8.9 ± 1.6 | 2.5 ± 0.8 | 2.5 ± 0.8 | 3.5 ± 0.8 |
| 18:1(11) | 1.8 ± 2.6 | 0.7 ± 1.2 | 2.9 ± 3.3 | 17.3 ± 2.2 | 1.7 ± 0.7 | 0.7 ± 0.7 | 0.6 ± 0.5 | 0.3 ± 0.2 | 2.9 ± 1.0 |
| 18:2(9,12) | 18.4 ± 8.6 | 16.7 ± 8.5 | 5.8 ± 3.1 | 5.1 ± 5.1 | 11.3 ± 4.4 | 13.6 ± 4.2 | 6.2 ± 3.5 | 10.3 ± 3.2 | 15.6 ± 8.5 |
| 18:3(6,9,12) | 8.2 ± 2.5 | 9.0 ± 2.1 | 23.4 ± 7.8 | 0.2 ± 0.3 | 0.0 ± 0.0 | 0.5 ± 0.1 | 1.2 ± 1.4 | 0.4 ± 0.0 | 2.8 ± 1.7 |
| 18:3(9,12,15) | 12.1 ± 2.6 | 14.9 ± 1.9 | 0.8 ± 0.2 | 1.4 ± 0.9 | 3.3 ± 2.1 | 11.2 ± 3.7 | 6.0 ± 3.6 | 38.0 ± 3.5 | 9.0 ± 3.4 |
| 18:4(6,9,12,15) | 0.8 ± 0.5 | 0.8 ± 0.5 | 1.0 ± 0.4 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.5 ± 0.7 | 0.1 ± 0.0 | 4.6 ± 4.0 |
- a Each value represents the average ± standard error of the results obtained from four independent experiments. Short names of lipid classes are defined in Table 1. Note that the names of fatty acids are shown as [number of carbon atoms]:[number of double bonds] according to convention. The position(s) of double bonds are indicated in parenthesis. 16:1(3t) represents 3-trans hexadecenoic acid, which is found in PG. PI contained 18:2(11,14) at 3.7 ± 3.8%.
The fatty acid compositions of PC and DGTS were largely similar in C. asymmetrica and C. sphaeroides, with 16:0, 18:2 and 18:3 as major components. 18:3(9,12,15) was present in PC and DGTS but not in PE in C. asymmetrica. 18:1(11) was detected as a fatty acid component in PI in both organisms, and also in PE in C. sphaeroides. Putative 18:2(11,14) was also detected in PI in both strains. An abundance of 16:4 in monogalactosyl diacylglycerol (MGDG) was a common characteristic in these strains as well as in C. reinhardtii.
Labeling of phospholipids in the two species of Chlamydomonas
The cells of C. asymmetrica and C. sphaeroides were pulse-labeled and chased with [32P]phosphate. The increase in the total amount of label was small during the chase period (Figure 3a,d). The major labeled lipid was PG (Figure 3b,e). In C. asymmetrica, significant amounts of radioactivity were detected in PC after the labeling, from a chase period of 2 h onwards. The radioactivity ratio of PC/PE increased rapidly during the chase period. In contrast, the labeling of PC or the radioactivity ratio of PC/PE increased slowly during the chase period in C. sphaeroides. The ratio of PC/PE on a molar basis (Table 1) was 0.54 and 0.24 for C. asymmetrica and C. sphaeroides, respectively. If PC and PE were labeled independently within the cell, they would be expected to be labeled at these molar ratios in the two strains, respectively. After labeling for 15 min, the radioactivity ratio was 0.14 ± 0.07 (n = 7) and 0.07 ± 0.06 (n = 4), respectively. After chasing for 2 h, the ratio increased to 0.28 ± 0.17 in C. asymmetrica, whereas it remained at 0.07 ± 0.07 in C. sphaeroides. After chasing for 22 h, the ratio increased to 0.42 ± 0.11 and 0.14 ± 0.10, respectively. We would expect that this PC/PE ratio could exceed the molar ratio during the chase if the conversion of PE to PC were very effective. These results suggest that the pathway of synthesis of PC might be different in these species. In C. asymmetrica there must be a pathway leading to a rapid labeling of PC. In C. sphaeroides (and perhaps in C. asymmetrica, too) PC might be labeled after a large pool of phosphate-containing compounds, possibly the PE pool.
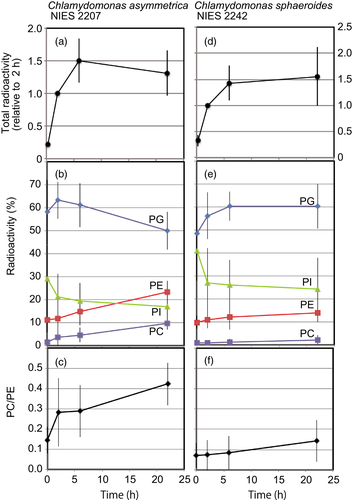
Phosphatidylcholine (PC) synthesis in the two Chlamydomonas species.
The cells of C. asymmetrica (a–c) and C. sphaeroides (d–f) were labeled with [32P]phosphate for 15 min, and then chased for 22 h. Cells were removed at time intervals and total lipids were extracted. Lipids were analyzed by 2D-TLC and then autoradiographed. The radioactive spots were scraped for liquid scintillation counting. (a), (d) Total radioactivity in the total lipids. (b), (e) Radioactivity in individual phospholipids. (c), (f) Radioactivity ratio of PC/PE. The average and standard deviation of seven (a–c) or four (d–f) independent experiments are shown. PI, phosphatidylinositol; PG, phosphatidylglycerol; PE, phosphatidylethanolamine.
Detection of a phosphoethanolamine methyltransferase gene
We analyzed the genomic sequences of these two species of Chlamydomonas. In C. asymmetrica, a putative coding sequence for PEAMT was detected. Based on the genomic data, we obtained a cDNA encoding a putative PEAMT (Figure S5). Curiously, a short extra sequence with a GS-rich tract and a histidine-rich region was found within the putative PEAMT of C. asymmetrica. Phylogenetic analysis indicated that the deduced amino acid sequence was closely related to the PEAMT of the charophyte Klebsormidium flaccidum (Figure 4). This PEAMT is, therefore, likely an indigenous enzyme in the genus Chlamydomonas but is unlikely to be a result of horizontal gene transfer.
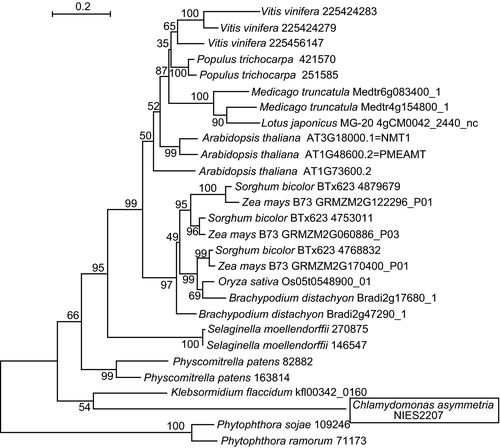
Phylogenetic tree of phosphoethanolamine methyltransferases (PEAMT).
The members of clusters 3827 and 28249 in the Gclust 2012_42 dataset, as well as the putative PEAMT of Chlamydomonas asymmetrica, were used to calculate the maximum likelihood tree as described in Sato (2010). The number on each major branch indicate the percentage confidence level.
If PEAMT provides phosphocholine, then CCT must be present. Ethanolamine-phosphate cytidylyltransferase (ECT) and CCT share the ‘CCT domain’ in their N-terminal halves. ECT has an extra domain specific to ECT. We found a putative CCT-encoding sequence in the genomic assembly of C. asymmetrica which contained only the CCT domain. Phylogenetic analysis (Figure 5) indicated that the putative CCT enzyme was near the root of plant CCT enzymes, which belong to a different clade from ECT enzymes. In addition, a putative CKI gene was also found. In the phylogenetic tree, the putative CKI was located again near the root of plant CKI enzymes, which are distinct from ethanolamine kinases (EKI) (Figure S6).
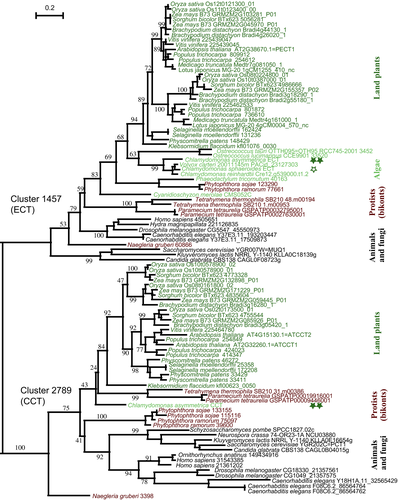
Phylogenetic tree of ethanolamine-phosphate cytidylyltransferases (ECT) and choline-phosphate cytidylyltransferases (CCT).
The members of the clusters 1457 and 2789 in the Gclust 2012_42 dataset, as well as the putative ECT and CCT of Chlamydomonas asymmetrica (double filled stars) and C. sphaeroides (star), were used to calculate the maximum likelihood tree. The number on each major branch indicates the confidence level as a percentage. Note the presence of C. asymmetrica CCT in the lower cluster representing CCT.
The final enzyme for the synthesis of PC from CDP-choline, cholinephosphotransferase (CPT), is identical to ethanolaminephosphotransferase (EPT), even though the two enzymes are distinguished in some organisms. Cyanidioschyzon merolae and various Chlamydomonas strains possess a single EPT/CPT enzyme (Figure S7). Curiously, all Chlamydomonas enzymes known to date were close relatives of animal enzymes but not plant enzymes.
We detected all enzymes for the synthesis of PE in the algae studied. We found EKI (Figure S6) and ECT (Figure 5) to be close relatives of the respective plant enzymes. Together with the aforementioned EPT/CPT, a complete pathway from ethanolamine to PE is present in C. merolae and the two Chlamydomonas strains. All these pieces of information are mapped on the pathways in Figure 1.
The genome of C. sphaeroides was carefully searched for the enzymes involved in PC synthesis. Neither CKI, CPT nor PEAMT were found encoded in the genome. But we found a short segment similar to the central region of PLMT in both C. sphaeroides and C. asymmetrica, but not in C. reinhardtii (Figure S8). It is difficult to judge if the detected fragments in the two Chlamydomonas species are part of the authentic PLMT and if the enzyme has the activity of three methylations, because the homology is limited to the indicated region (Figure S8c). Nevertheless, the results are consistent with the results of labeling studies, although we will have to determine the identity of these sequences in more detail in a future study.
Additional biochemical analyses
The pathway of synthesis of PC was tested by in vitro enzyme analysis. The activity of PEAMT was assayed in crude cell extracts. The activity was not detected in Cyanidioschyzon merolae, Chlamydomonas reinhardtii or C. sphaeroides. In C. asymmetrica, a low activity, dependent on the addition of phosphoethanolamine, was measured (2.1 pmol methyl h−1 μg−1 protein vs 0.3 without the addition of phosphoethanolamine). We still have to optimize the measuring condition, because another Chlamydomonas species having PC (C. applanata) showed a higher activity (10.8 and 0.5 pmol methyl h−1 μg−1 protein, with and without phosphoethanolamine, respectively).
We also added [14C]choline to the cells of Cyanidioschyzon merolae and Chlamydomonas asymmetrica, to test if choline is incorporated into PC. In C. asymmetrica, virtually no radioactivity was taken up by the cells. The putative CKI detected in C. asymmetrica is unlikely to act in utilizing choline in the environment. In Cyanidioschyzon merolae, choline was incorporated into the cells, but PC was not labeled. This result, as well as the absence of PEAMT activity in C. merolae, confirms that the PE methylation is the only currently identifiable pathway of PC synthesis in this alga. A simulation analysis also supports this argument (Appendix S1).
When [32P]-labeled PE was added to the cells of Chlamydomonas asymmetrica and C. sphaeroides as in the experiments in Cyanidioschyzon merolae (Figure 2g), PC was not efficiently labeled. The PC/PE ratio after 5 h of incubation was 0.03 ± 0.02 and 0.01 ± 0.00, respectively, whereas the ratio in the substrate [32P]PE was 0.01. The result suggests that exogenous PE could be utilized by these Chlamydomonas cells, but quite inefficiently. This could suggest the operation of a low activity of PEMT/PLMT as suggested by the genomic analysis.
Discussion
Complex metabolism of PC in Cyanidioschyzon merolae
In a previous study using [2-14C]acetate, we showed that PC was rapidly labeled in C. merolae (Sato and Moriyama, 2007). This is in clear contrast with the current results, in which the labeling of PC was slower than that of PE or other phospholipids. These contrasting results indicate that the acyl groups are rapidly recycling in PC as in many other organisms (for a recent review see Allen et al., 2015), whereas the head group of PC originates from PE by methylation. Comparison of the fatty acid compositions of PC and PE (Sato and Moriyama, 2007) indicated that more linoleic acid and less oleic acid are present in PC than in PE. This is consistent with the fact that PE is a precursor to PC and that desaturation (from oleic to linoleic) occurs on the lipid molecules (mainly PC). The presence of three desaturases (two stearoyl desaturases and one oleoyl desaturase) in the ER but not in the plastid suggested the importance of PC in desaturation. Other lipids are likely to acquire unsaturated acyl groups ultimately from PC. Toyoshima and Sato (2015) presented a model in which the acyl groups of PC are rapidly exchanged with the acyl CoA pool. This situation is specific to the red algae, in which plastidic stearoyl acyl-carrier protein (ACP) desaturase does not exist. The green algae such as Chlamydomonas have various desaturases within the chloroplast as well as in the ER for the production of highly unsaturated galactolipids and phospholipids. In plants, the DAG part of PC is known to be precursor to the DAG moiety of MGDG through the action of the enzyme cholinephosphotransferase (Slack et al., 1985). It is quite improbable that the whole DAG part of PC is a precursor to MGDG, because the positional distribution of 16:0 and 18:2 is opposite in PC and MGDG. A labeling experiment with glycerol would provide evidence for this, but virtually no labeled glycerol was incorporated into the lipids, maybe because glycerol is rapidly used as a substrate for respiration in these algae (Moriyama et al., 2015).
Another important question is raised with respect to the supply of ethanolamine in Cyanidioschyzon merolae. As shown in Figure 1(c), C. merolae lacks serine decarboxylase. Degradation of S1P can provide phosphoethanolamine, but this is unlikely to be the major pathway because the sphingolipid content is quite low in this alga. The results of simulation (Appendix S1) can be interpreted in both ways, but a pathway via S1P would retard the labeling of PE considerably. The question of the supply of ethanolamine or phosphoethanolamine will have to be solved in the future.
Diverse pathways for the synthesis of PC in algae
The three algae used in the present study, a red alga and two green algae, all synthesize PC, but the pathways for its synthesis are different. Methylation of PE is the sole pathway of synthesis of PC in C. merolae, whereas methylation of phosphoethanolamine provides phosphocholine for the synthesis of PC in Chlamydomonas asymmetrica. In the latter pathway, CCT is also present. Putative CKI could act in re-utilization of choline formed by degradation of PC by phospholipase D. All these pathways are present in land plants such as Arabidopsis thaliana except for the first methylation of PE. The genomic data of the charophyte K. flaccidum (Hori et al., 2014) suggest that this facultative terrestrial alga possesses the phosphoethanolamine pathway of PC synthesis and PE methylation pathway (Figures 4, 5, S1 and S6–S8). PLMT has not been annotated before, but the same partial sequence was detected in this alga (Figure S8). In this respect, the charophyte is similar to C. asymmetrica. The prasinophytes Ostreococcus tauri and O. lucimarinus are likely to possess PLMT (Figure S1), but not PEMT or PEAMT (Figure 4). The PC biosynthetic pathway in prasinophytes seems different from that in K. flaccidum and C. asymmetrica. A homology search in the genomic sequences of the red algae Porphyridium purpurea (Bhattacharya et al., 2013) and Pyropia yezoensis (Nakamura et al., 2013) detected putative sequences encoding PEMT and PLMT as in Cyanidioschyzon merolae. No homolog of PEAMT was detected in these red algae.
The mechanism of PC synthesis in Chlamydomonas sphaeroides is likely to involve methylation of PE by PLMT-like enzyme, as suggested by the detection of a fragment of a putative PLMT sequence (Figure S8). Chlamydomonas asymmetrica also possesses a similar sequence. The detected part corresponds to the active site, but we were not able to detect the N-terminal or C-terminal sequences by homology search with known PLMT/PEMT sequences of plants and animals. We will have to isolate the cDNA and determine the whole structure of the putative PLMT/PEMT in these organisms to draw a solid conclusion.
Evolutionary scenarios of PC biosynthesis in green algae
Phosphatidylcholine was detected in four strains of the genus Chlamydomonas (Sakurai et al., 2014b). These strains are distributed in various subgroups according to the phylogenetic tree based on 18S rRNA (Yumoto et al., 2013). Chlamydomonas sphaeroides is closely related to C. reinhardtii, whereas C. applanata belongs to the subgroup Polytominia. Chlamydomonas asymmetrica is placed between these subgroups. We examined two other strains that are placed between these subgroups, but found no PC. There is no simple phylogenetic relationship between the four strains having PC. In addition, the mechanism of PC synthesis is not identical even in these four strains. There are two types of explanation for this situation: divergent losses of PC or independent horizontal gene transfer events. In the first hypothesis, the ancestor of Chlamydomonas possessed multiple mechanisms of PC biosynthesis. During the diversification of Chlamydomonas species, the ability to biosynthesize PC was lost in various subgroups. Only some strains retain one or two pathway(s) of PC biosynthesis. In the second hypothesis, the ability to biosynthesize PC was independently acquired by horizontal gene transfer in various subgroups of Chlamydomonas. At least two different types of mechanisms for PC synthesis were thus acquired in different strains.
It is hard to determine which of these hypotheses is plausible. A combination of the two hypothesis is also possible, in which the ability to synthesize PC was lost in many subgroups while some strains acquired the ability by horizontal gene transfer. As pointed out above, the same set of PC biosynthetic enzymes are present in K. flaccidum and C. asymmetrica. The phylogenetic analysis in Figure 4, for example, indicated that the PEAMT in C. asymmetrica is closely related to the enzyme in K. flaccidum. This is evidence that the PEAMT was a product of vertical heritage, but not horizontal gene transfer. In this respect, the hypothesis of divergent loss of PC biosynthesis is likely. The putative CCT and CKI in C. asymmetrica are also related to the respective homologs in K. flaccidum, but in these cases the support values of the branching were low (Figures 5 and S6). We still have to wait for the full identification of PLMT/PEMT in the two species of Chlamydomonas to make a hypothesis about the evolution of this pathway.
We still have to analyze all four strains that have PC to obtain further knowledge about the mechanism of PC biosynthesis and the phylogenetic scenarios.
Concluding remarks
The available evidence suggests that there are different pathways for the synthesis of PC in different algae. Different types of PE methylation seem to work in the red algae and green algae such as K. flaccidum and Chlamydomonas. The phosphoethanolamine methylation pathway also acts in some Chlamydomonas species and the charophytes. Prasinophytes might possess the PE methylation pathway, but more work is needed on this.
Experimental procedures
Algal strains
Chlamydomonas asymmetrica NIES-2207 and C. sphaeroides NIES-2242 were obtained from the Microbial Culture Collection at the National Institute for Environmental Studies, Japan. These green algae were grown in C medium (Ichimura, 1971) with shaking at 25°C under illumination (50 μE m−2 sec−1), and used for radiolabeling experiments. They were also grown in the modified Bristol medium (MBM) with bubbling with air containing 1% CO2 for large-scale culture, as described previously for the growth of C. reinhardtii CC-1010 (Sakurai et al., 2014a). Cyanidioschyzon merolae was a laboratory stock. The cells of C. merolae were grown in 2 × Allen medium as described previously (Sato and Moriyama, 2007).
Lipid analysis
Total lipids were extracted from the algal cells by the method of Bligh and Dyer (1959). The 2D-TLC, fatty acid analysis and identification of double bond positions were performed as described previously (Sakurai et al., 2014a).
Radioactive experiments
Chlamydomonas cells that had been grown in C medium (typically 80 ml) to the mid-logarithmic phase were harvested by centrifugation (400 g, 5 min, 15°C), and then washed with MBM without phosphate. The cells were resuspended in 5 ml of MBM without phosphate. After the addition of 5 μl of [32P]phosphate (1.85 MBq at 300 TBq mmol−1, Perkin Elmer, http://www.perkinelmer.com/), the cells were incubated with shaking at 25°C under illumination (100 μE m−2 sec−1). Fifteen minutes later, 1 ml of MBM (with phosphate) was added and mixed. A 1-ml aliquot was withdrawn for lipid analysis. The remaining cells were harvested by centrifugation, and resuspended in 5 ml of C medium, which was then incubated with shaking under illumination. Aliquots (1 ml) were taken at time intervals for lipid analysis.
Cyanidioschyzon merolae cells that had been grown in the 2 × Allen medium at 40°C to the mid-logarithmic phase were harvested by centrifugation (500 g, 5 min, 30°C), and then washed with 2 × Allen medium without phosphate. The cells were resuspended in 5 ml of 2 × Allen medium without phosphate. After the addition of 5 μl of [32P]phosphate, the cells were incubated with shaking at 38°C under illumination (100 μE m−2 sec−1). Fifteen minutes later, 1 ml of 2 × Allen medium (with phosphate) was added and mixed. A 1-ml aliquot was withdrawn for lipid analysis. The remaining cells were harvested by centrifugation and resuspended in 5 ml of 2 × Allen medium. Culturing was continued, and aliquots were withdrawn at time intervals for lipid analysis.
For conversion of PE to PC, C. merolae cells in 10 ml of culture medium were incubated with [32P]PE [dissolved in a minimal amount of ethanol (50–100 μl)], which had been previously prepared by 2D-TLC from labeled C. merolae cells. Aliquots were taken and analyzed at various time intervals.
Lipids were extracted and analyzed by 2D-TLC. The first dimension was developed with acetone/benzene/methanol/water (80:30:20:10 by volume). The second dimension was developed with chloroform/methanol/ammonia water (65:35:5 by volume). In this system, PA co-migrated with PI, but we preferred this system (rather than one including acetic acid as in Sakurai et al., 2014a) for radioactive experiments because it is easier to remove solvents before autoradiography. After spraying the plates with 0.01% primuline in 80% aqueous acetone, the lipid spots were detected under UV light at 365 nm. Then the plates were autoradiographed with Fuji X-ray film RX. Radioactive lipid spots were scraped, and the radioactivity was measured by liquid scintillation counting in a toluene-based scintillation cocktail as described previously (Sato and Moriyama, 2007).
Genomic analysis
Genomic DNA was prepared from the cells of C. asymmetrica and C. sphaeroides according to established procedures (Tajima et al., 2011). Paired-end reads of these genomes were obtained by MiSeq (150 bases × 38 million reads) and HiSeq (100 bases × 341 million reads) sequencing, respectively, using the sequencing service of Takara Bio Inc. (http://www.takara-bio.com/). The reads were assembled with Velvet software v.1.2.08 (Zerbino, 2010) using a Linux workstation.
Homology search was performed with known protein sequences against the genomic contigs using the ‘tblastn’ program (Altschul et al., 1997). Manipulation of sequences was performed with siseq software v.1.59 (Sato, 2000). Comparative genomic analysis was performed with Gclust software (Sato, 2009). The Gclust2012_42 dataset was used for obtaining sequences of homolog clusters (http://gclust.c.u-tokyo.ac.jp/). Construction of multiple alignments and the phylogenetic tree was performed essentially as described by Sato (2010).
cDNA cloning
Total RNA of C. asymmetrica was purified with an RNeasy Plant Mini kit and RNase-Free DNase set (Qiagen, http://www.qiagen.com/) and cDNA was prepared by using the SMARTer RACE cDNA Amplification Kit (Clontech Laboratories, http://www.clontech.com/), according to the manufacturers’ protocols. The cDNA encoding a putative PEAMT was obtained by PCR with gene-specific primers (5′-ATGACGCACACAAGCGGAGGACTGGAGAGC-3′ and 5′-TCACTCAGGCTTGCGTGCAGTCACCAGGCC-3′). The PCR product was cloned into the pCR2.1-TOPO vector (Invitrogen, http://www.invitrogen.com/), and the DNA sequence was determined by the Fasmac sequencing service (http://www.fasmac.co.jp/English/).
Enzyme assay
The activity of PEAMT was measured in the crude cell extracts (0–70% saturated ammonium sulfate fraction) of Cyanidioschyzon merolae, Chlamydomonas reinhardtii, C. asymmetrica and C. sphaeroides according to the method of Smith et al. (2000). Phosphoethanolamine and [methyl-14C] S-adenosyl methionine were used as substrates.
Accession numbers
The nucleotide sequence data reported in the current study have been deposited in the DNA Data Bank of Japan (DDBJ) as LC068805, LC068979–LC068986.
Acknowledgements
The authors thank Drs Yuki Nakamura and Hung Chun-Hsien, Academia Sinica, Taipei for valuable discussions. The present work was supported in part by a Grant-in-Aid for Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Agency, and by a Grant-in-Aid for Scientific Research (no. 24570043) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The authors declare no conflicts of interest.




