Transfer RNA maturation in Chlamydomonas mitochondria, chloroplast and the nucleus by a single RNase P protein
Summary
The maturation of tRNA precursors involves the 5′ cleavage of leader sequences by an essential endonuclease called RNase P. Beyond the ancestral ribonucleoprotein (RNP) RNase P, a second type of RNase P called PRORP (protein-only RNase P) evolved in eukaryotes. The current view on the distribution of RNase P in cells is that multiple RNPs, multiple PRORPs or a combination of both, perform specialised RNase P activities in the different compartments where gene expression occurs. Here, we identify a single gene encoding PRORP in the green alga Chlamydomonas reinhardtii while no RNP is found. We show that its product, CrPRORP, is triple-localised to mitochondria, the chloroplast and the nucleus. Its downregulation results in impaired tRNA biogenesis in both organelles and the nucleus. CrPRORP, as a single-subunit RNase P for an entire organism, makes up the most compact and versatile RNase P machinery described in either prokaryotes or eukaryotes.
Introduction
Transfer RNAs (tRNAs) are essential adapter molecules required for translation in all domains of life. Similar to other RNA species, their biogenesis involves a number of maturation events. In particular, tRNA precursor transcripts are universally processed by an endonuclease called RNase P that removes 5′ leader sequences (Altman, 2007). A single exception is found in an archaean species where tRNAs are transcribed from position +1 without any leader sequence and RNase P is absent (Randau et al., 2008). RNase P was first characterised on a molecular level in bacteria, where it was found to occur as a ribonucleoprotein (RNP), with a catalytic RNA in complex with a protein subunit (Guerrier-Takada et al., 1983). Ribonucleoprotein RNase Ps were also identified in Archaea and eukaryotes, in both organelles and the nucleus (Hartmann and Hartmann, 2003). Throughout evolution, RNP RNase Ps have adopted very diverse architectures. Their catalytic RNA has significantly diverged, in particular those encoded in organelle genomes (Seif et al., 2003). Their protein content is even more diversified, with protein contents ranging from a single protein subunit in bacteria, four to five protein subunits in Archaea and up to ten protein subunits in nuclear enzymes (Hartmann and Hartmann, 2003). The prevalence of RNP RNase P has suggested for a long time that RNase P enzymes would universally contain a ribozyme, thus representing one of the very few conserved remnants of a prebiotic RNA world.
However, a second type of RNase P enzyme, evolutionarily independent of RNP RNase P and presenting a completely different architecture, has been found in eukaryotes (Holzmann et al., 2008; Gobert et al., 2010; Gutmann et al., 2012; Täschner et al., 2012; Pinker et al., 2013). These nuclear-encoded enzymes, called PRORPs (protein-only RNase Ps), are composed of a single polypeptide comprising two main domains: (i) an N-terminal domain containing pentatricopeptide repeat (PPR) motifs (Giegé, 2013; Cheng et al., 2016) believed to be responsible for RNA-binding and substrate specificity and (ii) a C-terminal catalytic domain belonging to the NYN (N4BP1, YacP-like nuclease) family (Anantharaman and Aravind, 2006). In all experimentally investigated PRORPs these two main domains are tridimensionally connected by a bipartite zinc-binding domain containing a CxxC signature in its first part and conserved H and C in its second part (Lechner et al., 2015). Exploration of the mode of action of PRORPs and comparison with RNP RNase P has suggested that the two types of enzyme have different catalytic mechanisms (Pavlova et al., 2012) but share similar substrate-binding processes (Gobert et al., 2013).
Analysis of all characterised RNase P systems has revealed that separate RNase P enzymes are always found in organelles and the nucleus. Although PRORP and RNP RNase P can coexist in the same eukaryote, their occurrence was found to be mutually exclusive in compartments where gene expression takes place. Nonetheless, in all cases, it was assumed that specialised RNase P enzymes (RNPs and/or PRORPs) would always be compartmentalised at specific sites where maturation of tRNA occurs (Lai et al., 2010; Lechner et al., 2015).
Interestingly, among plants, while multiple PRORP genes encode either organellar or nuclear PRORP proteins in Embryophyta such as Arabidopsis (Pinker et al., 2013), single PRORP genes are found in Chlorophyta. Unlike in animals, where a single PRORP is present in mitochondria and an RNP RNase P is found in the nucleus, no nuclear gene encodes a recognisable RNase P RNA in Chlorophyta. In some species of Prasinophytes, for example Ostreococcus tauri, RNP RNase P RNA genes have been retained in organelles; however, these genes are absent from most organelle genomes in Chlorophyta (Lechner et al., 2015). This led to the hypothesis that a single PRORP protein might be responsible for both organellar and nuclear RNase P activities in most Chlorophyta.
This hypothesis was investigated in the model chlorophyte Chlamydomonas reinhardtii: the product of the single CrPRORP gene has RNase P activity. It is found to localise to mitochondria, the chloroplast and the nucleus and its downregulation results in reduced accumulation of tRNA in both organelles and the nucleus.
Results
Identification and features defining CrPRORP
Putative PRORP sequences were searched for in the genome of C. reinhardtii using a conserved C-terminal NYN signature as a query that typifies bona fide PRORP enzymes (Lechner et al., 2015). A single candidate (Cre13.g563850) was identified. Tridimensional structure modelling using Phyre2 identified an N-terminal helix–turn–helix super-helical structure consistent with the conserved fold of the PPR domain of characterised PRORP proteins (Pinker et al., 2013; Hammani et al., 2014) (Figure 1). The product of this candidate gene has all the hallmarks of a PRORP protein and was thus termed CrPRORP. The gene is composed of nine exons. Some of the introns are present in the coding sequence of the PPR domain, unlike in PPR genes in land plants (Lurin et al., 2004). The GC content of the coding sequence is 70% (above the average genome GC content of 64%). The CrPRORP open reading frame has three predicted initiation codons, leading to possible protein lengths of 819, 783 and 718 amino acids, corresponding to calculated sizes of 87, 83 and 76 kDa, respectively, and thus significantly larger than HsPRORP, AtPRORP and TbPRORP (Gobert et al., 2010; Holzmann et al. 2008; Täschner et al. 2012). It is noticeable that stretches of alanines and glutamic acids (not found in higher plants) are present in the sequence. The connecting domain bridging the PPR and NYN domains of CrPRORP also differs from that of higher-plant PRORPs, with the conserved CxxC replaced by a CxxA motif in its first part and the conserved H residue in the second part replaced by M in Chlamydomonas (Figure 1 and Figure S1 in the Supporting Information).
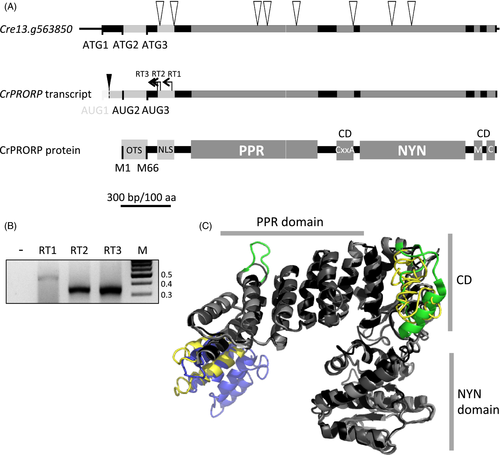
Features defining Chlamydomonas reinhardtii protein-only RNase P (CrPRORP).
(A) Schematic representation of the CrPRORP genomic locus, transcript and protein sequence. White triangles represent the positions of the eight introns. Three initiation codons are in frame with the CrPRORP sequence. The first one is not present in mature transcripts. The latter two result in methionine 1 and 66 (M1, M66) in CrPRORP. The two main domains, i.e. the RNA-binding pentatricopeptide repeat (PPR) domain and the catalytic NYN domain are bridged by a bipartite connecting domain (CD) involving a cysteine xx alanine (CxxA) signature in its first part as well as a methionine (M) and a cysteine (C) in its second part. OTS stands for organellar targeting sequence and NLS for nuclear localisation signal. The scale bar is represented in base pairs and amino acids. The black triangle represents the location of CrPRORP transcript 5′ ends mapped by 5′ rapid amplification of cDNA ends (B) as determined by reverse transcriptions initiated at three independent sites (RT1–RT3) resulting in single amplification products. Molecular weights are indicated in kilobase pairs.
(C) Tridimensional model of CrPRORP starting from M66 superimposed on the experimental structures of Arabidopsis PRORP1 and human PRORP. Grey parts represent structural elements common to the three proteins. Yellow parts are specific to CrPRORP while blue parts are specific to human PRORP and green parts to Arabidopsis PRORP1.
The three initiation codons found in frame in the 5′ part of CrPRORP gene suggest that various isoforms of CrPRORP might be produced from the single gene and potentially targeted to different compartments. Localisation prediction tools, i.e. TargetP, Predotar, MultiLoc2 and PredAlgo, were thus used to determine the potential compartment where the putative proteins could be targeted. Predictions for the longer putative protein were not consistent between algorithms. The four tools used here consistently predicted the second in-frame AUG codon (AUG2) to express a protein targeted to mitochondria and/or chloroplasts. The three organelle-specific tools predicted that the shorter version expressed from AUG3 would be localised outside organelles and MultiLoc2 predicted it to be localized in the cytosol or nucleus. Moreover NLS Mapper identified a putative monopartite nuclear localisation signal ten amino acids downstream of AUG3 (Figure S1). Taken together, these predictions led to the hypothesis that a longer CrPRORP expressed from AUG2 is targeted to organelles whereas a shorter CrPRORP is targeted to the nucleus.
CrPRORP is expressed from a single transcript
As a prerequisite to the characterisation of CrPRORP protein, the 5′ extremities of its transcript had to be determined in vivo. This would reveal if the three putative initiation codons are actually present in CrPRORP transcripts and if different forms of its mRNA accumulate in vivo. For this, the 5′ ends of CrPRORP mRNAs were mapped by 5′ rapid amplification of cDNA ends (5′-RACE). A single amplification product could be detected (Figure 1A, B), cloned and sequenced. Analysis of 180 sequences obtained from three independent reverse transcription sites revealed transcript ends at closely spaced discrete positions 26 and 44 nucleotides downstream of AUG1 (80 and 62 nucleotides upstream of AUG2). This shows that the longest conceivable isoform of CrPRORP is not produced and CrPRORP is expressed from a transcript comprising AUG2 and AUG3 in vivo.
The analysis of publicly available transcriptomic data, i.e. RNA-seq expression data from 133 different experiments compiled and analysed in the Chlamydomonas reinhardtii v5.5 resource from Phytozome 10.3 ( http://phytozome.jgi.doe.gov/) revealed that CrPRORP is co-expressed with many genes encoding proteins involved in gene expression processes. In particular, among the 100 genes displaying the same expression pattern with a Pearson correlated expression >0.89, more than half (59 genes) encode factors involved in processes such as rRNA and tRNA processing, transcription and RNA degradation (supplementary Table S1). This expression pattern is consistent with a gene having a housekeeping function required for RNA metabolism in Chlamydomonas.
CrPRORP localises in both organelles and nuclei in vivo
Recombinant CrPRORP proteins were used to raise specific antibodies that were assayed on total Chlamydomonas proteins. Antibodies detected a signal corresponding to a protein migrating at an apparent molecular weight of 100 kDa on SDS PAGE. Gel slices corresponding to the detected signals were analysed by tandem mass spectrometry, which unambiguously identified CrPRORP in the 100-kDa band (Figure S1). In order to evaluate the apparent molecular weight of CrPRORP, a cDNA starting from AUG3 was cloned, expressed in Escherichia coli and purified to homogeneity, leading to a protein of a calculated 76 kDa, pI of 5.0 and a grand average hydropathicity of −0.43, revealing a hydrophilic protein. Western blot analysis on protein extracts from E. coli cells where the expression of recombinant CrPRORP was induced also detected a 100-kDa signal (Figure 2A). Then, in order to determine the subcellular localisations of CrPRORP in vivo, subcompartments where gene expression takes place in Chlamydomonas, i.e. mitochondria, chloroplasts and nuclei, were purified and analysed by Western blot with the CrPRORP-specific antibodies. The CrPRORP-specific signal was detected in the three compartments (Figure 2B). The purity of the respective cell fractions was evaluated with antibodies specific for the mitochondrial Voltage Dependent Anion Channel I, the chloroplast cytochrome f and the nuclear histone 3 (Figure 2B). Taken together the results show that in vivo CrPRORP is present in Chlamydomonas in both organelles and the nucleus. The signals of equal size in E. coli cells expressing CrPRORP from AUG3 and in Chlamydomonas extracts suggest that shorter forms of CrPRORP are detected. These likely correspond (i) to the shorter CrPRORP expressed from AUG3 to achieve nuclear localisation and (ii) to a mature form of CrPRORP expressed from AUG2, where the organelle-targeting signal has been processed upon protein import. Indeed, localisation prediction tools such as TargetP predict that a mature isoform of CrPRORP expressed from AUG2 coincidentally has a size very close to that of the full-length isoform of CrPRORP expressed from AUG3.
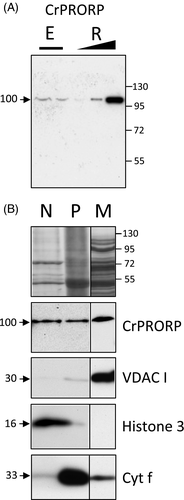
Chlamydomonas reinhardtii protein-only RNase P (CrPRORP) localises in organelles and the nucleus.
(A) CrPRORP is detected in vivo with specific antibodies in total protein extracts (E) from Chlamydomonas cells at an apparent molecular weight consistent with recombinant CrPRORP (R) translated from AUG3 in Escherichia coli extracts expressing increasing amounts (black triangle) of CrPRORP.
(B) Subcellular fractions were loaded on two 10–15% SDS-PAGE gels. After transfer, the filters were cut and incubated with antibodies directed against CrPRORP, mitochondrial Voltage Dependent Anion Channel I (VDACI) and nuclear histone 3 for the first blot and chloroplast cytochrome f (Cyt f) for the second blot. CrPRORP is immunodetected in subcellular fractions representing purified nuclei (N), chloroplasts (P) and mitochondria (M). The top panel is a composite image corresponding to the Coomassie staining of the first blot. For each antibody, the same exposure is shown for the two parts of the filter. Molecular weights are indicated in kDa.
CrPRORP carries out maturation of organellar and nuclear tRNAs in vitro
Protein-only RNase P enzymes carry out endonucleolytic 5′ maturation of precursor tRNAs either as single-subunit enzymes, as observed for characterised streptophyte PRORP enzymes (Gobert et al., 2010; Sugita et al., 2014), or require additional protein partners for their activity, as observed in humans (Holzmann et al., 2008). In order to test whether CrPRORP is indeed a bona fide RNase P enzyme and determine if it can act as a single-subunit enzyme, RNase P activity assays were performed on transcripts representing the diversity of tRNA precursors in Chlamydomonas. In vitro transcripts representing the three mitochondrial tRNA precursors, three chloroplast and three nuclear tRNA precursors containing 5′ leader and 3′ trailer sequences of about 50 and 20 nucleotides, respectively, were generated. Precursors for nuclear tRNATrp (CCA) and tRNAMet (CAU) also contained introns in their anticodon domain (Cognat et al., 2013). In the presence of recombinant CrPRORP, precursor tRNAs were processed to 5′ mature tRNAs and 5′ leader sequences were released (Figure 3). The characterisation of cleavage products by circular RT-PCR revealed that maturation had taken place immediately upstream of tRNA position +1, consistent with canonical RNase P activity (Figure S2). Maturation of intron-containing nuclear tRNA precursors had taken place similar to other tRNAs. This shows that CrPRORP is able to cleave precursor transcripts in the presence or absence of introns and confirms that the nature of the sequence and/or structure of the anticodon domain has no impact on RNase P activity as previously proposed (Gobert et al., 2013). Altogether, results show that Chlamydomonas PRORP has RNase P activity as a single-subunit enzyme.
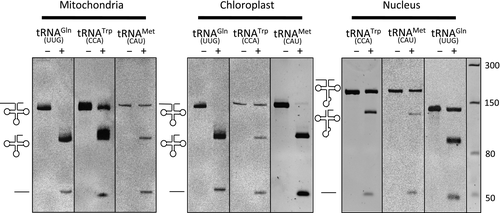
Chlamydomonas reinhardtii protein-only RNase P (CrPRORP) is an RNase P enzyme.
Activity was assayed using in vitro transcripts representing precursors of the three tRNAs encoded in the mitochondrial genome, three chloroplast tRNAs and three nuclear encoded tRNAs. Precursor transcripts are 142, 144, 142, 141, 140, 142, 168, 171 and 140 ribonucleotides long, respectively. Nuclear tRNATrp (CCA) and tRNAMet (CAU) contain introns in their anticodon domain. Transcripts were incubated alone (–) or in the presence of CrPRORP (+). RNase P endonucleolytic cleavages result in the release of 50-ribonucleotide-long leader sequences. Molecular weight markers are indicated in ribonucleotides.
CrPRORP is involved in the biogenesis of both organellar and nuclear encoded tRNAs in vivo
In order to investigate the function of CrPRORP in tRNA maturation in both organelles and the nucleus in vivo, a construct expressing an artificial microRNA (amiR) specifically targeting the CrPRORP transcript was stably transformed in Chlamydomonas. The 21-nucleotide long amiR targets a sequence starting 47 nucleotides downstream of AUG3 in CrPRORP mRNA. Downregulated levels of CrPRORP in individual transformants were evaluated by Western blot analysis of total protein extracts. Two specific lines were retained where CrPRORP was downregulated five- and ten-fold, respectively (Figure 4A). The effect of CrPRORP downregulation on the accumulation of tRNAs was monitored by gene-specific hybridisation of RNA gel blots, as described previously (Gutmann et al., 2012). Levels of tRNA were investigated for the same nine tRNAs already investigated in vitro (Figure 3). They include all the tRNAs encoded in the Chlamydomonas mitochondrial genome (three tRNAs) as well as identical numbers of tRNAs encoded in the chloroplast genome and the nuclear genome. Signals corresponding to the steady-state levels of the three mitochondrial mature tRNAs decreased 2.3-, 4.7- and 6.5-fold, respectively, in amiR lines 1 and 2 (average decrease for the two lines). Similarly, signals corresponding to the three chloroplast mature tRNAs decreased 5.8-, 6- and 17.8-fold, respectively, on average in amiR lines 1 and 2. Finally, signals corresponding to the three nuclear encoded tRNAs also decreased 5.3-, 5.6- and 5.9-fold, respectively, on average in amiR lines 1 and 2 (Figure 4B). Interestingly, for tRNATrp (CCA) two bands corresponding to the spliced and unspliced forms of the tRNA were detected. Both forms of the tRNA were downregulated in the amiR lines, thus showing that 5′ maturation of RNase P is independent of splicing in vivo. However, no signal corresponding to the accumulation of unprocessed tRNA 5′ precursors in the amiR lines could be observed. This suggests that degradation mechanisms and/or quality control processes might target unprocessed non-functional organellar and nuclear tRNAs in Chlamydomonas, contrary to Arabidopsis organelles where tRNA 5′ precursor accumulation could be observed in PRORP downregulation lines (Gutmann et al., 2012). As a control, the levels of mitochondrial L7 rRNA, chloroplast 16S rRNA and nuclear 18S rRNA were investigated in amiR lines, revealing no changes in steady-state levels of rRNA. Similarly, the levels of the mitochondrial Nd4 mRNA, chloroplast psaB mRNA and the nuclear MAA7 mRNA did not vary in amiR lines compared with the wild type (Figure 4C). Altogether, results show that the downregulation of CrPRORP in vivo results in decreased levels of both organellar and nuclear tRNAs, thus showing that its function is required for the biogenesis of both organellar and nuclear tRNAs.
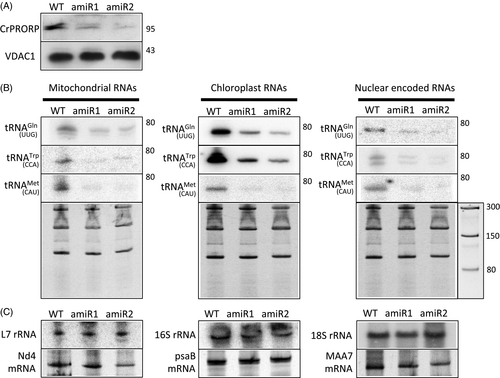
Molecular characterisation of Chlamydomonas reinhardtii protein-only RNase P (CrPRORP) downregulation mutants.
(A) Total proteins from wild type Chlamydomonas (WT) as well as from two independent lines expressing an artificial miRNA targeting CrPRORP (amiR1 and amiR2) were analysed by Western blot with specific antibodies. Molecular weights are indicated in kDa.
(B) Total RNA from the respective lines was separated on denaturing acrylamide gels, blotted and analysed by hybridisations using specific probes representing the nine tRNAs previously assayed for in vitro RNase P activity. Stained blots used for mitochondrial tRNAGln (UUG)-, chloroplast tRNAGln (UUG)- and nuclear tRNAGln (UUG)-specific hybridisations are shown as examples. Molecular weights are indicated in ribonucleotides.
(C) Control hybridisations were performed with rRNA- and mRNA-specific probes for the respective compartments.
Discussion
This study has identified CrPRORP, a protein with RNase P activity localised to both organelles and the nucleus in Chlamydomonas, but no recognisable RNase P RNA gene could be found. Isoforms of this protein are most probably the sole RNase P enzymes in Chlamydomonas because CrPRORP downregulation results in impaired tRNA biogenesis in both organelles and the nucleus.
CrPRORP has all the features that define canonical PRORP enzymes (Lechner et al., 2015). It seems to share a similar mode of action with other PRORPs, for example its activity does not seem to involve interaction with the tRNA anticodon domain similar to Arabidopsis PRORPs (Gobert et al., 2013). However, its architecture also involves specific features, in particular its connecting domain differs significantly from Arabidopsis PRORPs and possibly involves a weaker interaction with metals, as conserved residues involved in zinc coordination in Arabidopsis are absent in CrPRORP (Figures 1 and S1). Comparison of the structural model of CrPRORP with Arabidopsis PRORP1 and human PRORP experimental structures predicts that CrPRORP is more compact than Arabidopsis PRORP1 with a shorter loop in the PPR2 motif and a condensed, although disordered, connecting domain between the PPR and NYN domains. In contrast, CrPRORP appears to be less compact than human PRORP, which has an even more reduced connecting domain (Figure 1C). Taken together, modelling suggests that the core mature CrPRORP represents a structural intermediate between Arabidopsis and human PRORPs.
The observation of CrPRORP triple localisation raises the question of the nature of the mechanism responsible for this multi-addressing. While dual localisation of proteins to mitochondria and chloroplasts is well established and frequent in the green Archaeplastida lineage (Duchêne et al., 2005; von Braun et al., 2007), dual localisation of proteins to organelles and the nucleus has been less frequently described. Some reports show that it can take place between chloroplasts and the nucleus (Krause and Krupinska, 2009) and between mitochondria and the nucleus (Duchêne and Giegé, 2012). However, to our knowledge, triple localisation has never been reported. Published examples have revealed that multi-localisation could be achieved by: (i) alternative splicing, (ii) alternative initiation of transcription, (iii) alternative initiation of translation, and (iv) through the relocation of organellar mature proteins to the nucleus. In the case of CrPRORP, multi-addressing does not involve the production of alternative transcripts as experimentally shown here. It could thus be achieved by either process (iii) or (iv). However, because of its housekeeping function in both organelles and the nucleus, it is unlikely that CrPRORP is relocated from organelles to the nucleus as observed for regulatory proteins (Hammani et al., 2011). Multi-localisation is thus probably achieved by alternative initiation of translation, using AUG2 for the translation of the organellar CrPRORP and AUG3 for the translation of the nuclear isoform.
This situation, where an essential function such as RNase P is encoded by a single gene whose products are localised to multiple compartments, might not be unique to CrPRORP. Database analyses reveal that it could also take place for other essential functions, i.e. tRNA biogenesis. For example, similar to CrPRORP, a single gene encodes a tRNA CCA nucleotidyltransferase in Chlamydomonas, while CCA addition takes place in mitochondria, chloroplasts and the nucleus (Cognat et al., 2013).
The nuclear genomes of all Chlorophyta sequenced to date encode a single PRORP. The group Chlorophyta includes Prasinophytes such as Ostreococcus tauri, Trebouxiophyceae such as Chlorella variabillis and Chlorophyceae such as C. reinhardtii (Lemieux et al., 2014). In the Prasinophytae clade of Mamiellophyceae (including Ostreococcus), single PRORPs probably represent nuclear RNase P enzymes because RNase P RNA genes are present in mitochondrial and chloroplast genomes (Lai et al., 2011). In other Prasinophytae, PRORP might have acquired an additional organellar localisation because RNase P RNA genes were lost from either mitochondrial or chloroplast genomes. In all other Chlorophyta, RNase P RNA genes were seemingly entirely lost and single PRORP genes are likely to hold RNase P activity in mitochondria, chloroplasts and the nucleus, similar to Chlamydomonas. Phylogenetic analysis of PRORP proteins in Chlorophyta suggests a common origin for all PRORPs in this group (Figure 5, Table S2). This implies that the ancestral PRORP in this group might have been a nuclear RNase P enzyme that subsequently evolved additional organellar RNase P activities while RNase P RNA genes were lost from organellar genomes in Chlorophyta.
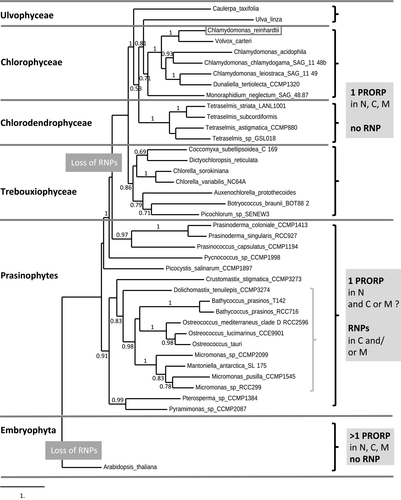
Distribution of protein-only RNase P (PRORP) proteins in Chlorophyta.
A phylogenetic tree of PRORP sequences representative of all the subgroups of Chlorophyta was constructed using the maximum likelihood method. Reliability for the internal branch was assessed using the aLRT test, with values above 0.5 indicated along branches. The grey brace on the right represents the Mamiellophyceae clade among Prasinophytes. The tree was rooted with the Embryophyta (Arabidopsis PRORP2). While multiple PRORPs are found in Embryophyta in the nucleus (N), chloroplasts (C) and mitochondria (M), a single PRORP is always found in Chlorophyta. In Prasinophytae, genes encoding putative ribonucleoprotein RNase P (RNP) RNAs have been kept in mitochondrial and/or chloroplast genomes. However, these genes were entirely lost in all the other chlorophyte subgroups, suggesting that organellar and nuclear RNase P enzymes are encoded by a single PRORP gene in Ulvophyceae, Chlorophyceae, Chlorodendrophyceae and Trebouxiophyceae.
This study contributes to our understanding of the evolutionary diversity of RNase P systems. In prokaryotes, the simplest form of RNase P enzyme consists of a ribozyme complexed with a single protein subunit. In eukaryotes, in contrast, fulfilment of RNase P activity can involve very complex machineries. For example, in yeast different ribonucleoproteins including ribozymes and different numbers of protein subunits have to be assembled in both mitochondria and the nucleus. Other eukaryotes such as Embryophyta have retained simpler machineries for RNase P activity with multiple PRORPs present in organelles and the nucleus. In Chlorophyta such as Chlamydomonas, the RNase P machinery is even simpler, with a unique gene encoding all the PRORP isoforms required for RNase P activity in an entire organism. Chlamydomonas thus holds the most compact RNase P machinery described to date in both prokaryotes and eukaryotes. Its occurrence contradicts the dogma of universal compartmentalisation and specialisation of RNase P systems in organelles and the nucleus.
Experimental procedures
Chlamydomonas strain
The C. reinhardtii cw15 arg7-8 mt+ strain used for this study was kindly provided by Professor C. Remacle (University of Liège, Belgium). This strain lacks a cell wall and the argininosuccinate lyase enzyme. Cells were routinely grown under continuous light (50 μmol photons m−2 sec−1) at 25°C in liquid or solid agar 2-amino-2-(hydroxymethyl)-1,3-propanediol (TRIS)-acetate phosphate (TAP) medium (Harris, 1989) and supplemented with arginine when necessary.
Bioinformatic analyses
Subcellular localisation predictions of CrPRORP were determined with TargetP ( http://www.cbs.dtu.dk/services/TargetP/), Predotar ( http://urgi.versailles.inra.fr/predotar/predotar.html), MultiLoc2 ( http://abi.inf.uni-tuebingen.de/Services/MultiLoc2) and PredAlgo ( https://giavap-genomes.ibpc.fr/cgi-bin/predalgodb.perl?page=main) (Small et al., 2004; Emanuelsson et al., 2007; Blum et al., 2009; Tardif et al., 2012). Prediction of the nuclear localisation signal was performed with NLS Mapper (Kosugi et al., 2009).
Protein structures were predicted using the Phyre2 algorithm (Protein homology/analogy recognition engine v.2.0) in the intensive modelling mode (Kelley and Sternberg, 2009).
Phylogenetic analysis of 38 PRORP sequences from various chlorophyte species was performed (Dereeper et al., 2008). Sequences were aligned with ProbCons (v.1.12) (Do et al., 2005) and the phylogenetic tree was reconstructed using the maximum likelihood method implemented in the PhyML program (v.3.1/3.0 aLRT) (Guindon and Gascuel, 2003); reliability for internal branching was assessed using the aLRT test (minimum of SH-like and χ2-based parametric) (Anisimova and Gascuel, 2006). The graphical representation was performed using TreeDyn (v.198.3) (Chevenet et al., 2006).
Transcript 5′ termini mapping experiments
5′-RACE was performed as described by Peltier et al. (2012) with total RNA extracted from 7-day Chlamydomonas agar plate cultures. In brief, after treatment with DNase I, RNA was dephosphorylated and decapped with decapping pyrophosphoydrolase. A RNA oligonucleotide (5′-GAACACUGCGUUUGCUGGCUUUGAUGAAA-3′) was ligated in 5′ and cDNA was produced with SuperScript III reverse transcriptase using three alternative CrPRORP-specific primers (5′-TCGCGTGTCTGCCGCCTCGCC-3′, 5′-CTGCGTGTCCGGCGACTCGTCG-3′ and 5′-GGGATACTAGCCGTTCGACGTGG-3′). CrPRORP cDNAs were amplified by nested PCR, cloned and sequenced.
Protein expression, antibody production and immunodetection analyses
Two cDNAs corresponding to CrPRORP M66 (AUG3) to A783 and to CrPRORP M261 to E663 were cloned in pET28b to express recombinant proteins fused to C-terminal polyhistidine tags in E. coli BL21 cells. Proteins were purified in non-denaturing conditions by HisTrap affinity chromatography. The longer protein was used in enzyme activity assays while the shorter protein was injected into rabbits to raise polyclonal antibodies. The sera were purified against the fusion protein CrPRORP-His coupled to HiTrap NHS-activated sepharose and used at 1/5000 dilution for Western blot analysis.
Chlamydomonas subcellular fractionations
Chlamydomonas cells were incubated in 100 mm HEPES (pH 7.5), 4 mm EDTA, 5 mm DTT, 0.4 mm PMSF, 10% (v/v) glycerol and 0.5% (v/v) Triton X-100, homogenised using a Potter homogeniser and centrifuged for 10 min at 12 000 g to obtain a total protein extract.
To purify the mitochondria cells were lysed using digitonin. After obtaining a cytoplasmic aggregate (Klein et al., 1983), separation of organelles was performed in the presence of Mg2+ by differential centrifugation (Cardol et al., 2002). The mitochondria-enriched fraction was further purified on a Percoll step gradient (45%/21%/13%).
To purify the chloroplasts, Chlamydomonas cells were lysed with 0.25% (w/v) saponin in 50 mm HEPES-KOH (pH 7.5), 5 mm MgCl2 and 0.3 m sorbitol (Henderson et al., 2003). The 2300-g pellet was washed and loaded on a two-step Percoll gradient (40% and 80%) as described previously (Bienvenut et al., 2011). Intact chloroplasts were harvested from the interphase.
Nuclei were isolated using a protocol designed for Arabidopsis and optimised for Chlamydomonas (Winck et al., 2012). In brief, cells in early log phase (3 × 106 cells ml−1) were harvested, washed and disrupted by grinding in a mortar cooled with liquid nitrogen. Lysis of the cellular membrane was obtained after incubation in a buffer containing 1% (v/v) Triton X-100. The 1000-g pellet was washed by resuspension and a fraction enriched in nuclei obtained after centrifugation at 600 g.
In vitro RNase P activity assays
The cDNAs representing nine Chlamydomonas tRNA precursors were cloned in pUC19, transcribed in vitro by T7 RNA polymerase. The RNase P cleavage assays were performed in three replicates using 0.5 mm of transcript and 0.15 mm of recombinant CrPRORP protein for 15 min at 25°C, as described previously (Gobert et al., 2010). RNA fragments were separated by denaturing PAGE and visualised by ethidium bromide staining.
Downregulation lines and RNA blot analyses
The artificial miRNA targeting CrPRORP was designed according to Molnár et al. (2009) using the wmd3 tool at http://wmd3.weigelworld.org/(Ossowski et al., 2008). The resulting oligonucleotides 5′-ctagtTCGGACAGGCGAGGGGGCATAtctcgctgatcggcaccatgggggtggtggtgatcagcgctaTATGTTCCCTCGCCTGTCCGAg-3′ and 5′-ctagcTCGGACAGGCGAGGGAACATAtagcgctgatcaccaccacccccatggtgccgatcagcgagaTATGCCCCCTCGCCTGTCCGAa-3′ (uppercase letters indicate miRNA*/miRNA sequences) were annealed by boiling and cooling down gradually overnight and then ligated into SpeI-digested pChlamiRNA2. Screening for correct clones was done as described (Molnár et al., 2009). pChlamiRNA2-CrPRORP was linearised by digestion with PvuI and transformed into the Chlamydomonas cw15 arg7-8 mt+ strain by electroporation according to Shimogawara et al. (1998). In brief, cells grown to mid-log phase [(3–6) × 106 cells ml−1] in arginine-supplemented TAP medium were collected by centrifugation (1500 g for 5 min) and resuspended in TAP medium with 40 mm sucrose at 108 cells ml−1. In a sterile 4-mm gap cuvette, 250 μl of cells, 2.5 μ; of herring sperm DNA (10 mg ml−1) in water and 2 μl of pChlamiRNA2-CrPRORP construct (1 mg ml−1) were added and electroporation was performed under standard parameters, i.e. 800 V, 800 Ω and 25 μF. Cells were plated in TAP agar medium and incubated at 50 μmol photons m−2 sec−1 until transformants appeared (about 1–2 weeks).
RNA blot analyses of CrPRORP downregulation lines were performed as described previously (Gutmann et al., 2012). Blots were hybridised with radiolabelled gene-specific oligonucleotides and the signal revealed with a FLA-7000 PhosphorImager and quantified with ImageGauge (Fujifilm, http://www.fujifilm.com/).
Acknowledgements
We thank the Strasbourg Esplanade proteomics platform for mass spectrometry analyses. This work was supported by the Centre National de la Recherche Scientifique, the University of Strasbourg, by Agence Nationale de la Recherche (ANR) grant (PRO-RNase P, ANR 11 BSV8 008 01) to PG and by the LabEx consortium ‘MitoCross’.




