Functional analysis of chimeric lysin motif domain receptors mediating Nod factor-induced defense signaling in Arabidopsis thaliana and chitin-induced nodulation signaling in Lotus japonicus
Summary
The expression of chimeric receptors in plants is a way to activate specific signaling pathways by corresponding signal molecules. Defense signaling induced by chitin from pathogens and nodulation signaling of legumes induced by rhizobial Nod factors (NFs) depend on receptors with extracellular lysin motif (LysM) domains. Here, we constructed chimeras by replacing the ectodomain of chitin elicitor receptor kinase 1 (AtCERK1) of Arabidopsis thaliana with ectodomains of NF receptors of Lotus japonicus (LjNFR1 and LjNFR5). The hybrid constructs, named LjNFR1–AtCERK1 and LjNFR5–AtCERK1, were expressed in cerk1-2, an A. thaliana CERK1 mutant lacking chitin-induced defense signaling. When treated with NFs from Rhizobium sp. NGR234, cerk1-2 expressing both chimeras accumulated reactive oxygen species, expressed chitin-responsive defense genes and showed increased resistance to Fusarium oxysporum. In contrast, expression of a single chimera showed no effects. Likewise, the ectodomains of LjNFR1 and LjNFR5 were replaced by those of OsCERK1 (Oryza sativa chitin elicitor receptor kinase 1) and OsCEBiP (O. sativa chitin elicitor-binding protein), respectively. The chimeras, named OsCERK1–LjNFR1 and OsCEBiP–LjNFR5, were expressed in L. japonicus NF receptor mutants (nfr1-1; nfr5-2) carrying a GUS (β-glucuronidase) gene under the control of the NIN (nodule inception) promoter. Upon chitin treatment, GUS activation reflecting nodulation signaling was observed in the roots of NF receptor mutants expressing both chimeras, whereas a single construct was not sufficient for activation. Hence, replacement of ectodomains in LysM domain receptors provides a way to specifically trigger NF-induced defense signaling in non-legumes and chitin-induced nodulation signaling in legumes.
Introduction
The activation of plant defense responses against the unwanted invasion of bacteria or fungi (innate immunity) includes recognition of microbe-associated molecular patterns (MAMPs) by pattern recognition receptors (PRRs). Microbe-associated molecular patterns are conserved molecular components from a specific class of microbes that function as elicitors of defense signaling, resulting in immunity to fungi and bacteria (Boller and Felix, 2009). A typical MAMP is chitin (a polymer with β-1,4-linked N-acetyl-d-glucosamine residues; poly-GlcNAc), which is an important structural component in fungi. Chitin fragments (chitooligosaccharides) released from fungal cell walls by plant chitinase function as MAMPs in a similar way (Felix et al., 1993; Boller and Felix, 2009). An example of a bacterial MAMP is peptidoglycan, which is a conserved structural part of the bacterial cell wall and contains alternating residues of β-1,4-linked N-acetylglucosamine and N-acetylmuramic acid residues (Gust et al., 2007). Chitin, chitooligosaccharides and peptidoglycan are recognized by PRRs containing extracellular lysin motif (LysM) domains, which are known to bind to GlcNAc-containing molecules. Ligand-mediated receptor activation triggers defense signaling, which culminates in accumulation of reactive oxygen species (ROS), mitogen-activated protein (MAP) kinase signaling, activation of defense genes and ultimately in immunity against invading microbes (Hamel and Beaudoin, 2010; Gust et al., 2012).
In rice (Oryza sativa L.), OsCEBiP (chitin elicitor-binding protein) and OsCERK1 (chitin elicitor receptor kinase 1) are LysM domain PRRs required for the induction of chitin-induced defense signaling. The chitin-binding OsCEBiP is a glycosylphosphatidylinositol-anchored protein, whereas OsCERK1 possesses an intracellular kinase domain. Biochemical studies suggest that the two proteins form heterodimers in a ligand-dependent manner to initiate defense signaling (Kaku et al., 2006; Shimizu et al., 2010). Moreover, rice possesses additional LysM domain PRRs, namely OsLYP4 and OsLYP6 (lysin motif-containing proteins 4 and 6), which seem to play a dual role in perception of chitin and peptidoglycan (Liu et al., 2012a). The OsCERK1 and OsCEBiP receptors appear to interact with the Rho-like small GTPase OsRac1 and the guanine nucleotide exchange factor OsRacGEF1, forming a so-called ‘defensome’. Phosphorylation of OsRacGEF1 by the kinase domain of OsCERK1 was found to be required for downstream defense signaling (Akamatsu et al., 2013). Furthermore, OsCERK1 phosphorylates the receptor-like cytoplasmic kinase OsRLCK185, which is a downstream signaling component required for activation of the chitin-induced MAP kinase cascade OsMKK4–OsMPK3/OsMPK6 (Kishi-Kaboshi et al., 2010; Yamaguchi et al., 2013).
In Arabidopsis thaliana, AtCERK1 (also called LYK1; LysM-containing receptor-like kinase 1) is a similar LysM domain PRR required for chitin perception and activation of chitin-responsive defense genes (Miya et al., 2007; Wan et al., 2008). AtCERK1 possesses a functional intracellular kinase domain and its activation does not require interaction with an OsCEBiP-related protein (Shinya et al., 2012; Wan et al., 2012). Biochemical data and crystal structure analysis showed that chitin and chitooligosaccharides [degree of polymerization (DP) = 4–8] bind directly to the ectodomain of AtCERK1. Long-chain chitooligosaccharides [namely chitooctaose, (GlcNAc)8] and chitin act as bivalent ligands, having the capacity to trigger dimerization of two AtCERK1 ectodomains and subsequent phosphorylation of the kinase domain (Iizasa et al., 2010; Petutschnig et al., 2010; Liu et al., 2012b). AtLYK4, an AtCERK1-related receptor with a kinase domain, may assist AtCERK1-dependent chitin signaling (Wan et al., 2012). The OsCEBiP-related protein AtLYM2 (lysin motif domain-containing glycosylphosphatidylinositol-anchored protein 2) probably represents a component of an additional chitin perception system in A. thaliana. Mutant analysis revealed that AtLYM2 is required for a chitin-induced reduction in molecular flux through plasmodesmata, a chitin response that is independent of AtCERK1-regulated defense gene activation (Faulkner et al., 2013). The related AtLYM1 and AtLYM3 proteins are involved in peptidoglycan perception and presumably form a receptor complex with AtCERK1 (Willmann et al., 2011). Similar to rice, activated AtCERK1 initiates MAP kinase signaling (via AtMPK3/AtMPK6), which triggers the expression of chitin-responsive defense genes (Wan et al., 2004; Miya et al., 2007).
Nod factors (nodulation factors; NFs) are chitin-related bacterial signals that are secreted by nitrogen-fixing rhizobia to initiate nodule symbiosis with host legumes. Nod factors are lipo-chitooligosaccharides, i.e. they consist of a carbohydrate moiety usually containing four or five β-1,4-linked GlcNAc oligomers and an acyl residue (fatty acid) at the non-reducing end. Furthermore, the terminal sugars of NFs carry strain-specific modifications, which may be required for nodulation of certain host plants (Perret et al., 2000). Arbuscular mycorrhizal fungi produce structurally related lipo-chitooligosaccharides, the so-called Myc factors, but their role in establishing symbiosis with host roots remains elusive (Maillet et al., 2011; Genre et al., 2013). Similar to the PRRs perceiving chitin, chitooligosaccharides and peptidoglycan, specific LysM domain receptors of host plants recognize NFs and Myc factors to initiate symbiotic signaling (Ferguson et al., 2010; Oldroyd, 2013). LjNFR1 and LjNFR5 (Nod factor receptors 1 and 5) of Lotus japonicus are prime examples of NF receptor genes, which have been also characterized in other legumes such as soybean (Glycine max) and Medicago truncatula (Limpens et al., 2003; Madsen et al., 2003; Radutoiu et al., 2003; Arrighi et al., 2006; Indrasumunar et al., 2010, 2011). Nod factors of Mesorhizobium loti bind to LjNFR5 and LjNFR1 at nanomolar concentrations and the two receptors seem to form a ligand-dependent heterodimerization complex (Madsen et al., 2011; Broghammer et al., 2012). LjNFR5 interacts with the Rho-like small GTPase LjROP6, which positively regulates the development of infection threads (Ke et al., 2012). In contrast to LjNFR5, which lacks protein kinase activity, the kinase activity of LjNFR1 was found to be essential for autophosphorylation and downstream signaling (Madsen et al., 2011). A specific component of nodulation signaling is the transcription factor NIN (nodule inception), which is required for expression of early nodulin genes and subsequent initiation of nodule organogenesis (Schauser et al., 1999). The NIN protein of L. japonicus binds to the promoters of two NF-Y (nuclear factor-Y) subunit genes, which are thought to regulate cell division in nodule primordia (Soyano et al., 2013).
Chimeric receptors can be designed by swapping specific domains from two different receptors. Plants expressing such chimeras are useful tools for characterization or identification of receptor–ligand interactions (e.g. Albert et al., 2010; Brutus et al., 2010; Nakagawa et al., 2011; Mueller et al., 2012). In L. japonicus, for example, chimeras containing the ectodomain of LjNFR1 and various modifications of the protein kinase domain of AtCERK1 were expressed in a non-nodulating nfr1 mutant to analyze functionality, i.e. establishment of symbiosis with M. loti. The complementation tests showed that the kinase domain of AtCERK1 gains specificity for nodulation signaling if short amino acid sequences in the region around the activation loop are replaced by those of LjNFR1 (Nakagawa et al., 2011). Plants can also be engineered that express chimeric receptors with completely different ectodomains and therefore activate signaling pathways in response to different ligands. Such altered signal output may improve the plant's responsiveness to environmental stimuli. In rice, for example, a chimeric receptor, consisting of the ectodomain of BRI1 (recognizing the brassinosteroid hormone) and the intracellular domain of the disease resistance protein XA21 (a leucine-rich repeat receptor kinase), can mediate induction of a hypersensitive response (rapid cell death) in response to brassinosteroids (He et al., 2000). More recently, the ectodomain of the chitin-binding protein OsCEBiP was fused to the intracellular kinase domain of the resistance proteins XA21 and Pi-d2. Rice plants expressing these constructs and treated with chitoheptaose, (GlcNAc)7, showed a similar hypersensitive response. Consequently, increased resistance was observed when the plants were inoculated with the rice blast fungus Magnaporthe oryzae (Kishimoto et al., 2010; Kouzai et al., 2013).
In the present study, we examined whether hybrid LysM domain receptors with exchanged ectodomains can mediate NF-induced defense signaling in a non-leguminous plant and chitin-induced nodulation signaling in a legume. Chimeras were constructed in which the ectodomain of AtCERK1 from A. thaliana was replaced by the ectodomain of LjNFR1 or LjNFR5 from L. japonicus. Mutant plants of A. thaliana (cerk1-2) expressing the chimeras were treated with NFs and characterized with respect to known AtCERK1-mediated responses, namely the formation of reactive oxygen species (ROS), expression of defense genes and resistance to the fungus F. oxysporum. Likewise, the ectodomains of the NF receptors LjNFR1 and LjNFR5 were substituted by ectodomains of the chitin perception system of rice (OsCERK1 and OsCEBiP). The chimeras were expressed in hairy roots of two NF receptor mutants of L. japonicus that express a β-glucuronidase (GUS) reporter gene under the control of the NIN promoter. Activation of symbiotic signaling in response to chitin or chitooligosaccharides was analyzed by GUS staining and by microscopic observations of root-hair deformation responses.
Results
Chimeric LysM domain receptor constructs
A first set of chimeric LysM domain receptors was designed to test whether defense signaling in A. thaliana can be specifically activated by rhizobial NFs. The N-terminal ectodomains of the NF receptor genes LjNFR1 and LjNFR5 of L. japonicus were fused to the transmembrane and cytoplasmic domains of the chitin receptor gene AtCERK1 of A. thaliana, forming the chimeras LjNFR1–AtCERK1 and LjNFR5–AtCERK1 (Figure 1). The domains in these hybrid receptor constructs were not further modified, in order to maintain correct ligand-induced conformational changes and an unaltered interaction with cytoplasmic partners. The chimeras were placed under the control of the cauliflower mosaic virus (CaMV) 35S promoter and then used for Agrobacterium tumefaciens-mediated transformation of A. thaliana cerk1-2 (mutated in the AtCERK1 gene). Reverse transcription polymerase chain reaction (RT-PCR) indicated expression of the LjNFR1–AtCERK1 and/or LjNFR5–AtCERK1 chimeras in stably transformed plants (Figure S1).
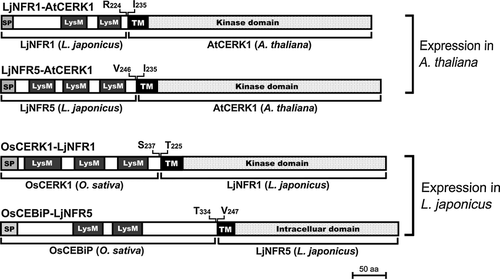
A second set of chimeric LysM domain receptors was designed to examine whether nodulation signaling in L. japonicus roots can be specifically activated by application of chitin. Chimeras, named OsCERK1–LjNFR1 and OsCEBiP–LjNFR5, were constructed by fusing the ectodomains of the chitin receptor genes OsCERK1 and OsCEBiP from rice to the transmembrane and intracellular domains of LjNFR1 and LjNFR5, respectively. The CaMV 35S promoter sequence was inserted upstream of these hybrid receptor constructs (Figure 1). Agrobacterium rhizogenes was used to obtain transgenic hairy roots of L. japonicus NF receptor mutants (nfr1-1 and nfr5-2 derivatives). Transformed hairy roots were subjected to RT-PCR analysis with primers specific for OsCERK1 and OsCEBiP. As shown in Figure S1, corresponding amplicons were detected, indicating expression of the OsCERK1–LjNFR1 and OsCEBiP–LjNFR5 chimeras.
Co-expression of LjNFR1–AtCERK1 and LjNFR5–AtCERK1 in the A. thaliana cerk1-2 mutant does not result in resistance to F. oxysporum
Arabidopsis thaliana cerk1 mutants lack a functional chitin perception system and consequently do not accumulate ROS in response to chitin or chitooligosaccharides. Such mutants are more susceptible to infection by biotrophic fungal pathogens (Miya et al., 2007; Wan et al., 2008). To examine whether constitutive expression of LjNFR1–AtCERK1 and/or LjNFR5–AtCERK1 in the cerk1-2 mutant affects pathogen-induced ROS formation, 10 μl (about 1 × 103 spores) of a suspension of F. oxysporum f. sp. cubense race 4 were injected into the upper left quarter of a given leaf. As shown in Figure 2(a,b), cerk1-2 plants expressing either one or both chimeras did not show increased ROS formation as determined by staining with 3′,3′-diaminobenzidine (DAB). Accordingly, the transformed plants were susceptible to F. oxysporum infection in a similar way to the non-transformed cerk1-2 mutant as analyzed by the quantification of leaf chlorosis. In contrast, wild-type (Col-0) plants and the cerk1-2 mutant complemented with the full-length AtCERK1 gene showed an apparent increased resistance to F. oxysporum, which was accompanied by strong ROS accumulation and weak leaf chlorosis. As determined by trypan blue staining, no obvious cell death was detected 24 h post-inoculation in any of the inoculated plants.
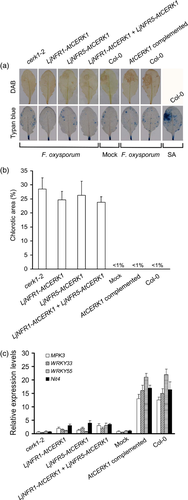
Quantitative reverse transcription (qRT)-PCR was further performed to determine transcript levels of chitin-responsive defense genes encoding MAP kinase 3 (MPK3), WRKY transcription factors (WRKY33, WRKY53) and a nitrilase (Nit4) (Wan et al., 2004). RNA was extracted from leaves 2-h after F. oxysporum inoculation. Transcript levels of the four genes were remarkably upregulated in inoculated wild-type (Col-0) and AtCERK1 complemented plants. On the other hand, only low transcript levels of these genes were found in cerk1-2 mutant derivatives expressing either one or both chimeras (Figure 2c). These data indicate that the LjNFR1/LjNFR5 ectodomains of the constructed chimeras were obviously unable to perceive fungal chitin elicitors.
The cerk1-2 mutant line co-expressing LjNFR1-AtCERK1 and LjNFR5-AtCERK1 perceives NFs
To test whether the constructed cerk1-2 derivatives show responsiveness to rhizobial NFs, preparations of purified NFs were infiltrated into test leaves with a syringe. The NFs were obtained from cultures of Rhizobium sp. NGR234, a strain with a broad host-range that nodulates L. japonicus (Hussain et al., 1999). Upon stimulation with NFs, cerk1-2 lines co-expressing LjNFR1–AtCERK1 and LjNFR5–AtCERK1 showed rapid generation of ROS as determined by DAB staining and a chemiluminescence assay with luminol (Figure 3a,b). No cell death was seen when the NF-treated leaves were stained with trypan blue. Leaves of cerk1-2 lines expressing only one or no chimeras that have been treated with NFs did not exhibit increased ROS generation. Control material from NGRΔnodABC, a mutant deficient in NF synthesis (Price et al., 1992), showed no effects on any tested plants. In contrast to NFs, chitin and chitooligosaccharides were inactive in inducing a ROS response in cerk1-2 lines co-expressing LjNFR1–AtCERK1 and LjNFR5–AtCERK1. Interestingly, NF also enhanced ROS levels in wild-type (Col-0) plants as well as in the cerk1-2 mutant complemented with AtCERK1 (Figure S2), indicating that A. thaliana can recognize NFs in an AtCERK1-dependent manner.
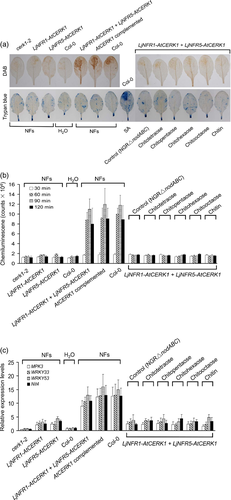
Transgenic cerk1-2 lines expressing LjNFR1–AtCERK1 and/or LjNFR5–AtCERK1 were further used to test whether the expressed chimeras mediate expression of chitin-responsive genes in response to NFs. Transcript levels in leaves were determined by qRT-PCR 2 h after infiltration with NFs or water. When treated with NFs, the cerk1-2 line expressing both chimeras showed increased transcript levels of the examined genes. However, no obvious stimulatory effects were seen for treatments with chitin, chitooligosaccharides and control material from NGRΔnodABC. Transcript levels also remained low in NF-infiltrated leaves of cerk1-2 lines expressing LjNFR1–AtCERK1 or LjNFR5–AtCERK1 alone, although a slight increase was observed compared to the non-transformed cerk1-2 mutant. Similarly to the ROS response, the NF treatment also induced transcript levels of the chitin-responsive genes in wild-type (Col-0) plants and in the cerk1-2 mutant complemented with AtCERK1 (Figure 3c). Taken together, these findings indicate that the LjNFR1–AtCERK1 and LjNFR5–AtCERK1 chimeras can perceive NFs and mediate defense signaling, resulting in ROS generation and the induction of defense genes.
Pre-treatment of the cerk1-2 line co-expressing LjNFR1–AtCERK1 and LjNFR5–AtCERK1 with Nod factors results in resistance to F. oxysporum
To examine effects of pre-treatment with NFs on fungal infection, the different A. thaliana lines were first treated with NFs or NGR234 bacteria and then inoculated with F. oxysporum. Leaf chlorosis was used as a measure of disease severity. Compared with the non-transformed cerk1-2 mutant, leaves of the cerk1-2 line co-expressing LjNFR1–AtCERK1 and LjNFR5–AtCERK1 showed nearly no chlorosis when pre-treated with NFs. Pre-treatment with water or control material from NGRΔnodABC showed no obvious effects and chlorotic disease symptoms were clearly visible (Figure 4a,b). Reduced leaf chlorosis was seen when the cerk1-2 line expressing both chimeras was treated with NGR234 bacteria prior to fungal inoculation, whereas NGRΔnodABC bacteria or mock inoculation with MgSO4 did not prevent formation of chlorotic disease symptoms (Figures 4a,b). Pre-treatment with chitin or chitooligosaccharides also did not reduce leaf chlorosis in the cerk1-2 line expressing both chimeras (Figure S3a). Similar to the non-transformed cerk1-2 mutant, cerk1-2 derivatives expressing a single chimeric receptor developed chlorotic disease symptoms that were not reduced by rhizobial pre-inoculation (Figure S3b,c). Wild-type (Col-0) plants were included in the experiment as controls and showed no visible leaf chlorosis.
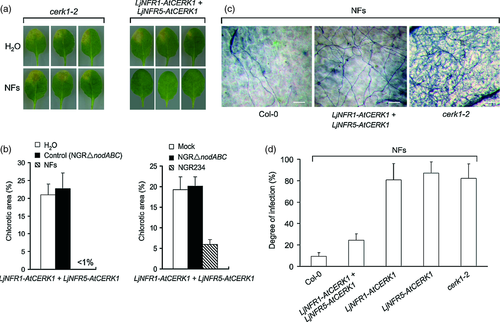
Furthermore, F. oxysporum-inoculated leaves pre-treated with NFs from NGR234 were stained with trypan blue to quantify differences in fungal colonization among the different genotypes. The cerk1-2 line co-expressing LjNFR1–AtCERK1 and LjNFR5–AtCERK1 pre-treated with NFs as well as wild-type (Col-0) plants showed reduced growth of hyphae. By contrast, fungal colonization was significantly increased in cerk1-2 lines expressing only one or no chimera (Figure 4c,d). Hence, NFs can specifically induce resistance to F. oxysporum in the cerk1-2 line expressing both chimeras, which is in accordance with the observed NF-induced defense responses in this line.
Chitin activates nodulation signaling in L. japonicus NF receptor mutants that co-express OsCERK1–LjNFR1 and OsCEBiP–LjNFR5
Expression of the NIN gene is a specific marker for activation of NF-triggered nodulation signaling in L. japonicus roots (Schauser et al., 1999). Accordingly, the non-nodulating NF receptor mutant nfr1-1 (pNIN::GUS) (mutated in LjNFR1 and carrying a NIN promoter–GUS gene construct) does not show any GUS activity in response to either M. loti inoculation or purified NFs (Radutoiu et al., 2003). Here, we tested whether nodulation signaling in L. japonicus nfr1-1 (pNIN::GUS) co-expressing the chimeras OsCERK1–LjNFR1 and OsCEBiP–LjNFR5 can be induced by chitin (Figure 1). Agrobacterium rhizogenes-transformed hairy roots were treated with chitin or chitooligosaccharides and activity of the NIN promoter was visualized by GUS staining. No GUS activity was detected in roots of nfr1-1 (pNIN::GUS) expressing a single chimeric receptor or the empty vector in response to chitin or chitooctaose (Figure 5a). However, roots of nfr1-1 (pNIN::GUS) co-expressing OsCERK1–LjNFR1 and OsCEBiP–LjNFR5 showed blue staining at the treatment site. In contrast to chitooctaose, a local treatment with short chitooligosaccharides (DP = 4, 5 and 6) or NFs from Rhizobium sp. NGR234 did not induce GUS staining (Figure 5b). We also observed GUS staining for nfr5-2 (pNIN::GUS) plants (mutated in the LjNFR5 gene) co-expressing OsCERK1–LjNFR1 and OsCEBiP–LjNFR5 when roots were spot-treated with chitin or chitooctaose. In contrast, such treatments did not induce any GUS activity in roots of nfr5-2 (pNIN::GUS) transformed with one chimera or without chimera (Figure S4a,b). As expected, ‘wild-type’ plants [Gifu (pNIN::GUS)] showed GUS activity in response to locally applied NFs from Rhizobium sp. NGR234, whereas a treatment with chitin or chitooligosaccharides had no visible effects (Figure S4c). Thus, these data indicate that chitin or chitooctaose induce nodulation signaling in hairy roots of test plants that express both chimeras.
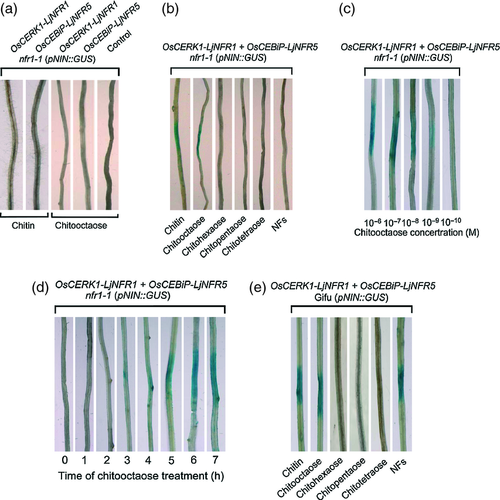
Further experiments were conducted with different concentrations of chitooctaose and different incubation periods before analysis by GUS staining. In roots of nfr1-1 (pNIN::GUS) expressing both chimeras, local GUS staining was visible at a threshold level of 10–9 m chitooctaose when plants were analyzed 7 h after the treatment (Figure 5c). The incubation time with chitooctaose also influenced NIN promoter activity. Maximal GUS staining was reached at 6 h post-treatment, whereas an incubation of 2 h or less resulted in no visible detection of GUS activity (Figure 5d).
For comparison, we also performed spot-treatment experiments with Gifu (pNIN::GUS) ‘wild-type’ plants, which were transformed with OsCERK1–LjNFR1 and OsCEBiP–LjNFR5. Non-transformed Gifu (pNIN::GUS) plants were included in the analysis. The GUS activity in hairy roots expressing both chimeras was locally activated by NFs as well as by application of chitin or chitooctaose (Figure 5e). In contrast, normal ‘wild-type’ (pNIN::GUS) roots were only responsive to NFs and did not show any GUS activity when treated with chitin or chitooligosaccharides (Figure S4c). These results substantiate our finding that OsCERK1–LjNFR1 and OsCEBiP–LjNFR5 can mediate activation of nodulation signaling in response to chitin or chitooctaose.
We further examined whether a mixture of M. loti (strain MAFF303099) and chitin triggers nodulation responses in our test plants. A bacterial suspension supplemented with chitin (100 μg ml−1) was added to nfr1-1 (pNIN::GUS) expressing one or both chimeras. Root-hair responses in GUS-stained hairy roots were microscopically examined 24 h after treatment (Figure S5a–d). In roots of nfr1-1 (pNIN::GUS) co-expressing OsCERK1–LjNFR1 and OsCEBiP–LjNFR5, we observed balloon-like swelling of the root-hair tips, which showed strong GUS staining (Figure S5e). Such root-hair responses were not seen for nfr1-1 (pNIN::GUS) plants expressing a single chimera (Figure S5c,d). Mutant plants expressing both chimeras showed a similar swelling of root-hair tips and GUS staining when analyzed 7 h after chitin application (Figure S5 g,h). Histochemical analysis revealed GUS-stained cells in the rhizodermis and the root cortex, whereas cells in the pericycle and the central cylinder showed no staining (Figure S5f).
Although root-hair responses were visible, no infection threads or nodules were observed for nfr1-1 (pNIN::GUS) co-expressing OsCERK1–LjNFR1 and OsCEBiP–LjNFR5 after inoculation with a M. loti suspension containing chitin. Hence, exogenously applied chitin did not rescue the non-nodulation phenotype of this NF receptor mutant. ‘Wild-type’ Gifu (pNIN::GUS) plants inoculated with the mixture of bacteria and chitin formed about five nodules per plant under the test conditions used. This prompted us to investigate to what extent defense genes are activated in our test plants. Based on previous reports (Wan et al., 2008; Nakagawa et al., 2011), qRT-PCR analysis was performed for four defense gene markers (LjPRp27b, Chitinase, MPK3 and WRKY33). RNA was isolated from roots harvested 2 h after application of chitin, water (mock treatment), M. loti bacteria or a mixture of bacteria and chitin. Interestingly, test plants [namely nfr1-1 (pNIN::GUS) expressing OsCERK1–LjNFR1 and/or OsCEBiP–LjNFR5; wild-type plants] showed a similar pattern of defense gene expression. Compared with the control, treatments with chitin as well as the mixture containing bacteria and chitin strongly induced transcript levels of the examined genes (up to 30-fold for Chitinase), whereas inoculation with bacteria alone resulted in a weak induction (about five-fold) (Figure S6). In addition, we analyzed symbiotic signaling in this experiment by quantifying transcripts of NSP1 and NSP2, which encode GRAS domain transcriptional regulators required for NF-induced gene expression (Heckmann et al., 2006). Similar to the NIN promoter activity analyzed in the previous experiments, transcript levels of NSP1 and NSP2 were induced in nfr1-1 (pNIN::GUS) co-expressing OsCERK1–LjNFR1 and OsCEBiP–LjNFR5 in response to a chitin treatment. Inoculation of these plants with M. loti mixed with chitin resulted in a similar increase in transcripts. In contrast, nfr1-1 (pNIN::GUS) expressing one or no chimeras did not show elevated gene expression. In wild-type plants inoculated with M. loti, however, NSP1 and NSP2 expression was increased, whereas the mixture containing bacteria and chitin was nearly inactive in inducing expression of these genes (Figure S7a,b). Hence, chitin elicited the expression of defense genes in all examined test plants and had a negative effect on expression of M. loti-induced GRAS domain transcriptional regulators in wild-type plants.
Discussion
The expression of receptors with replaced ectodomains in plants is a strategy to activate specific signaling pathways by applied stimuli and thus to regulate cellular properties. In our study, the chimeric receptor genes LjNFR1–AtCERK1 and LjNFR5–AtCERK1 were expressed in the cerk1-2 mutant of A. thaliana in order to induce AtCERK1-mediated plant defense reactions by NF signals. When pre-treated with NFs or rhizobia, plants expressing both chimeras showed increased resistance to F. oxysporum. Induced resistance was accompanied by the formation of ROS and elevated transcript levels of chitin-responsive genes. We also show that chitin or chitooctaose can trigger expression of a GUS reporter gene driven by the NIN promoter in NF receptor mutants of L. japonicus that express the chimeras OsCERK1–LjNFR1 and OsCEBiP–LjNFR5. Our results indicate that the chimeric receptors constructed in this study were expressed as functional proteins and that the ectodomains of the chimeras possess recognition properties that are congruent to the original LysM domain receptors. Upon ligand binding, the non-modified protein kinase domains of the chimeras seem to phosphorylate downstream targets, i.e. the substrates for AtCERK1 and LjNFR1, respectively.
Arabidopsis thaliana cerk1-2 mutant plants expressing LjNFR1–AtCERK1 and LjNFR5–AtCERK1 showed defense signaling in response to NFs, whereas treatment with chitin or simple chitooligosaccharides had nearly no effect (Figures 2 and 3). Wild-type (Col-0) A. thaliana plants, however, seem to recognize NFs in an AtCERK1-dependent manner (Figure S2). This is reminiscent of the chitin perception system of tomato cells that can perceive NFs at nanomolar concentrations as measured by an extracellular alkalinization response (Staehelin et al., 1994). It is worth noting in this context that defense responses induced by the flagellin peptide flg22 in A. thaliana and other plants were partially suppressed by application of a NF from Bradyrhizobium japonicum as well as by chitotetraose. Further analysis of A. thaliana mutants revealed that the observed effects depended on AtLYK3 (LysM-containing receptor-like kinase 3), whereas plants defective in AtCERK1 were no different from wild-type plants in the performed experiments (Liang et al., 2013).
The NF receptor mutants nfr1-1 (pNIN::GUS) or nfr5-2 (pNIN::GUS) of L. japonicus expressing OsCERK1–LjNFR1 and OsCEBiP–LjNFR5 remained insensitive to NFs as determined by GUS staining of plants carrying the pNIN::GUS construct (Figures 5 and S4). However, GUS activity reflecting nodulation signaling was induced when transformed hairy roots of these NF receptor mutants were treated with chitin or chitooctaose. Shorter chitooligosaccharides were inactive in inducing nodulation signaling, indicating that the chain length of the oligosaccharide is crucial for elicitor activity (Figures 5b,e and S4b). This is in accordance to the findings of previous studies, in which long-chain chitooligosaccharides were used to study OsCERK1/OsCEBiP-mediated chitin signaling in rice (e.g. Kaku et al., 2006; Iizasa et al., 2010).
When only a single chimeric receptor was expressed, ligand-induced responses were not observed in either the A. thaliana cerk1-2 mutant or the NF receptor mutants of L. japonicus. These findings indicate that co-expression of the constructed chimeric receptor pairs is essential for their function. We suggest that a heterodimeric interaction occurs between the ectodomains of the constructed chimeras. Our findings support recently published biochemical data that provided evidence for the formation of a protein complex of the OsCERK1/OsCEBiP pair in rice (Shimizu et al., 2010) and the LjNFR1/LjNFR5 pair in L. japonicus (Madsen et al., 2011; Broghammer et al., 2012).
The correct conformational changes of receptors are essential for ligand-induced activation of their kinase domains. Transmembrane domains in receptors may play a crucial role in the ligand-dependent dimerization processes. Charged amino acid residues located in juxtamembrane and transmembrane regions can be particularly critical for the correct function of a given receptor (Constantinescu et al., 2001; Davis et al., 2008). Therefore, we conserved the intracellular and transmembrane domains in our constructed chimeras to ensure optimal ligand-induced conformational changes and efficient activation of the kinase domain. We fused the ectodomain of OsCEBiP to the transmembrane/intracellular domain of LjNFR5 even though LjNFR5 has been described as lacking kinase activity (Madsen et al., 2011). In future studies, it would be interesting to examine the expression, stability and interactions of the constructed chimeras at the protein level.
In the A. thaliana cerk1-2 mutant line expressing LjNFR1–AtCERK1 and LjNFR5–AtCERK1, pre-treatment with NFs or rhizobia induced defense signaling and subsequent increased resistance to F. oxysporum. Nod factors seem to be the only elicitors secreted by strain NGR234, because the NF-deficient mutant NGRΔnodABC showed no effects in our experiments. Remarkably, treatment with NFs was more efficient than rhizobial inoculation (Figure 4b). This might be due to the limited amounts of NFs produced by bacteria under the test conditions used. As NF production by NGR234 largely depends on legume flavonoids (Staehelin et al., 1994), we supplemented the rhizobial inoculum with apigenin. Rhizobia that constitutively synthesize NFs in the absence of flavonoids can be engineered (Spaink et al., 1989) and NGR234 modified in a similar way could be used in future tests. Hence, our experiments suggest the possibility of using NF-producing rhizobia as biocontrol bacteria in agriculture to enhance resistance to certain pathogens in non-legumes.
Co-expression of the NF receptor genes LjNFR1 and LjNFR5 in the non-legumes Nicotiana benthamiana and leek (Allium ampeloprasum) caused a cell death response, which was also observed in the absence of NFs. Analysis of mutant proteins revealed that cell death was only observed when the kinase domain of LjNFR1 was functional (Nakagawa et al., 2011). Similarly, NF-independent rapid cell death was observed in N. benthamiana co-expressing the NF receptor genes MtNFP and MtLYK3 of M. truncatula (Pietraszewska-Bogiel et al., 2013). These effects are perhaps due to constitutive activation of downstream MAP kinase signaling. Indeed, over-expression of genes involved in MAP kinase signaling was reported to cause cell death in tobacco plants (Yang et al., 2001; Zhang and Liu, 2001). In our study, however, no cell death was found in the A. thaliana cerk1-2 mutant co-transformed with LjNFR1–AtCERK1 and LjNFR5–AtCERK1, whereas ROS generation and defense gene activation were detected specifically in response to NFs. These findings suggest that the constructed chimeric receptors showed no or low constitutive kinase activity and that they were only activated upon ligand binding.
Our experiments with L. japonicus NF receptor mutants indicate that nodulation signaling in the nfr1-1 (pNIN::GUS) mutant co-expressing OsCERK1–LjNFR1 and OsCEBiP–LjNFR5 was activated in response to chitin or chitooctaose. These findings suggest that OsCERK1–LjNFR1 interacts with OsCEBiP–LjNFR5, providing support for ligand-dependent conformational changes of the hybrid receptors. These results are in agreement with previous findings that the OsCERK1/OsCEBiP proteins are essential components of the chitin receptor complex in rice (Shimizu et al., 2010).
Although the constructed chimeras seem to be fully functional, the nfr1-1 (pNIN::GUS) mutant co-expressing OsCERK1–LjNFR1 and OsCEBiP–LjNFR5 showed root-hair swelling but did not form nodules after inoculation with a mixture of M. loti and chitin. Hence, induction of nodulation signaling by chitin probably induced feedback responses that prevented bacterial infection. A well-known response is the so-called autoregulation phenomenon, in which nodule formation and mycorrhization are controlled by long-distance signals. This regulatory circuit enables the host plant to suppress further rhizobial or mycorrhizal infection once symbiosis has been established (Ferguson et al., 2010; Staehelin et al., 2011). Furthermore, activation of chitin-induced defense reactions in hairy roots (Figure S6) probably suppressed rhizobial infection. It is worth noting in this context that M. loti-inoculated wild-type L. japonicus formed only about five nodules in the presence of chitin. This is reminiscent of effects caused by application of the flagellin peptide flg22, which resulted in significantly reduced nodule formation in L. japonicus (Lopez-Gomez et al., 2012). Future work is required to examine chitin perception and the effect of chitin-induced defense gene activation on nodulation signaling. The chitin receptor genes of L. japonicus have not been identified but probably exist among the 17 sequenced LysM domain receptor genes (Lohmann et al., 2010).
In conclusion, our work shows that non-legumes can be modified to initiate defense reactions in response to NFs instead of chitin. This opens the possibility of using harmless rhizobia as biocontrol bacteria. We also found that NF-insensitive L. japonicus mutants expressing receptors with altered ectodomains are able to initiate nodulation signaling in response to chitin. Although rhizobial infection was blocked in these mutants, such engineered plants could be used in future to study the relationship between defense reactions and nodule formation. Furthermore, plants expressing chimeric LysM domain receptors could be constructed to examine to what extent the establishment of symbiosis with mycorrhizal fungi is stimulated by application of NFs, related fungal Myc factors or chitin. Indeed, induction of symbiotic signaling by application of NFs promoted mycorrhization in various legumes (Xie et al., 1995, 1997; Oláh et al., 2005).
Experimental Procedures
Biological material, plasmids and primers
The chitin receptor mutant cerk1-2 of Arabidopsis thaliana ecotype Columbia (Col-0) (SALK_007193C; donated by Dr Joseph Ecker, The Salk Institute for Biological Studies, La Jolla, CA, USA) was obtained from the Arabidopsis Biological Resource Center at Ohio State University (http://abrc.osu.edu/). This mutant contains a T-DNA insertion that renders AtCERK1 non-functional as described previously (Miya et al., 2007). Homozygous plants were identified by RT-PCR with gene-specific primers (Table S2). Arabidopsis thaliana plants were grown under long-day conditions (16-h light/8-h dark, 90–110 μE m−2 sec−1, 22°C). Mutants of the L. japonicus (ecotype Gifu) nfr1-1 (pNIN::GUS), nfr5-2 (pNIN::GUS) and Gifu (pNIN::GUS) expressing the GUS gene uidA under the control of the symbiotic NIN gene promoter (Radutoiu et al., 2003) were obtained from Dr Jens Stougaard (University of Aarhus, Aarhus, Denmark) and confirmed by nodulation tests (non-nodulation phenotype) with M. loti strain MAFF303099 (Japan Collection of Microorganisms; RIKEN BioResource Center, http://www.brc.riken.jp/inf/en/). Lotus japonicus seedlings were placed on nitrogen-free half-strength B&D medium (Broughton and Dilworth, 1971) plates and cultivated at 24°C in a temperature-controlled growth room with a 16-h light/8-h dark cycle. Rice seedlings (O. sativa L. cv. Nipponbare) were kept at 30°C in a growth chamber with a 16-h light/8-h dark cycle. All plasmids and bacterial strains used in this study are listed in Table S1. Fusarium oxysporum f. sp. cubense race 4 was obtained from Dr Jianghui Xie (Chinese Academy of Tropical Agricultural Sciences, Zhanjiang, China). Standard protocols were used for plasmid construction. The PCR primers are listed in Table S2.
Construction of chimeric receptor genes and plant transformation
Plasmid DNAs containing the coding sequences of LjNFR1 (AJ575248) and LjNFR5 (AJ575255) from L. japonicus were kindly provided by Dr Zhong-Ming Zhang (Huazhong Agricultural University, Wuhan, China). The AtCERK1 (AB367524) gene from A. thaliana ecotype Col-0 as well as OsCERK1 (AK111766) and OsCEBiP (AB206975) from O. sativa were PCR-cloned into plasmids using cDNA derived from total plant RNA isolated with TRIzol reagent (Invitrogen, http://www.invitrogen.com/).
For construction of the chimeric receptor gene LjNFR1–AtCERK1, the ectodomain of AtCERK1 (amino acid residues 1–234) was replaced by the ectodomain of LjNFR1 (amino acid residues 1–224). Similarly, the ectodomain of AtCERK1 was replaced by the ectodomain of LjNFR5 (amino acid residues 1–234) to generate LjNFR5–AtCERK1 (see Figure 1). The chimeras were then inserted into pRT104 (Töpfer et al., 1987), which carries the CaMV 35S promoter and a poly-A signal. Finally, all the expression cassettes were cloned into pCAMBIA1305.1 (http://www.cambia.org), which contains a GUS gene under the control of the CaMV 35S promoter. The plasmids (see Table S1) were then introduced into A. tumefaciens EHA105 by electroporation. Transgenic A. thaliana plants (cerk1-2 derivatives) were obtained by the floral-dip method and selected on 1% (w/v) agar containing MS medium (Duchefa, http://www.duchefa-biochemie.nl/) and 50 mg l−1 kanamycin (Zhang et al., 2006).
For construction of OsCERK1–LjNFR1, the ectodomain of LjNFR1 (amino acid residues 1–224) was replaced by the ectodomain of OsCERK1 (amino acid residues 1–237). Similarly, OsCEBiP–LjNFR5 was constructed by replacing the ectodomain of LjNFR5 (amino acid residues 1–246) with the ectodomain of OsCEBiP (amino acid residues 1–334). After insertion into pRT104, the expression cassettes with CaMV 35S promoters were cloned into pCAMBIA1305ΔCaMV35S, which is a pCAMBIA1305 derivative lacking the CaMV 35S promoter upstream of the GUS gene sequence (no constitutive GUS expression in transformed plants). Plasmids (Table S1) were then transferred into A. rhizogenes LBA9402 by electroporation. Transgenic hairy roots on L. japonicus mutant plants (grown on Fahraeus medium plates) were obtained according to previously described procedures (Chabaud et al., 2006; Nakagawa et al., 2011).
Chitin, chitooligosaccharides and NFs
Details of the chitin, chitooligosaccharides, NFs from Rhizobium sp. strain NGR234 and the control material from NGRΔnodABC (Price et al., 1992) used in this work are described in Methods S1.
Treatment of A. thaliana plants
Four-week-old A. thaliana plants were used for all treatments with chemicals and inoculation experiments as described in Methods S2.
Treatment of L. japonicus plants
Lotus japonicus genotypes with a pNIN::GUS construct were locally treated with NFs, chitooligosaccharides, chitin and M. loti strain MAFF303099 as summarized in Methods S3.
Cell death and ROS measurements
Methods used for cell death staining with trypan blue and for ROS measurement with luminol or DAB are described in Methods S4.
Quantitative reverse transcription (qRT)-PCR
Quantitative RT-PCR was performed as summarized in Methods S5. The primers used are listed in Table S2.
Acknowledgements
We are grateful to Dr Jens Stougaard (University of Aarhus, Aarhus, Denmark) for L. japonicus mutants and Dr Zhong-Ming Zhang (Huazhong Agricultural University, Wuhan, China) for plasmids containing LjNFR1 and LjNFR5 genes. We also thank Dr Jianghui Xie (Chinese Academy of Tropical Agricultural Sciences, Zhanjiang, China) for F. oxysporum and Dr William J. Broughton (University of Geneva, Geneva, Switzerland) for strain NGRΔnodABC. We thank Qi Sun, Li-Ming Liang, Feng Yang and Dr Christian Wagner (Sun Yat-sen University) for their help with various aspects of this work. This study was supported by the National Basic Research Program of China (973 program, no. 2010CB126501), by the National Natural Science Foundation of China (grant 31070219), by the Guangdong Natural Science Foundation (grant 10251027501000014), by the Science Foundation of the State Key Laboratory of Biocontrol (grant SKLBC201123) and by the Guangdong Key Laboratory of Plant Resources (grant plant01k19).




