Sustained high fatality during TB therapy amid rapid decline in TB mortality at population level: A retrospective cohort and ecological analysis from Shiselweni, Eswatini
Sustainable Development Goal: Good Health and Wellbeing
Abstract
Objectives
Despite declining TB notifications in Southern Africa, TB-related deaths remain high. We describe patient- and population-level trends in TB-related deaths in Eswatini over a period of 11 years.
Methods
Patient-level (retrospective cohort, from 2009 to 2019) and population-level (ecological analysis, 2009–2017) predictors and rates of TB-related deaths were analysed in HIV-negative and HIV-coinfected first-line TB treatment cases and the population of the Shiselweni region. Patient-level TB treatment data, and population and HIV prevalence estimates were combined to obtain stratified annual mortality rates. Multivariable Poisson regressions models were fitted to identify patient-level and population-level predictors of deaths.
Results
Of 11,883 TB treatment cases, 1302 (11.0%) patients died during treatment: 210/2798 (7.5%) HIV-negative patients, 984/8443 (11.7%) people living with HIV (PLHIV), and 108/642 (16.8%) patients with unknown HIV-status. The treatment case fatality ratio remained above 10% in most years. At patient-level, fatality risk was higher in PLHIV (aRR 1.74, 1.51–2.02), and for older age and extra-pulmonary TB irrespective of HIV-status. For PLHIV, fatality risk was higher for TB retreatment cases (aRR 1.38, 1.18–1.61) and patients without antiretroviral therapy (aRR 1.70, 1.47–1.97). It decreases with increasing higher CD4 strata and the programmatic availability of TB-LAM testing (aRR 0.65, 0.35–0.90). At population-level, mortality rates decreased 6.4-fold (−147/100,000 population) between 2009 (174/100,000) and 2017 (27/100,000), coinciding with a decline in TB treatment cases (2785 in 2009 to 497 in 2017). Although the absolute decline in mortality rates was most pronounced in PLHIV (−826/100,000 vs. HIV-negative: −23/100,000), the relative population-level mortality risk remained higher in PLHIV (aRR 4.68, 3.25–6.72) compared to the HIV-negative population.
Conclusions
TB-related mortality rapidly decreased at population-level and most pronounced in PLHIV. However, case fatality among TB treatment cases remained high. Further strategies to reduce active TB disease and introduce improved TB therapies are warranted.
INTRODUCTION
About 2.5 million cases of tuberculosis (TB) and 377,000 TB-related deaths were estimated in Sub-Saharan Africa in 2019 [1]. Southern Africa is particularly affected because of the intertwined HIV epidemic, with more than half of all TB treatment cases co-infected with HIV [1]. The risk of TB disease is higher in people living with HIV (PLHIV) mainly due to reactivation of latent TB [1], with the highest death rates reported in patients with advanced HIV disease [2-4].
Strategies addressing the interlinked HIV and TB epidemics are various [5-9]: reducing the risk of developing TB disease (e.g., TB preventive therapy), infection control, intensified TB case finding (e.g., index case investigation), scale-up of sensitive and closer to the patient diagnostics (e.g., Xpert MTB/RIF assay, urine lateral flow lipoarabinomannan assay [TB-LAM]), and provision of antiretroviral therapy (ART) at time of HIV diagnosis (treat-all). Access to TB care has also improved in remote locations and for vulnerable populations through provision of decentralised and integrated HIV/TB services at routine clinics and community settings [6, 10-12].
For instance, these concerted efforts improved the quality and comprehensiveness of TB care, resulting in a decrease in TB notifications and improved treatment outcomes in Eswatini between 2009 and 2016 [13, 14]. However, many resource-limited settings (RLS) sustained high TB mortality at population level (e.g., >20 deaths per 100,000 population in Southern Africa in 2019) [1], and particular in TB patients co-infected with HIV [1, 13, 15]. Sustained high TB-related deaths could jeopardise achievement of the targets of the End TB strategy aiming at 95% reduction in TB deaths between 2015 and 2030 [16].
Data from Eswatini revealed possible divergent trends concerning TB-related deaths. First, despite an improvement in TB treatment success from 73% in 2012 to 83% in 2017 [17, 18], there was a consistently high proportion of deaths reported in patients undergoing treatment, notably among PLHIV (13% in 2016) [18]. Second, annual TB incidence declined rapidly from 1350 cases per 100,000 population in 2012 to 363/100,000 population in 2019 [1, 19], thus likely contributing to an overall decrease in TB-related deaths in the population. These contrasting patterns between sustained high mortality during treatment and the probable swift decline in TB-related deaths at population-level requires further understanding, especially in resource-poor settings where HIV and TB prevalence is high and both epidemics are intertwined. Thus, we aim to provide a detailed comparison of trends and predictors of TB-related deaths in Eswatini, spanning over an 11-year period, from both individual and population perspectives.
METHODS
Setting
The predominantly rural Shiselweni region (203,376 inhabitants in 2017) in Eswatini comprises Nhlangano, Hlathikulu and Matsanjeni health zones [20]. The region had a high HIV prevalence of 26% in ≥15-year-olds in 2017 [21]. Although regional drug-sensitive and drug-resistant TB notifications declined fivefold from 1341 to 269 cases per 100,000 population between 2009 and 2016, HIV co-infection among TB cases remained high at ~71% [14]. Figure 1 summarises the main programmatic interventions implemented in the region [14, 22, 23]. HIV and TB care initially centralised at three secondary care facilities in 2008, was—with the support of Médecins sans Frontières (MSF)—decentralised to 22 nurse-led and medical doctor-supported primary care clinics between 2009 and 2011. The public sector primarily delivered TB care in the region, while the private sector played a minor role. Laboratory capacity was expanded with the introduction of Xpert MTB/RIF testing in the three health centres in 2009 and TB-LAM testing in all clinics of Nhlangano health zone in 2018. ART eligibility criteria evolved from 2009 onwards, initially targeting HIV-coinfected TB patients and PLHIV who had a CD4 ≤200 cells/mm3 (Figure 1). Eventually, universal ART (treat-all) was piloted in Nhlangano zone in 2014 and extended to the other health zones 2 years later.
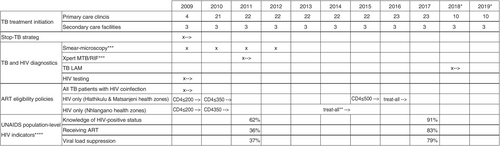
Study design, data management and definitions
We analysed patient-level (retrospective cohort analysis, from 2009 to 2019) and population-level (ecological analysis, from 2009 to 2017) trends and predictors of TB-related deaths in first-line TB treatment cases in Shiselweni.
Patients registered in routine paper-based drug-sensitive TB registers at facilities in the region were considered first-line TB treatment cases. The TB-related variables were defined in accordance with WHO recommendations [24]. The date of registration—corresponding to the date of prescription of TB drugs—was considered as time zero (start of observation period). Final TB treatment outcomes were assigned ~1 year after registration. Patients dying during treatment were considered a TB-related death. TB treatment cases that were transferred in were excluded from analyses to avoid duplications. These registers were the only data source for this study.
For the cohort analysis, we used treatment data from Shiselweni from January 2009 to December 2017, as well as from Nhlangano health zone from 2018 to 2019. Patient-level treatment data were unavailable for the other two zones for 2018–2019. For the ecological analysis, we considered TB cases from the entire region for the period from 2009 to 2017 only, lacking sex- and age-stratified population-level data for Nhlangano zone thereafter.
Assumptions behind the relationship of variables for the cohort analysis were summarised in a directed acyclic graph (Figure S1). We identified factors that may be (in)directly associated with timely TB care registration and death during therapy, which were then included in analysis. All available variables were incorporated into the final fitted models for multiple imputation and Poisson regression.
Statistical analysis
Analyses were performed with Stata 16.1. Frequencies and proportions describe baseline and crude patient-level and population-level outcome data overall and by HIV status. Annual treatment outcomes were reported in the same year of TB treatment initiation (cohort specific).
Multiple imputations by chained equation was used to account for missing baseline values, with 10 imputed datasets created and imputation diagnostics being satisfied (Figures S2 and S3). We also accounted for undocumented deaths. Some treatment outcomes (loss to follow-up [LTFU], treatment failure, outcome not evaluated, transfer out) may contain undocumented deaths, possibly resulting in underestimation of TB-related deaths. Therefore, these outcomes (17.7%) were assumed missing, with multiple imputations used to obtain the final binary outcome of treatment success and death (Table 1). Interactions among variables were not evaluated in the process of multiple imputations. However, interactions of HIV status with other variables were assessed for the cohort and ecological analysis.
| Complete values (n) | Incomplete values (n, %) | |
|---|---|---|
| HIV negative (n = 2798) | ||
| TB classification | 2780 | 18 (0.6) |
| TB site | 2790 | 8 (0.3) |
| Age | 2778 | 20 (0.7) |
| Outcome (potential undocumented deaths)a | 2425 | 373 (13.3) |
| HIV positive (n = 8443) | ||
| TB classification | 8386 | 57 (0.7) |
| TB site | 8411 | 32 (0.4) |
| Sex | 8440 | 3 (<0.1) |
| Age | 8413 | 30 (0.4) |
| CD4 cell count | 3311 | 5132 (60.8) |
| Outcome (potential undocumented deaths)a | 6963 | 1480 (17.5) |
| HIV status missing (n = 642) | ||
| TB classification | 631 | 11 (1.7) |
| TB site | 636 | 6 (0.9) |
| Sex | 638 | 4 (0.6) |
| Age | 634 | 8 (1.2) |
| Outcome (potential undocumented deaths)a | 386 | 256 (39.9) |
- Note: Multiple imputation by chained equation accounted for missing baseline values in sex (0.1%), age (0.5%), TB classification (0.7%), TB site (0.4%), and CD4 cell count in HIV-positive patients (60.8%). Missing values for bacteriological status and HIV-status were considered to be missing not at random. We therefore decided to categorise the data of these variables and added a missing data indicator category. According to guidance of the World Health Organisation at that time [57], initiation of empiric TB treatment was encouraged for individuals with HIV coinfection and suspected TB disease, which could lead to instances of missing bacteriological status when access to TB testing was impractical or delayed [14]. Additionally, missing HIV status is likely not at random (conditional on measured covariates), as seen in other studies [58, 59] with missingness associated with a higher probability of incomplete treatment, treatment failure, and death [60, 61]—that is, the missing HIV status values affect the missingness probability. Ten imputations were performed separately by HIV-status, using identical imputation methods for missing baseline and outcome values. The imputation model, utilising all available variables (see Table 2), employed logistic regression for binary variables and predictive mean matching for continuous variables, and did not incorporate interactions.
- a We assumed that undocumented deaths can occur in patients with the following outcomes as recorded in the TB treatment register: loss to follow-up, treatment failure, outcome not evaluated, transfer out.
Cohort analysis (2009–2019)
We used multivariable Poisson regression on the imputed datasets to describe associations with death. One model was fitted for the entire cohort, and—as HIV-status interacted with some covariates—separate models were fitted for HIV-negative, positive and missing status.
Ecological analysis (2009–2017)
Population-level denominators were calculated by combining mid-year sex- and age-stratified 2007 and 2017 housing census population estimates from Shiselweni [25, 26]—with an annual negative linear population growth assumed for the years between 2007 and 2017 [26]—with the corresponding regional sex- and age-stratified HIV prevalence estimates [27-29]. Lacking disaggregated HIV prevalence estimates in older people, we combined people aged ≥60 years into one age category. Then, in each imputed dataset, we divided annual numbers of deaths stratified by age, sex and HIV status (numerator) by the corresponding population denominators and multiplied by 100,000 to obtain stratified mortality rates per 100,000 populations. These stratified mortality rates were averaged across all imputed datasets to obtain mortality rates adjusted for undocumented deaths. The peak year for TB-related deaths was identified as the year with the highest number of deaths or highest mortality rate between 2009 and 2017.
Using the same imputed stratified population-level data, multivariable Poisson regression models with robust standard errors were built to describe associations between population-level factors and TB-related deaths. Models were fitted for the entire Shiselweni population, and separately for the HIV-positive and negative populations. Given the potential association between changing rates of TB infection in the population that may result in changes in population-level mortality, we used the annual number of treatment cases as a proxy factor for the risk of population-level TB infections.
Ethics
This analysis was approved by the Eswatini Health and Human Research Review Board. It also fulfilled the exemption criteria set by the MSF Ethics Review Board (ERB) for a posteriori analysis of routinely collected clinical data and thus did not require MSF ERB review.
RESULTS
Cohort analysis (2009–2019)
Baseline factors
Of 11,883 TB treatment cases, 10,257 (86.7%) presented with pulmonary TB alone. A total of 2798 (23.6%) patients were HIV negative, 8443 (71.1%) were PLHIV, and 642 (5.4%) had an unknown HIV status. PLHIV (vs HIV negative) were more likely in the 20–49 years age group, women, patients presenting at secondary care level, those with extra-pulmonary TB, retreatment cases and those with negative bacteriological status (Table 2). In PLHIV, most initiated TB treatment before universal ART (treat-all) and TB-LAM became programmatically available. Most PLHIV received ART during TB treatment and had CD4 cell counts below 200 cells/mm3.
| HIV-negative patients (n = 2798, 23.6%) | HIV-positive patients (n = 8443, 71.1%) | Unknown HIV status (n = 642, 5.4%) | Total (n = 11,883) | |
|---|---|---|---|---|
| Xpert programmatic availability | ||||
| No | 1199 (42.9) | 4618 (54.7) | 570 (88.8) | 6387 (53.7) |
| By referral | 951 (34.0) | 1936 (22.9) | 45 (7.0) | 2932 (24.7) |
| On-site | 648 (23.2) | 1889 (22.4) | 27 (4.2) | 2564 (21.6) |
| Health care level | ||||
| Primary care | 1317 (47.1) | 2997 (35.5) | 125 (19.5) | 4439 (37.4) |
| Secondary care | 1481 (52.9) | 5446 (64.5) | 517 (80.5) | 7444 (62.6) |
| Sex | ||||
| Female | 1118 (40.0) | 4298 (50.9) | 250 (39.2) | 5666 (47.7) |
| Male | 1680 (60.0) | 4142 (49.1) | 388 (60.8) | 6210 (52.3) |
| Age, years | ||||
| 0–19 | 689 (24.8) | 886 (10.5) | 143 (22.6) | 1718 (14.5) |
| 20–49 | 1127 (40.6) | 6450 (76.7) | 330 (52.1) | 7907 (66.9) |
| ≥50 | 962 (34.6) | 1077 (12.8) | 161 (25.4) | 2200 (18.6) |
| TB site | ||||
| Pulmonary | 2461 (88.2) | 7233 (86.0) | 563 (88.5) | 10,257 (86.7) |
| Extra-pulmonary/both | 329 (11.8) | 1178 (14.0) | 73 (11.5) | 1580 (13.3) |
| Bacteriological status | ||||
| Positive | 1373 (49.1) | 3838 (45.5) | 164 (25.5) | 5375 (45.2) |
| Negative | 872 (31.2) | 3169 (37.5) | 236 (36.8) | 4277 (36.0) |
| Missing | 553 (19.8) | 1436 (17.0) | 242 (37.7) | 2231 (18.8) |
| TB case classification | ||||
| New | 2534 (91.2) | 7287 (86.9) | 561 (88.9) | 10,382 (88.0) |
| Retreatment | 246 (8.8) | 1099 (13.1) | 70 (11.1) | 1415 (12.0) |
| ART eligibility criteriaa, cells/mm3 | ||||
| ≤200 | — | 2849 (33.7) | — | 2849 (33.7) |
| ≤350 | — | 4349 (51.5) | — | 4349 (51.5) |
| ≤500 | — | 200 (2.4) | — | 200 (2.4) |
| Treat-all | — | 1045 (12.4) | — | 1045 (12.4) |
| TB-LAM programmatic availability | ||||
| No | — | 8171 (96.8) | — | 8171 (96.8) |
| Yes | — | 272 (3.2) | — | 272 (3.2) |
| ART during treatmentb | ||||
| Yes | — | 6004 (71.1) | — | 6004 (71.1) |
| No | — | 2439 (28.9) | — | 2439 (28.9) |
| CD4 cell count, cells/mm3 | ||||
| 0–100 | — | 1151 (34.8) | — | 1151 (34.8) |
| 101–200 | — | 767 (23.2) | — | 767 (23.2) |
| 201–350 | — | 721 (21.8) | — | 721 (21.8) |
| ≥351 | — | 672 (20.3) | — | 672 (20.3) |
- Note: Percentages may not add up to 100% due to rounding.
- Abbreviation: ART, antiretroviral therapy.
- a ART eligibility criteria defined as CD4 cell count based eligibility thresholds for ART initiation for all people living with HIV who do not present with TB disease. All patients presenting with TB disease were eligible for lifelong ART initiation.
- b In the absence of ART initiation dates, the variable ART during treatment encompasses patients who had already started ART before TB treatment and those who commenced it during therapy.
Crude TB treatment outcomes
Treatment success increased from 65.7% to 81.5% between 2009 and 2019 and was highest in 2018 (84.8%) (Figure 2, Table S1). Treatment success tended to be higher for HIV-negative patients in all years; in 2019 it was 86.4% in HIV-negative patients versus 80.5% in PLHIV.
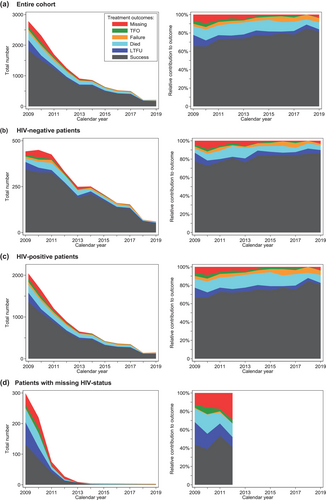
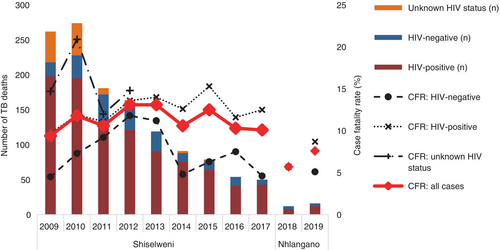
Of 11,883 TB treatment cases, 1302 (11.0%) patients were registered as dead during treatment. By HIV-status, there were 210/2798 (7.5%) deaths in HIV-negative patients, 984/8443 (11.7%) deaths in PLHIV, and 108/642 (16.8%) in patients with unknown HIV status. While the absolute number of TB treatment cases and deaths declined each consecutive year (Table S1), PLHIV sustained higher case fatality ratios (CFRs) in all years (Figure 3). The CFR remained above 10% in most years (other than 2009, 2017 and 2018), with the highest CFR recorded in 2015 (15.3%) and the lowest in 2018 (5.7%). For HIV-negative patients, only the years 2012 and 2013 had reported CFRs at 11.8%–11.2%, while it ranged between 4.5% and 9.2% for the remaining years.
Potential undocumented deaths
A total of 2109 (17.7%) patients had an outcome recorded that could include undocumented deaths (LTFU, treatment failure, outcome not evaluated, transfer out). For the entire cohort, this was highest in 2009 (24.9%) and decreased to 10.9% in 2019. It was highest in patients with missing HIV status (39.9%), followed by PLHIV (17.5%) and HIV-negative patients (13.3%).
Accounting for undocumented deaths using multiple imputation, the overall proportion of deaths increased from 11.0% in the crude dataset to a mean proportion of 13.5% (n = 1610) across the 10 imputed datasets (minimum: n = 1582, 13.3%; maximum: n = 1650, 13.9%).
Multivariable analyses
For the entire cohort, the fatality risk was higher in PLHIV (adjusted risk ratio [aRR] 1.75, 95% CI 1.51–2.02) and missing HIV status (aRR 3.35, 2.78–4.04) versus HIV-negative patients (Table S2).
In separate analyses by HIV status (Table 3), for both PLHIV and HIV-negative patients, increasing older age groups (20–49 and ≥50 years) had a higher fatality risk compared with ≤19 years, as did patients with extra-pulmonary TB (vs. pulmonary TB) and missing bacteriological status (vs. bacteriologically confirmed TB). Associations appeared more pronounced for HIV-negative patients. In addition, for HIV-negative patients only, a negative bacteriological TB status increased the fatality risk, and programmatic availability of on-site Xpert possibly did (aRR 1.60, 1.00–2.55).
| HIV-negative patients (n = 2798)a | HIV-positive patients (n = 8443)a | |||
|---|---|---|---|---|
| Univariate analysis (cRR, 95% CI) | Multivariate analysis (aRR, 95% CI) | Univariate analysis (cRR, 95% CI) | Multivariate analysis (aRR, 95% CI) | |
| Calendar year | 0.98 (0.94–1.02) | 0.99 (0.92–1.08) | 1.00 (0.98–1.02) | 1.07 (1.00–1.14) |
| Xpert programmatic availability | ||||
| No | 1 | 1 | 1 | 1 |
| By referral | 1.00 (0.74–1.35) | 1.09 (0.69–1.72) | 1.13 (0.99–1.29) | 1.14 (0.90–1.44) |
| On-site | 1.21 (0.87–1.67) | 1.60 (1.00–2.55) | 0.98 (0.85–1.13) | 1.04 (0.80–1.35) |
| Health care level | ||||
| Primary care | 1 | 1 | 1 | 1 |
| Secondary care | 1.03 (0.80–1.32) | 0.84 (0.54–1.30) | 0.90 (0.81–1.01) | 1.01 (0.82–1.25) |
| Sex | ||||
| Female | 1 | 1 | 1 | 1 |
| Male | 1.56 (1.17–2.09) | 1.23 (0.92–1.63) | 1.12 (1.00–1.26) | 1.02 (0.91–1.14) |
| Age, years | ||||
| 0–19 | 1 | 1 | 1 | 1 |
| 20–49 | 1.94 (1.09–3.45) | 2.33 (1.31–4.11) | 1.66 (1.30–2.13) | 1.80 (1.39–2.32) |
| ≥50 | 5.55 (3.29–9.38) | 5.45 (3.19–9.32) | 2.21 (1.69–2.89) | 2.32 (1.76–3.06) |
| TB site | ||||
| Pulmonary | 1 | 1 | 1 | 1 |
| Extra-pulmonary/both | 2.53 (1.84–3.46) | 1.83 (1.32–2.52) | 1.33 (1.16–1.53) | 1.22 (1.06–1.41) |
| Bacteriological status | ||||
| Positive | 1 | 1 | 1 | 1 |
| Negative | 3.34 (2.37–4.71) | 2.51 (1.71–3.68) | 1.05 (0.92–1.19) | 1.07 (0.92–1.24) |
| Missing | 2.26 (1.48–3.43) | 2.51 (1.58–4.00) | 1.52 (1.31–1.75) | 1.67 (1.42–1.97) |
| TB case classification | ||||
| New | 1 | 1 | 1 | 1 |
| Retreatment | 1.59 (1.08–2.33) | 1.20 (0.83–1.76) | 1.38 (1.18–1.60) | 1.38 (1.18–1.61) |
| ART eligibility criteriab, cells/mm3 | ||||
| ≤200 | — | — | 1 | 1 |
| ≤350 | — | — | 1.11 (0.98–1.26) | 1.06 (0.86–1.30) |
| ≤500 | — | — | 1.09 (0.75–1.58) | 0.89 (0.54–1.45) |
| Treat-all | — | — | 0.90 (0.74–1.09) | 0.82 (0.54–1.25) |
| TB-LAM programmatic availability | ||||
| No | — | — | 1 | 1 |
| Yes | — | — | 0.60 (0.39–0.90) | 0.56 (0.35–0.90) |
| ART during treatmentc | ||||
| Yes | — | — | 1 | 1 |
| No | — | — | 1.47 (1.30–1.66) | 1.70 (1.47–1.97) |
| CD4 cell count, cells/mm3 | ||||
| 0–100 | — | — | 1 | 1 |
| 101–200 | — | — | 0.79 (0.67–0.94) | 0.78 (0.66–0.93) |
| 201–350 | — | — | 0.63 (0.49–0.81) | 0.63 (0.49–0.81) |
| ≥351 | — | — | 0.56 (0.44–0.70) | 0.54 (0.43–0.67) |
- Note: Percentages may not add up to 100% due to rounding. Calendar year was treated as a continuous variable, as categorising it did not reveal associations with the outcome.
- Abbreviations: aRR, adjusted risk ratio; ART, antiretroviral therapy; cRR, crude incidence risk ratio.
- a Analyses were performed on 10 imputed datasets to account for missing baseline values and undocumented deaths.
- b ART eligibility criteria defined as CD4 cell count based eligibility thresholds for ART initiation for all people living with HIV who do not present with TB disease. All patients presenting with TB disease were eligible for lifelong ART initiation.
- c In the absence of ART initiation dates, the variable ART during treatment encompasses patients who had already started ART before TB treatment and those who commenced it during therapy.
For PLHIV (Table 3), the fatality risk was higher in TB retreatment cases (aRR 1.38, 1.18–1.61) and possibly for each increase in calendar year (aRR 1.07, 1.00–1.14). The fatality risk was increased for patients without ART during TB therapy (aRR 1.70, 1.47–1.97), while it decreased for consecutive higher CD4 cell count strata, being almost half for CD4 ≥350 cells/mm3 (aRR 0.54, 0.43–0.67) versus CD4 ≤100. Finally, the programmatic availability of TB-LAM also decreased the fatality risk by almost half (aRR 0.65, 0.35–0.90).
In patients with missing HIV status, only older age and TB retreatment were clearly associated with an increased fatality risk (Table S3).
The sensitivity analyses, which involved treating calendar year as a categorical variable with and without inclusion of the variable ART eligibility criteria, applying varied age categories, excluding data from the Nhlangano health zone for the years 2018 and 2019, and restricting the analysis to the years 2013–2019 (due to a substantial proportion of missing HIV and CD4 values in earlier years) confirmed the results obtained from the main models.
Ecological analyses (2009–2017)
Mortality rates
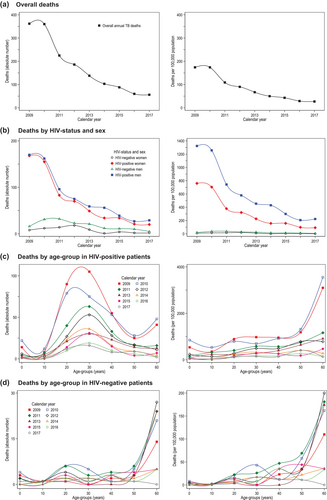
TB treatment cases (2785 in 2009 vs. 497 in 2017) (Table S1) and annual TB-related mortality rates per 100,000 population decreased in consecutive years (see Table 4, Figure 4). In summary, the peak mortality rate was 174/100,000 population in 2009 and 2010, and it decreased 6.4-fold (−147/100,000 population) to 27/100,000 population in 2017. In all years, men (peak year 2010: 200/100,000 population) had a higher mortality rate than women (peak year 2009: 158/100,000 population). PLHIV sustained a many times higher mortality rate (peak year 2009: 967/100,000 population) versus the HIV-negative population (peak year 2011: 27/100,000 population). The absolute decline between peak years and 2017 was most pronounced in PLHIV (6.9-fold; −826/100,000 population) versus the HIV-negative population (6.8-fold; −23/100,000 population) while the relative decline was of comparable magnitude. By age, mortality rates were highest in the 30 to 39 years age group (peak year 2009: 469/100,000 population) and the ≥60 years group (peak year 2010: 481/100,000 population) while the decline was highest in the ≥60 years group (37.0-fold; −468/100,000 population).
| Annual mortality rates per 100,000 populationa | Relative decline in mortality rates until 2017 | Absolute decline in deaths per 100,000 population until 2017 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | From 2009 | From peak year | From 2009 | From peak year | |
| Overall | 174 | 174 | 109 | 90 | 67 | 50 | 43 | 29 | 27 | 6.4 | b | 147 | b |
| By sex | |||||||||||||
| Women | 158 | 151 | 88 | 80 | 53 | 32 | 35 | 21 | 20 | 7.9 | b | 138 | b |
| Men | 193 | 200 | 133 | 102 | 83 | 71 | 52 | 39 | 35 | 5.5 | 5.7 | 158 | 165 |
| By HIV status | |||||||||||||
| HIV positive | 967 | 908 | 514 | 417 | 311 | 260 | 211 | 139 | 141 | 6.9 | b | 826 | b |
| HIV negative | 14 | 25 | 27 | 24 | 18 | 8 | 9 | 7 | 4 | 3.5 | 6.8 | 10 | 23 |
| By HIV status and sex | |||||||||||||
| HIV-negative women | 9 | 14 | 16 | 20 | 10 | 1 | 5 | 2 | 2 | 4.5 | 10.0 | 7 | 18 |
| HIV-negative men | 19 | 37 | 38 | 28 | 25 | 14 | 13 | 12 | 6 | 3.2 | 6.3 | 13 | 32 |
| HIV-positive women | 760 | 704 | 379 | 321 | 226 | 157 | 158 | 98 | 92 | 8.3 | b | 668 | b |
| HIV-positive men | 1323 | 1257 | 743 | 579 | 454 | 430 | 299 | 206 | 223 | 5.9 | b | 1100 | b |
| By age group, years | |||||||||||||
| 0–9 | 30 | 47 | 11 | 15 | 4 | 2 | 4 | 2 | 4 | 7.5 | 11.8 | 26 | 43 |
| 10–19 | 17 | 25 | 15 | 8 | 12 | 4 | 0 | 0 | 4 | 4.3 | 6.3 | 13 | 21 |
| 20–29 | 244 | 230 | 126 | 85 | 64 | 70 | 48 | 34 | 37 | 6.6 | b | 207 | b |
| 30–39 | 469 | 356 | 292 | 238 | 132 | 167 | 135 | 87 | 79 | 5.9 | b | 390 | b |
| 40–49 | 398 | 377 | 258 | 216 | 160 | 119 | 125 | 91 | 63 | 6.3 | b | 335 | b |
| 50–59 | 264 | 307 | 203 | 182 | 153 | 98 | 71 | 61 | 89 | 3 | 3.4 | 175 | 218 |
| ≥60 | 391 | 481 | 273 | 255 | 243 | 58 | 76 | 43 | 13 | 30.1 | 37.0 | 378 | 468 |
- a The peak years of mortality rate are in bold.
- b Data not presented as peak year was 2009.
Multivariable analyses
The overall TB-related mortality risk was 4.68 times higher in PLHIV (aRR 4.68, 3.25–6.72) versus the HIV-negative population (Table S4).
In the HIV-negative population (Table 5), the mortality risk was higher for people aged ≥60 years (aRR 5.15, 2.70–9.82) versus 30–39 years, and increased between 1.86 and 1.93 for the years 2010–2012 (vs. 2009). The mortality risk decreased by 17% (aRR 0.83, 0.75–0.93) for each 200 per 100,000 population decrease in TB treatment notifications.
| HIV-negative populationa | HIV-positive populationa | |||
|---|---|---|---|---|
| aRR | 95% CI | aRR | 95% CI | |
| Calendar year | ||||
| 2009 | 1 | 1 | ||
| 2010 | 1.86 | (1.22–2.82) | 1.16 | (0.86–1.56) |
| 2011 | 1.93 | (1.30–2.88) | 0.82 | (0.56–1.20) |
| 2012 | 1.92 | (1.20–3.07) | 0.76 | (0.49–1.17) |
| 2013 | 1.63 | (0.85–3.13) | 0.62 | (0.38–1.03) |
| 2014 | 0.81 | (0.42–1.58) | 0.53 | (0.32–0.90) |
| 2015 | 0.92 | (0.49–1.75) | 0.47 | (0.26–0.84) |
| 2016 | 0.81 | (0.38–1.73) | 0.31 | (0.18–0.54) |
| 2017 | 0.46 | (0.15–1.42) | 0.32 | (0.18–0.58) |
| Sex | ||||
| Women | 1 | 1 | ||
| Men | 1.41 | (0.97–2.06) | 0.87 | (0.75–1.02) |
| Age groups, years | ||||
| 0–9 | 1.03 | (0.46–2.27) | 0.12 | (0.08–0.18) |
| 10–20 | 0.61 | (0.28–1.32) | 0.09 | (0.06–0.12) |
| 20–29 | 1.99 | (1.09–3.63) | 0.75 | (0.57–0.97) |
| 30–39 | 1 | 1 | ||
| 40–49 | 1.11 | (0.57–2.16) | 0.61 | (0.53–0.70) |
| 50–59 | 1.55 | (0.85–2.82) | 0.27 | (0.21–0.34) |
| ≥60 | 5.15 | (2.70–9.82) | 0.26 | (0.21–0.33) |
| Decrease in TB cases, per 200/100,000 populationb | 0.83 | (0.75–0.93) | 0.98 | (0.96–0.99) |
- Note: In sensitivity analyses omitting the covariate TB case decrease, effect estimate trends remained consistent across covariate factors for the HIV-positive population, though a more pronounced association was noted for calendar year compared to the main model. In the HIV-negative population, a similar trend emerged, and with men exhibiting an increased mortality risk (aRR 2.30, 1.78–2.97).
- Abbreviations: aRR, adjusted risk ratio, CI, confidence interval.
- a Analyses were performed on 10 imputed datasets to account for missing baseline values and undocumented deaths.
- b This is a proxy variable for the risk of TB infection in the population.
In PLHIV (Table 5), the mortality risk tended to decrease in consecutive years from 2011 onwards, but the decrease was statistically significant only from 2014 (aRR 0.53, 0.32–0.90) to 2017 (aRR 0.32, 0.18–0.58). The mortality risk was also decreased for all younger and older age groups (vs. 30–39 years), and by 2% (aRR 0.98, 0.96–0.99) for each 200 per 100,000 population decrease in TB treatment notifications.
All analyses showed no clear associations for sex.
DISCUSSION
This study from a high HIV and TB burden setting showed differentiated trends in TB-related deaths. Although overall treatment success increased over time, the CFR remained high and was mainly driven by PLHIV. In contrast, at population level, the absolute number of TB-related deaths and mortality rates declined rapidly over a decade, particularly in PLHIV.
Treatment fatality in treatment cases
The overall crude annual treatment fatality remained high throughout the years. Despite a decline in CFR in HIV-negative patients from 2014 onwards, the overall high CFR was driven and sustained by PLHIV and comparable to previously reported trends from Eswatini and other RLS [15, 30].
Similar to other settings [31-34], older age increased the fatality risk irrespective of HIV status, possibly related to factors increasing biological vulnerability to TB disease and unmeasured competing causes of death (e.g., cardiovascular disease, cancer) in older people. Interestingly, sex did not show an obvious association with case fatality, but estimates may have been distorted by lack of adjustment for other socio-demographic factors or correlations between covariates.
Comparable to other studies [30], case fatality was higher in HIV-negative patients and PLHIV who presented with extra-pulmonary TB and missing bacteriological status, while a negative bacteriological status increased the fatality risk in HIV-negative patients only. Possible explanations are (undiagnosed) underlying infectious (e.g., pneumocystis pneumonia in PLHIV) and other chronic lung diseases (e.g., lung cancer, silicosis) associated with high case fatality, delay in diagnosis and treatment (e.g., ruling out other infectious diseases), unrecognised drug-resistant TB disease, and possibly more disseminated and severe disease in patients with extra-pulmonary TB disease. Importantly, negative bacteriological status—which is more common in immunosuppressed patients and a risk factor for death in advanced HIV disease [30, 35]—was not clearly associated with increased case fatality in PLHIV. In our setting, health workers were capacitated to start TB treatment in bacteriologically negative cases irrespective of HIV status, potentially reducing delays in treatment initiation in those at greatest need for treatment.
Previous TB therapy was clearly associated with higher fatality risk in HIV co-infected patients while the association with HIV-negative patients was not obvious. In Eswatini, retreatment cases and PLHIV are more likely to present with drug-resistant TB disease [36] that is associated with increased case fatality. Accurate and timely diagnosis of drug-resistant TB was compromised in our setting because of suboptimal access to culture-based drug-resistance testing, and the inability of the Xpert and line probe assays to detect the locally prevalent RpoB I491F rifampicin resistance mutation [36-38], thus possibly resulting in misclassification of drug-sensitive and drug-resistant TB disease. These challenges suggest a need for faster and more accurate tests for rifampicin-resistant TB disease as well as more potent first-line TB treatment regimens that could cover undiagnosed drug-resistant TB disease.
Similar to other studies [2-4], higher CD4 cell counts and ART decreased the fatality risk, possibly due to less severe HIV disease and improved immunity. However, the overall impact of programmatic and policy factors mainly targeted at PLHIV remained inconclusive. While the programmatic availability of Xpert testing and the expansion of ART eligibility criteria lacked strong associations, the availability of TB-LAM testing was associated with a lower fatality risk. Although Xpert and TB-LAM testing may improve diagnosis and result in more timely treatment [39-43], the effect of Xpert on case fatality remains inconclusive, as does the population-level impact of TB-LAM [44-46]. Patients only benefit from improved diagnostics if these are available and used at sites where treatment decisions are made, health workers correctly perform and interpret them, and administrative delays are minimised (e.g., reporting back of test results). Although health policies promoting earlier access to ART may reduce time periods of increased risk for (severe) TB disease, a patient-level benefit may only be achieved if patients actually initiate ART at the appropriate time and remain virally suppressed. Notably—and as suggested by a 7% increased fatality risk for each consecutive calendar year in PLHIV—other temporal opposing factors may hide possible patient-level benefits of policy and programmatic interventions—for instance, when they are related to unmeasured temporal changes in access to care (e.g., hard to reach and to treat patients are possibly proportionally overrepresented in recent years). Overall, findings suggest that theoretically valuable programme and policy interventions at population level may not always translate to obvious patient-level health benefits, or that effects of new interventions may be difficult to isolate in observational studies.
TB-related mortality at population level
The absolute crude number of TB-related deaths was highest in 2010 and decreased each consecutive year thereafter. Although mortality rates remained higher in PLHIV, as also reported elsewhere [2-4], the absolute decline was most pronounced in PLHIV and was seen in all age groups and both sexes. This decline coincided with rapidly falling numbers of TB treatment cases in this region [14], suggesting that changes in TB notifications and treatment cases may be an important driver for changes in TB-related mortality at population level. With a lower prevalence of active TB in the population, the absolute number of TB cases resulting in death decreases. This inference is supported by the multivariate analysis, indicating an association between declining TB notifications and a lowered mortality risk. However, this risk reduction may primarily signify a decreased risk of TB infection in the population rather than a risk reduction in mortality alone. Although a spurious decline in TB treatment notifications may have resulted in more undiagnosed TB disease and undocumented deaths, the decline in TB is likely real in our setting due to higher ART coverage in PLHIV as well as increased case finding activities and better TB diagnostics in recent years, in addition to a similar decline reported from other parts of the country and settings in Southern Africa [13, 47-49]. Future decline in TB-related mortality may be achieved by further reducing active TB disease—for instance, by reducing the pool of people with increased susceptibility to TB infection (e.g., reducing HIV infections, early ART) and decreasing the risk of activation of latent TB infection with TB preventive therapy.
Overall, population-level multivariable analysis confirmed crude trends with a rapid mortality risk reduction in PLHIV but less obvious mortality risk reduction in the HIV-negative population when compared with 2009. The crude decline in HIV-negative cases may have been too small to detect an obvious temporal trend in adjusted analysis. In the HIV-negative population, the elderly population had the highest mortality risk, surprisingly followed by the 20–29 years group (vs. 30–39 years). The latter may be a spurious finding or explained by unaccounted HIV infections. For instance, mortality risk in PLHIV was also high in young adults (e.g., 15–34 years) who were known to have high levels of undiagnosed, untreated and virally elevated HIV infections [21, 28].
Notably, the relative mortality risk was comparable between sexes. It may be explained by complex differentiated temporal trends in sex-related and HIV-associated TB factors with regards to epidemiology, behaviour and exposure, biological and genetic determinants, and access to care [50-54], possibly balancing crude differences between sexes.
Limitations and strengths
First, we accessed first-line TB treatment data, and were thus unable to account for undiagnosed TB or pre-treatment mortality (38% in some African settings [53]), and deaths related to drug-resistant TB therapy. Our estimates of TB-related mortality rates were therefore at the lower range (27/100,000 in 2017) compared with WHO estimates (55/100,000 in 2017) [55]. In contrast, all deaths during TB treatment were assumed to be related to TB disease, which may overestimate mortality rates if some deaths were caused by other conditions.
Second, although, population-adjusted estimates of TB mortality depended on the accuracy of disaggregated population-level denominators, overall trends in mortality rates appeared reasonable, with higher rates and risks of death in PLHIV, older people, and the mid-year age group for PLHIV.
Third, our dataset contained missing values. Under the assumption that values were missing at random (MAR), multiple imputation was used to impute missing baseline values as well as treatment outcomes that could clinically not be assigned to the binary outcome of death and treatment success. Multiple imputation allows all observations to be retained, thus increasing power and precision [56]. As patients lost to follow-up and transferred during TB treatment have a risk of death [32], the omission of non-binary outcomes would have disregarded undocumented deaths and likely resulted in an underestimation of TB-related deaths. Importantly, the assumption of MAR may have been violated and TB-related deaths underestimated if, for instance, LTFU and treatment failure have a disproportionally higher risk of undocumented deaths, or if missing categories have a meaning (e.g., sicker patients are less likely to have measurements). Omitting these outcomes from analysis might have resulted in an underestimation of TB-related deaths. The absence of contextualised information about reasons for and the extent of deaths in LTFU prevented informed assumptions and adjustments in multiple imputation. Alternative correction methods, such as confirming undocumented deaths with the National Population Register, were not feasible due to limited access. As a result, our imputation model might have contributed to underestimating TB-related deaths. Finally, despite a high proportion of missing values for CD4 cell count, we retained it in analyses due to the absence of alternative immune-suppression proxy variables. Avoiding additional assumptions about missingness, we primarily attributed it to inadequate documentation, given the availability of point-of-care CD4 testing in clinics since the early years. Anticipated associations with deaths were consistent with findings in comparable studies [2-4].
Fourth, we could not adjust for some unmeasured factors that were identified in the directed acyclic graph (DAG) to be associated with TB treatment initiation and the outcome, thus possibly resulting in biased effect estimates. They included communicable (e.g., lower respiratory infections due to pathogens other than TB) and non-communicable (e.g., diabetes mellitus) comorbidities, disease severity and socio-economic determinants (e.g., employment status, income) as well as factors influencing HIV and TB treatment (e.g., delayed TB diagnosis and treatment, viral load suppression in HIV coinfected patients, other concurrent treatments).
A strength was that that the study was conducted in a routine public sector setting, generalizable to many rural contexts in Southern Africa with an intertwined HIV/TB epidemic. In addition, the long observation period allowed us to explore temporal trends amid the rapid expansion of TB and HIV care.
CONCLUSIONS
TB-related CFR remained high in this high HIV/TB burden setting, specifically among PLHIV who also had more risk factors associated with death during treatment. In contrast, population estimates indicated a rapid decline in TB-related mortality, mainly driven by a decrease in TB treatment notifications in PLHIV. Continued concerted efforts are required to further reduce active TB disease in high HIV/TB burden settings.
ACKNOWLEDGEMENTS
We would like to thank all health workers and patients of the Shiselweni region.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.




