Disease mapping for informing targeted health interventions: childhood pneumonia in Bohol, Philippines
Abstract
enBackground
Acute lower respiratory tract infections (ALRI) are the leading cause of childhood mortality worldwide. Currently, most developing countries assign resources at a district level, and yet District Medical Officers have few tools for directing targeted interventions to high mortality or morbidity areas. Mapping of ALRI at the local level can guide more efficient allocation of resources, coordination of efforts and targeted interventions, which are particularly relevant for health management in resource-scarce settings.
Methods
An efficacy study of 11-valent pneumococcal vaccine was conducted in six municipalities in the Bohol Province of central Philippines from July 2000 to December 2004. Geocoded under-five pneumonia cases (using WHO classifications) were mapped to create spatial patterns of pneumonia at the local health unit (barangay) level.
Results
There were 2951 children with WHO-defined clinical pneumonia, of whom 1074 were severe or very severely ill, 278 were radiographic, and 219 were hypoxaemic. While most children with pneumonia were from urban barangays, there was a disproportionately higher distribution of severe/very severe pneumonia in rural barangays and the most severe hypoxaemic children were concentrated in the northern barangays most distant from the regional hospital.
Conclusions
Mapping of ALRI at the local administrative health level can be performed relatively simply. If these principles are applied to routinely collected IMCI classification of disease at the district level in developing countries, such efforts can form the basis for directing public health and healthcare delivery efforts in a targeted manner.
Abstract
frContexte
Les infections aiguës des voies respiratoires inférieures (IAVRI) sont la principale cause de mortalité infantile à travers le monde. Actuellement, la plupart des pays en développement allouent des ressources au niveau du district, pourtant les médecins de district ont peu d'outils pour guider des interventions ciblées dans les zones à mortalité ou morbidité élevées. La cartographie des IAVRI au niveau local peut guider l'allocation plus efficace des ressources, la coordination des efforts et des interventions ciblées, qui sont particulièrement pertinentes pour la gestion de la santé dans les milieux à faibles ressources.
Méthodes
Une étude d'efficacité du vaccin antipneumococcique 11-valent a été menée dans six municipalités de la province de Bohol dans le centre des Philippines de juillet 2000 à décembre 2004. Des cas de pneumonie chez les moins de cinq ans géocodés selon la classification de l’OMS ont été cartographiés pour créer des profils spatiaux de la pneumonie au niveau de l'unité locale de santé (Barangay).
Résultats
Il y avait 2951 enfants atteints de pneumonie clinique selon la définition de l’OMS, dont 1074 étaient des cas sévères ou très gravement malades, 278 cas étaient radiographiques et 219 étaient hypoxémiques. Alors que la plupart des enfants atteints de pneumonie provenaient de barangays urbaines, il y avait une distribution anormalement élevée de la pneumonie sévère/très sévère dans les barangays rurales et les cas hypoxémiques les plus sévères étaient concentrés dans les barangays nordiques les plus éloignées de l'hôpital régional.
Conclusions
La cartographie des IAVRI au niveau administratif local de la santé peut être réalisée de façon relativement simple. Si ces principes sont appliqués à la classification (dans la Prise en Charge Intégrée des Maladies de l'Enfant) des maladies collectées systématiquement au niveau du district dans les pays en développement, ces efforts peuvent constituer la base pour guider la délivrance de la santé publique et des soins de santé de manière ciblée.
Abstract
esAntecedentes
Las infecciones agudas del tracto respiratorio inferior (IATRI) son la principal causa de mortalidad infantil a nivel mundial. Actualmente, la mayoría de los países en vías de desarrollo destinan recursos a nivel distrital, y sin embargo los Secretarios Distritales de Salud tienen pocas herramientas para llevar a cabo intervenciones dirigidas en áreas con una alta mortalidad y morbilidad. Mapear las IATRI a nivel local puede servir de guía para mejorar la eficiencia en la distribución de recursos, la coordinación de esfuerzos y las intervenciones dirigidas, los cuales son en particular relevantes para el manejo sanitario en lugares con pocos recursos.
Métodos
Se llevó a cabo un estudio de eficacia de la vacuna neumocócica de 11 serotipos en 6 municipios de la provincia de Bohol en el centro de Filipinas entre Julio del 2000 y Diciembre del 2004. Se mapearon de forma geocodificada los casos de menores de cinco años con neumonía (utilizando la clasificación de la OMS) para crear patrones espaciales de neumonía a nivel de la unidad sanitaria local (barangay).
Resultados
Había 2,951 niños con neumonía clínica (según la definición de la OMS), de los cuales 1,074 estaban severa o muy severamente enfermos, 278 tenían diagnóstico radiográfico, y 219 estaban hipoxémicos. Mientras que la mayoría de los niños con neumonía provenían de barangays urbanos, había una distribución desproporcionadamente mayor de casos severos/muy severos de neumonía en barangays rurales y los niños con hipoxemias más severas estaban concentrados en barangays del norte, los más distantes del hospital regional.
Conclusiones
Mapear IATRI a nivel de administraciones sanitarias locales puede ser relativamente simple. Si se aplican estos principios a la clasificación rutinaria de enfermedades en la AIEPI a nivel distrital en países en vías de desarrollo, el esfuerzo puede convertirse en la base para dirigir la salud pública y la atención sanitaria de una objetiva.
Introduction
Although global child mortality rates have declined consistently over the last several decades, preventable infectious diseases continue to require substantial attention. Infectious diseases persist as the most significant cause of childhood mortality, responsible for an estimated 68% of deaths among children under the age of five 1, and acute lower respiratory tract infections (ALRI) are the leading cause of death 2, 3. An estimated 1.3 million children died of pneumonia in 2011, 81% of whom were under 2 years of age 2.
Pneumonia mortality can be substantially reduced through less fragmented service provision, greater equity in access that reaches the most vulnerable populations and integrated care 4. While the solution is multifaceted, surveillance and transfer of information from the community level to decision-makers at higher levels has been identified as a key requirement for preventing mortality and morbidity 4. Surveillance systems support decision-making and assist in targeting and monitoring interventions 5. Geographic information systems (GIS) have emerged as a useful tool for assessing and displaying the geographic patterns of a variety of infectious diseases 6-8 and can extend surveillance systems to a mapping platform that conveys complex data in a graphical form 9.
Geographic analyses of ALRIs have examined linkages between disease outcomes and poverty, access to care and climate conditions. Pneumonia occurs in clusters or ‘hotspots’ in both high-income 10-12 and low-income countries 7, 13, 14. Further, pneumonia is associated with income and across different geographic settings, both at the household 13 and regional levels of analyses 10. In Indonesia, temporal and spatial clusters of pneumonia were associated with monsoon-related weather conditions, including higher temperatures and rainfall 14. In Kenya, Moïsi et al. observed that the probability of dying from pneumonia was associated with distance to hospitals 15. In addition to improving understanding of underlying risk factors, exploration of spatial patterns has the potential to guide focused interventions and support community-level decision-making.
As in many low- and mid-income countries, the healthcare system in the Philippines is decentralised 16, 17 and the local level is primarily responsible for delivery of health care and public health initiatives. While local health facilities collect and retain a variety of patient and clinic data, these usually remain unexplored or analysed beyond monthly or annual tabular reports and are rarely utilised for targeting community-level interventions. The study was designed to use data collected for a pneumococcal vaccine trial for mapping pneumonia severity. The ultimate goal is to illustrate a simple approach that can be used by District Medical Officers/District Health Management Teams in low-income countries for directing appropriate resources to places with high need.
Using point-level geographic data from a randomised, double-blind and placebo-controlled study of the 11-valent pneumococcal conjugate vaccine (PCV) collected between 2000 and 2004, we conducted an exploratory spatial data analysis of childhood pneumonia in Bohol, Philippines to identify areas with high concentrations of pneumonia. Importantly, these data, collected from health facility visits, are similar to data that are commonly available at the district level in most low- and middle-income countries.
Methods
Study setting
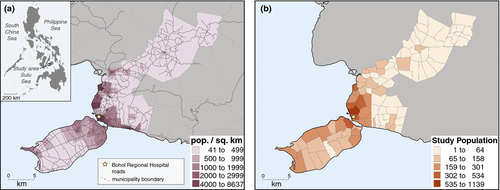
The study occurred in six municipalities in Bohol Province in the central Philippines (Figure 1a) with an area of 357 km2 and a population of 149 000 in 2000 18. The Philippines had an average family income of ₱235 000 (approximately 5200 US$) in 2012 19. Each municipality is divided into barangays, each containing between 400 and 2000 residents. The infant mortality rate in Bohol between 1999 and 2002 was 28 per 1000 live births, and the primary causes of infant death were diarrhoea and pneumonia 20. The Philippines has a decentralised health structure, with the Department of Health guiding the Local Government Units (LGUs) that are comprised of provincial and municipal/city hospitals, rural health units and barangay health stations. About 40% of the hospitals are run by the government, and the remainder are private 17, 21.
Pneumococcal vaccine trial design
This is a secondary analysis of a randomised, placebo-controlled and double-blind vaccine trial that examined the efficacy of an 11-valent pneumococcal vaccine (PCV 11) among children younger than 2 years of age between July 2000 and December 2004. Enrolment and vaccination of infants took place in 48 government-administered primary healthcare centres when infants were brought in for their first vaccination. Study endpoints were monitored at three private hospitals and Bohol Regional Hospital. During the original study period, 12 191 children were enrolled of 15 593 births in the region. Extensive data were collected on all children between enrolment and 2 years of age, including hospital and clinic data for all child pneumonia visits. A detailed description of recruitment procedures, the vaccine, vaccine administration procedures, definition of study endpoints and trial results have been published elsewhere 22.
Mapping of study subjects in the PCV 11 trial
The geographic location of each child's household of residence, along with other demographic information, was collected with geographic positioning systems (GPS) by trained health personnel from November 2008 to June 2009 23. The birthplace for 383 children could not be found. The locations of 11 832 (97.0%) participants' households were utilised and mapped for this study (Figure 1b). All occurrences of pneumonia with a located household were included from the intent-to-treat population (all participants were included in the analysis regardless of compliance). Although the data for this study were acquired by GPS of households, typically collected health facility/clinic records include a geographic identifier for patient residence, such as a town/village. These can be mapped in a similar fashion as described in this study.
Acute lower respiratory tract infection definitions
WHO-defined pneumonia in infants and children who presented with a cough and/or difficult breathing was classified as non-severe, severe and very severe using standard definitions 24, 25. Radiographic pneumonia was defined as the presence of dense or diffuse infiltrates on a chest radiograph, using the Pneumococcal Trialist Group's definitions 25, 26, and hypoxaemic pneumonia was defined as clinical or radiographic pneumonia with a pulse oximetry measurement of less than 90%.
Mapping methods
Each record was linked to a household location and then aggregated by each study endpoint to the 48 barangays. In turn, incidence rates were calculated per 1000 person-years of observation; a rate ratio was calculated using the overall rate for the study area as the denominator. To explore patterns of childhood pneumonia, maps were produced by barangay of raw numbers and rate ratios using ArcGIS 10.1 27 for: (i) WHO-defined pneumonia, (ii) WHO-defined severe/very severe pneumonia, (iii) radiographic pneumonia and (iv) hypoxaemic pneumonia.
Although maps hold far-reaching promise as a tool for guiding decision-making, mapped results should also be interpreted critically. Like with any data analysis, maps can reveal varying patterns depending upon the quality and source of underlying data. For example, when ALRI cases are passively identified at health facilities, rather than through active systematic monitoring of children in the community, health-seeking behaviour and access to care may directly influence ALRI incidence estimates 28. This, in turn, would subsequently influence geographic relationships. Along with data quality, decisions about how to represent data on the map, such as classification schemes or aggregation approaches, can also produce varying patterns. In this study, previous work in health mapping guided format and design 29, 30.
Ethical review
The ethics review boards from the Research Institute for Tropical Medicine (Philippines) and the National Institute for Health and Welfare (Finland) approved the core trial and associated data collection. This trial is also registered with ISRCTN (62323832). This study was performed at the University of Colorado Denver in Denver and Aurora, Colorado, and was approved by the Colorado Multiple Institution Review Board.
Results
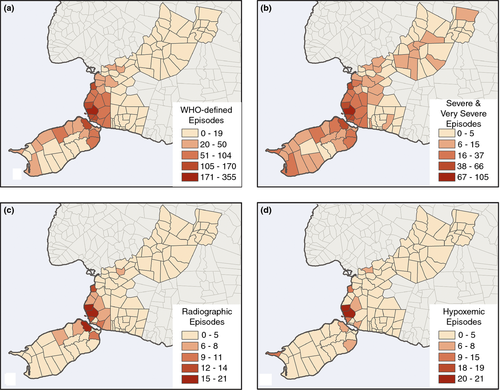
There were 2951 episodes of WHO-defined clinical pneumonia (147.6 per 1000 person-years), of which 1074 were classified as severe or very severe (53.7 per 1000), 278 radiographic pneumonia (13.9 per 1000) and 219 hypoxaemic pneumonia (11.0 per 1000). Figure 2 displays the barangay count for each of the ALRI study endpoints. These maps consistently indicate, unsurprisingly, that the more urbanised areas near Bohol Regional Hospital experienced the highest number of episodes across all endpoints. However, some variation existed between the severe/very severe, radiographic and hypoxaemic diagnoses. The pattern disperses somewhat from the urban core to the surrounding areas for severe/very severe occurrences, extending to some of the more rural parts of the study area in the north-east and south-west. Table 1 shows that only one rural barangay (Doljo) ranks in the highest groupings.
| WHO, all severities | WHO, severe/very severe | ||||
|---|---|---|---|---|---|
| Barangay | Count | Urban/Rural | Barangay | Count | Urban/Rural |
| Cogon | 355 | Urban | Cogon | 105 | Urban |
| Booy | 170 | Urban | Booy | 66 | Urban |
| Poblacion II | 169 | Urban | Totolan | 57 | Urban |
| Poblacion III | 141 | Urban | Manga | 54 | Urban |
| Manga | 139 | Urban | Poblacion II | 54 | Urban |
| Hypoxaemic | Radiographic | ||||
|---|---|---|---|---|---|
| Barangay | Count | Urban/Rural | Barangay | Count | Urban/Rural |
| Booy | 21 | Urban | Cogon | 21 | Urban |
| Cogon | 19 | Urban | Booy | 19 | Urban |
| Poblacion II | 15 | Urban | Totolan | 19 | Urban |
| Manga | 9 | Urban | Manga | 13 | Urban |
| Doljo | 9 | Rural | Poblacion II | 12 | Urban |
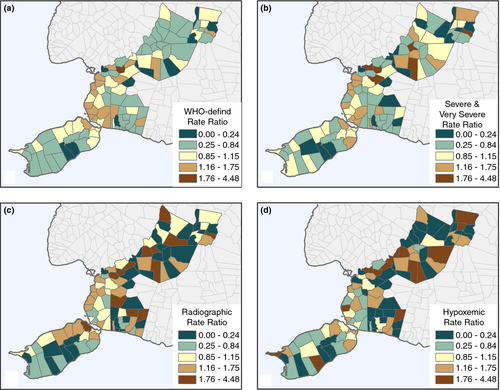
When the rate ratio (barangay rate divided by the overall study area rate) is mapped, the spatial patterns change substantially from the raw numbers. Across all endpoints, the pattern of ALRI shifts to include many of the more rural areas, the dark brown colour highlighting barangay revealing a higher rate than the overall study area average and the dark blue highlighting the lower than average rates. Children with WHO-defined pneumonia were generally concentrated in the more densely populated area (Figure 3a), although severe/very severe pneumonia was more concentrated in rural areas. The highest and lowest ratios of radiographic pneumonia (Figure 3c) and hypoxaemia (Figure 3d) were concentrated in the rural, north-central portion of the region, distant from the regional hospital. Areas in the more rural, north-central portion of the study region reveal a higher ratio of severe/very severe cases than other portions of the study area (Table 2). Nearly, all of the barangay with a ratio of more than 1.4 times the overall rate are rural. Although the pattern shifts substantially for rate ratio maps, the numbers also become small in rural areas, particularly for radiographic and hypoxaemic pneumonia, and as such are unstable requiring careful interpretation and needing further surveillance over time.
| WHO, all severities | WHO, severe/very severe | ||||
|---|---|---|---|---|---|
| Barangay | Ratio to overall area | Urban/Rural | Barangay | Ratio to overall area | Urban/Rural |
| San Roque | 1.99 | Rural | New Lourdes | 2.64 | Rural |
| Monserrat | 1.75 | Rural | Santo Nino | 2.47 | Rural |
| Cambanac | 1.75 | Rural | Cantalid | 2.33 | Rural |
| Malayo Norte | 1.70 | Rural | San Roque | 2.22 | Rural |
| Ubujan | 1.69 | Urban | Malayo Norte | 2.11 | Rural |
| Hypoxaemic | Radiographic | ||||
|---|---|---|---|---|---|
| Barangay | Ratio to overall area | Urban/Rural | Barangay | Ratio to overall area | Urban/Rural |
| Cabad | 7.62 | Rural | Tanday | 4.48 | Rural |
| Cantalid | 4.57 | Rural | San Roque | 3.48 | Rural |
| Malayo Norte | 4.44 | Rural | Santo Nino | 3.18 | Rural |
| Datag Sur | 3.59 | Rural | Haguilanan Grande | 2.58 | Rural |
| Libaong | 3.40 | Rural | Monserrat | 2.32 | Rural |
Discussion
Our findings were similar to those of previous studies exploring spatial hotspots of ALRI in Portugal 12 and Canada 11, where higher rates were concentrated in rural areas with relatively poor access to health care. Very little previous work, however, has examined spatial patterns of childhood ALRI in low-income countries, and existing work makes no distinction between different diagnostic endpoints. Two separate studies of pneumonia in Brazil 7, 12 identified distinct areas with high rates of childhood ALRI. In both studies, high pneumonia incidence was associated with impoverished areas located in mountainous regions.
Consistent with the rate ratio severity maps, the relatively high rate of hypoxaemic pneumonia in the north-central portion of the study region could be explained by poor access to medical care. Children who reside in rural areas further from the main hospitals may be more likely to access care when symptoms become severe because of the resource-intensive nature (in time and money) of seeking health care at Bohol Regional Hospital in the city. In other words, when people are more distant from a clinic, where the costs of seeking health care are higher, they are less likely to seek care for mild cases than residents closer to clinics. This observation is consistent with health-seeking behaviour for different medical needs observed in other geographic contexts 31-35. In Africa, for example, Moïsi et al. found that distance from hospitals was inversely associated with the ‘access to care ratio’, the probability that children in need of care would have access to hospital care 15. As a result, children who reside farther from healthcare facilities may be more likely to develop severe or hypoxaemic pneumonia.
One of the main goals for this analysis was to present a simple approach that can be used by District Medical Officers/District Health Management Teams to identify areas for more efficiently allocating resources, coordinating efforts and targeting interventions. Most first-level facility records in low-income countries document the address and Integrated Management of Childhood Illness (IMCI) classification of disease for children under age five. At a minimum, the village of residence is usually recorded. While we used accurately geocoded data for identifying households, we analysed data aggregated at the barangay level to reflect what would more likely be available at the health facility in these settings. Mapping barangays by numbers and rates of pneumonia can guide where to target educational initiatives, outreach services, additional resources for treatment or other locally identified efforts for improving access to health care.
The pattern of disease burden (revealed by raw numbers), centred in the more densely populated areas near the regional hospital, was distinct from the concentration of severe disease in the outlying rural areas (revealed by the ratio maps). This finding suggests an opportunity for setting priorities. Services may need to be increased in areas with high numbers and low severity, whereas more resources (such as injectable antibiotics or oxygen) may need to be placed in first-level health facilities located within high concentrations of severe or very severe pneumonia. Further, recent research findings suggest that home treatment with oral amoxicillin is as effective for severe pneumonia as hospital treatment 36, which may also provide a viable option in rural settings, given the apparent delay in health-seeking behaviour. However, the management of very severe cases pneumonia still requires referral to a hospital, often an expensive proposition for many people in rural areas. Detection of hypoxaemia requires a pulse oximeter, and its treatment requires the provision of oxygen. Upgrading selected health centres with these provisions could be a viable proposition, in contrast to upgrading all health centres in a district. Disease mapping can guide these decisions in a cost-effective and demand-driven manner.
The Integrated Management of Childhood Illness [IMCI] was introduced as a global strategy by WHO in 1996 27, 37, 38 for improving child survival. The strategy involves three components: a community component, recognition and management of common childhood illnesses at the first-level health facility and improvement of services at referral facilities. The strategy was rapidly implemented in most countries in Africa, Asia and Latin America. At the community level, healthcare workers were trained to encourage and improve community involvement; at the first-level health facility, they were trained in the appropriate care management for children under the age of five; and at referral facilities, training involved quality management of the referred patient, including emergency treatment and triage and management at the referral facility 39-41. All of these IMCI components require both pre-service and in-service training.
WHO initially introduced training to areas of high morbidity and mortality and then, in most cases, subsequently extended it to the rest of the target countries 42, 43. Unfortunately, several problems arose with the strategy, including the length of training and the necessity for continuous training in districts, where health workers' turnover is high 44. A more prudent approach might have been to focus on districts or health centres with the highest under-five mortality. Disease mapping, such as we have illustrated in this study, could be a cost-effective option for identifying areas with the highest under-five mortality, enabling programme officers for IMCI and DMOs to focus training and/or provision of supplies and resources. Further, particularly important in the context of rising vaccines costs for preventing disease, mapping facilitates a cost-effective method of targeting interventions in these low resource settings.
Mapping approaches can be extended to guide integrated community case management [iCCM], a strategy designed to train community health workers in the management of childhood pneumonia, diarrhoea and malaria in the community, and to provide appropriate supervision and support with an uninterrupted supply of medicines and equipment required to manage these conditions. 45, 46. Since being introduced into Africa in 2008 by UNICEF and WHO, at least 38 countries will have implemented some component of iCCM by 2014. While the strategy was primarily designed to be administered in areas without any access to care, it has been expanded to include the training of thousands of community health workers across all districts in many countries 47, 48. In fact, in some countries, donor-supported training has occurred in most districts, except the ones with the highest mortality 49.
This study illustrates the potential for using existing hospital and clinic data to create relevant maps for informing targeted interventions and for providing a baseline for monitoring at an administrative level that is relevant to health decision-makers. While the data in this study were collected as part of a vaccine trial, the endpoint data were collected during visits to the four area hospitals, which are routinely collected in most contexts. Additionally, community attributes, such as proximity to major hospitals or smaller clinics, road networks and transportation, could be used to further refine guidance for targeting interventions.
Conclusions
Analytical geographic analysis of disease morbidity and mortality should be conducted at district and local levels to increase understanding context-specific aetiologies and to prioritise public health and healthcare delivery efforts. This work offers an example of how mapping could direct public health policy in low-income countries.
Acknowledgements
This study is part of the research of the Acute Respiratory Infection Vaccine (ARIVAC) consortium. We are indebted to the Consortium study team and the following collaborators – The Data Safety Monitoring Board: Kim Mulholland (chair), Keith Klugman, Mary Ann Lansang (local safety monitor), David Sack, Pratap Singhashivanon, Peter Smith and Chongsuphajaisiddhi Tan; Research Institute for Tropical Medicine (RITM): Socorro Lupisan, Beatriz Quimbao, Diozele Sanvictores, Erma Abucejo-Ladesma, Juanita Ugpo, Marites Lechago, Leilani T. Nillos, Vernoni Ermata Dulalia; National Institute of Health and Welfare (formerly National Institute of Public Health KTL): Taneli Puumalainen, Antti Nissinen, Anu Soininen, Petri Ruutu and P. Helen Mäkelä; and University of Colorado: Adriana Weinberg; University of Queensland: Ian Riley, Margaret de Campo, sanofi pasteur, Emmanuel Feroldi, Dirk Teuwen and James Maleckar.
The ARIVAC Consortium thanks and acknowledges the participation of the infants, parents, staff, Local Government of the Province of Bohol and Local Government Units (LGUs) of Baclayon, Balilihan, Cortes, Dauis, Panglao and Tagbilaran City; staff of the Pathology and Pediatric Departments of the Bohol Regional Hospital, and the private hospitals Tagbilaran Community Hospital, Borja Family Clinic, Medical Mission Group of Hospitals, Ramiro Community Hospital, St. Jude Hospital, Englewood Hospital, and Tagbilaran Puericulture Center. Support for research to enable this publication was provided by the European Commission DG Research INCO programme (contracts IC18-CY97-2019, ICA4-CT- 1999-10008, ICA4-CT-2002-10062), Academy of Finland (contracts 206283, 106974, 108873 and 108878), Finnish Ministry of Foreign Affairs (bilateral contracts 75502901 and 327/412/2000), Finnish Physicians for Social Responsibility, GAVI ADIP Pneumo, sanofi pasteur, Research Institute for Tropical Medicine of the Philippines, National Public Health Institute Finland, University of Queensland, University of Colorado, National Health and Medical Research Council of Australia and PATH.




