Serologic evaluation of vaccine preventable infections and vaccination rates in kidney transplant candidates
Abstract
Introduction
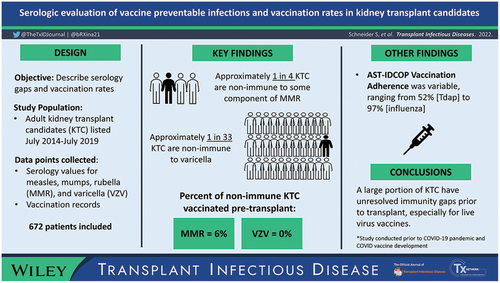
Assessing vaccine serologic status presents opportunities to provide live vaccinations to kidney transplant candidates (KTC). This is especially important given the increased risk of infection while taking lifelong immunosuppression following transplant and the inability to routinely provide live vaccines to patients on immunosuppressive medications. In March 2019, the American Society of Transplantation Infectious Disease Community of Practice (AST-IDCOP) released updated guidelines for vaccination of KTC, which emphasize pretransplant viral serology screening and live vaccine administration prior to transplant.
Primary Endpoint
The primary endpoint of this study was to determine adherence to AST-IDCOP guidelines for live measles, mumps, and rubella (MMR) and VZV vaccination prior to transplant in KTC non-immune by serology.
Methods
This retrospective, descriptive study examined serologic status and rates of live vaccination in 672 patients listed for kidney transplant at our center between July 2014 and July 2019. Secondary endpoints included subgroup analysis of adherence to full AST-IDCOP vaccination recommendations and validation of CDC presumed immunity definitions for measles and VZV.
Results
Seventeen patients (2.7%) were nonimmune by serology for VZV, while 182 (27.1%) were nonimmune by serology to MMR. In a subgroup analysis of the seronegative KTC, none received VZV vaccination, and 6% received MMR vaccination prior to transplant or last follow-up.
Conclusions
CONFLICT OF INTEREST
The authors of this manuscript declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.