Randomised, split-face study of a dermocosmetic cream containing Sphingobioma xenophaga extract and Neurosensine® in subjects with rosacea associated with erythema and sensitive skin
Abstract
Introduction
Rosacea is a chronic inflammatory skin condition associated with erythema, inflammation and skin sensitivity.
Objectives
To assess the benefit of a dermocosmetic cream (DC cream) containing Sphingobioma xenophaga extract and soothing agent in adult females with rosacea-associated erythema and sensitive skin.
Materials and Methods
During phase 1, DC was applied twice daily on the randomized half-face and compared to usual-skincare (USC) for 28 days. During phase 2, DC was applied on the full face twice daily for 56 days. Clinical, instrumental and skin sensitivity assessments were performed at all visits; demodex density (standardized skin surface biopsy (SSSB) method) was performed at baseline and D28, quality of life (QoL) was assessed using the stigmatization questionnaire (SQ), Rosacea Quality of Life index (ROSAQoL) and Dermatology Life Quality Index (DLQI) at baseline and D84.
Results
At D28, a significant benefit of DC over USC was observed for erythema, tightness, burning and stinging (all p ≤ 0.05), erythema measured by chromameter (p < 0.01), corneometry and transepidermal water loss (p < 0.0001 and p < 0.05, respectively), skin sensitivity (p < 0.001) and significant reduction of mean demodex density (p < 0.05) on the DC side. At D84, DC significantly (all p < 0.05) improved clinical signs and symptoms on both sides of the face compared to baseline; SQ, ROSAQoL and DLQI scores improved by 40.4%, 25.0% and 55.7%, respectively compared to baseline. Tolerance was excellent.
Conclusion
DC significantly improved erythema, skin sensitivity, demodex count, QoL and feeling of stigmatization of subjects with rosacea and is very well tolerated.
1 INTRODUCTION
Rosacea is a chronic inflammatory skin condition. It is commonly associated with an altered skin barrier, a dysbalanced microbiome, inflammation and vasodilation.1 Persistent erythema and telangiectasia are frequent primary features, and can be associated with flushing and papules, as well as sensitive skin symptoms.2, 3 The course of rosacea is fluctuant, with periods of flares and remission.4
In subjects with rosacea, the skin barrier is damaged and more sensitive towards external aggressions such as heat, cold and pollution.7, 8 These factors may lead to flares of rosacea and further skin discomfort.9-11 Symptoms of sensitive skin are frequently reported in rosacea and include skin burning, itching, stinging and tingling. Moreover, patients often feel uncomfortable and stigmatized, adding a psychosocial burden to the visible features of rosacea.2, 3 Several of these symptoms have been described as the secondary symptoms of rosacea.1
Rosacea is associated with a microbiome dysbiosis with increase in Demodex mites and expression of transient receptor potential cation channel subfamily V member 1 TRPV1, tropomyosin receptor kinase A (TrkA) and nerve growth factor (NGF), triggering cutaneous neurogenic inflammation.5 Rosacea associated with erythema and flushing and burning sensation refractory to traditional treatment may be designated as neurogenic rosacea.6
A specific dermocosmetic cream (DC cream, Toleriane Rosaliac® AR concentrate cream, La Roche-Posay Laboratoire Dermatologique, France) containing acetyl dipeptide-1 cetyl ester (Neurosensine®), a soothing compound that acts on skin sensitivity by decreasing erythema and irritation as well as reducing signs related to neurogenic inflammation, and shea butter to moisturise the skin barrier, has been developed for rosacea patients.12 It also contains an extract of a flagellated bacterial strain of Sphingomonas xenophaga isolated from the endogenous flora component of La Roche-Posay thermal spring water. It improves skin barrier function, decreases inflammation and inhibits, with a dose-response effect, the activity of pre-Kallikrein which plays an important role in inflammation.13 The increase in quantity and the magnitude of biological activity of Kallikrein 5 leads to the increased production of cathelicidins such as LL-37. LL-37 is an antimicrobial peptide that increases innate cutaneous inflammation, vasodilation and vascular proliferation, which are underlying pathogenic features of rosacea.14
This study assessed the benefit and local tolerance of DC cream in patients with rosacea associated with erythema and sensitive skin.
2 MATERIALS AND METHODS
This, randomized, controlled, double phase (phase 1: split-face, phase 2: full face) study was conducted between January and May 2023 at one single site in Italy. The study adhered to the principles of Good Clinical Practices and the declaration of Helsinki. According to local regulatory guidelines, this type of study testing marketed cosmetics did not require approval from local ethics committees. However, the local ethics committee was informed about this study and subjects provided written informed consent prior to participation.
Female subjects > 18 years of age with a phototypes I–IV, almost clear (very few small papules/pustules, very mild erythema) to mild (few small or large papules/pustules, moderate erythema) rosacea (IGA 1–2), and sensitive skin (positive skin stinging test with 15% lactic acid) were recruited.15
During phase 1 and according to a randomisation schema, subjects applied DC cream on one half-face and their commercially purchased usual skin care on the other half-face (USC) twice daily, in the morning and evening, for 28 days. This comparative and randomised period was followed by a 56-day period during which all subjects applied DC cream twice daily on the full face, up to visit D84.
Clinical evaluations at baseline, D15, D28 and D84 included erythema, skin tightness, burning sensation, stinging and pain, according to the VAS scale (0 = none to 10 = very much), rosacea severity (0 = none to 4 = severe; modified IGA Scale), stinging test and local tolerability.
Instrumental evaluations at all visits included chromametry (Chromameter CR400®, Konica Minolta, Japan) assessing erythema, corneometry (Corneometer CM825®, Courage & Khazaka, Germany) evaluating skin hydration, and transepidermal water loss assessments (TEWL; Tewameter TM 300® MDD 4, Courage & Khazaka Germany).
Demodex density was assessed at Baseline and Day 28 on both sides of the face using the standardized skin surface biopsy (SSSB) method; microscopy pictures were taken using Dinolite® AM, Dunwell Tech, USA with DinoCapture® 2.0 software.16
Quality of life (QoL) was rated using the stigmatization questionnaire (SQ), ROSAQoL and DLQI at baseline and D84.17-19
Global tolerance was assessed at all post-baseline visits on a scale from 0 = none to 3 = excellent tolerability.
Digital image capture was performed using ColorFace® (Newtone Technologies, France) and Skincam® (Newtone Technologies, France).
Mean values, standard deviations and variations were calculated. The ANOVA and the Bonferroni Tests were used to compare instrumental data at baseline, D15, D28 and D84. The t-test was used to compare variations at D15-baseline, D28-baseline and D84-baseline in the DC cream-treated areas and in those having received USC. The Friedman ANOVA and Kendall's Concordance Coefficient Scores were used to compare clinical evaluations and the stinging test at baseline, D15, D28 and D84. The Wilcoxon test for non-parametric and dependent data was used to compare variations at D15-baseline, D28-baseline, D84-baseline and the Demodex density on both half-face sides, as well as for the scores of stigmatization, DLQI and RosaQoL questionnaire at baseline and D84.
3 RESULTS
Twenty-two women aged between 38 and 71 years, phototype II (11 subjects) or III (11 subjects) and an IGA grade of 1 (9 subjects) or 2 (13 subjects) were included in this study.
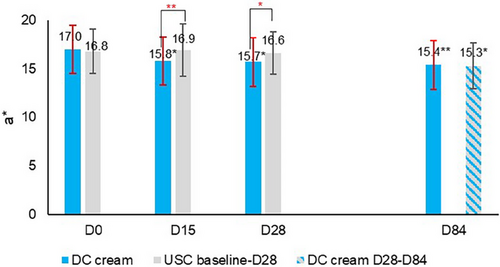
The clinical evaluation of erythema (Figure 1) showed that DC cream (D15: −7.5%, D28: −11.9%) compared to USC (D15: −2.9%, D28: −1.4%) significantly reduced erythema after 15 days (p < 0.001) and 28 days (p < 0.01) of use. At D84, erythema had significantly (p < 0.05) decreased on both sides of the face, with no between-side difference. Chromameter assessments (Figure 2) showed a statistically significantly higher decrease with DC (D15: −7.0%, D28: −7.6%) than with the USC (D15: 0.5%, D28: −1.3%) at both time points (D15: p < 0.001; D28: p < 0.01), confirming clinical observations for erythema. Again, at the end of the study, erythema had significantly improved on both face sides (DC cream: p < 0.001, USC: p < 0.01) with no between-side difference.
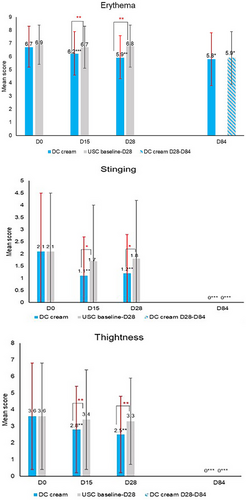
Skin tightness significantly (p < 0.01) decreased with DC cream by 22.2% and 30.6%, compared to 5.6% and 8.3% with the USC at D15 and D28, respectively. Similar findings were noted for burning (DC: −72.7% at D15 and D28, both p < 0.05 vs. USC: D15: −11.1%, D28: −22.2%) and stinging (DC: −47.6% at D15 and −42.9% at D28, both p < 0.01 vs. USC: D15:−19.0% and D28: −14.3%). Reductions were all significantly (p < 0.05) higher with DC cream compared to USC at both D15 and D28 time points, for which no significant difference from baseline was observed (Figure 1). At D84, all clinical symptoms had significantly improved on both sides compared to baseline (all p < 0.05) with no event of skin tightness, burning or stinging on both half-faces.
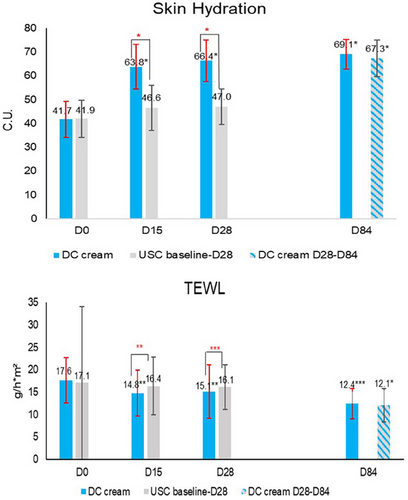
Corneometer measurements showed a statistically significant (p < 0.0001) improvement of skin hydration with DC cream at D15 (+53.0% vs. +11.2% with USC), D28 (+59.2% vs. +12.2% with USC), continuing with DC cream until D84 (+65.7% vs. +67.3% with previous USC use). Improvements were significantly (p < 0.0001) higher with DC than with USC, at both D15 and D28, and similar at D84.
TEWL (Figure 3) significantly decreased from baseline with DC cream at D15 (−16.2%; p < 0.01 vs. −4.2% with USC) and D28 (−14.5%; p < 0.05 vs. −5.6%), and continued to decrease with DC cream until D84. The decrease was significantly (p < 0.05) higher with DC cream than with USC at both D15 and D28. At D84, TEWL had further decreased on both face sides (p < 0.0001), with no between-side difference.
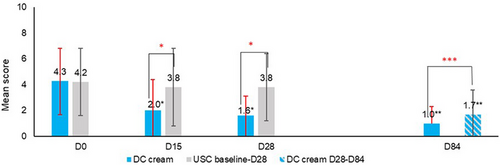
Skin sensitivity (Figure 4) significantly decreased with DC cream at D15 (−53.5%; p < 0.0001 vs. −9.5% with USC) and at D28 (−62.8%; p < 0.001 vs. −9.5% with USC) from baseline. DC cream did significantly (p < 0.001) better than USC as early as D15. At D84, DC cream provided additional relief over the entire face (p < 0.0001).
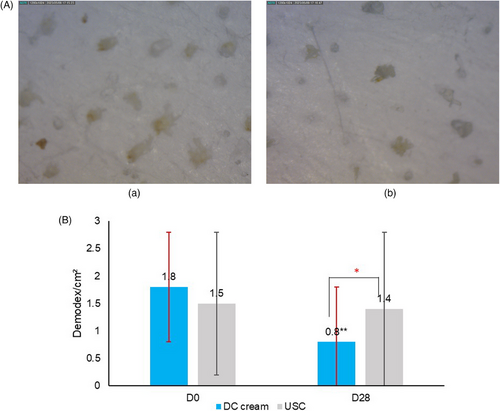
A significant (p < 0.05) reduction of the mean Demodex density was observed on the DC cream side (−56.0%), compared to the USC side (−7%) at D28. Figure 5a provides microscopy pictures of the Demodex density and Figure 5b shows the evolution of the Demodex density at D0 and D28.
Results from the stigmatization questionnaire showed that the total score significantly (p < 0.001) decreased from 10.4 points at D0 to 6.2 points at D84 (−40.4% compared to baseline). So did the ROSAQoL (baseline: 2.8 points vs. D84: 2.1 points (−25.0%), p < 0.0001) and the DLQI (from 7.0 points to 3.1 points (−55.7%), p < 0.001).
Tolerance to DC cream was excellent in all subjects.
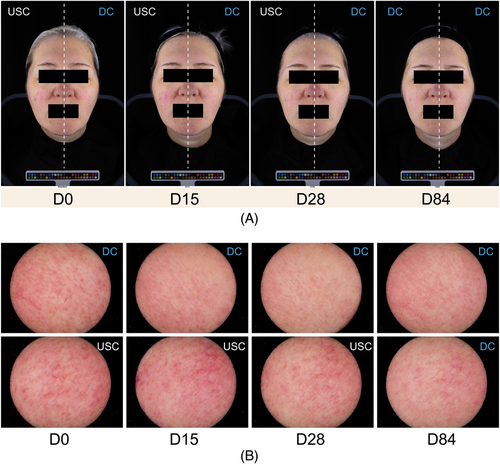
Figure 6 provides comparative ColorFace® and Skincam® images at D0, D15, D28 and D84.
4 DISCUSSION
Results from this study conducted in women with almost clear or mild rosacea associated with erythema, and sensitive skin showed that the tested dermocosmetic cream compared to the patients’ usual skin care significantly (all p < 0.05) improved erythema, rosacea signs and symptoms as well as skin sensitivity as early as after 15 days of daily use. Moreover, when switching after 28 days from USC to the DC cream, clinical and instrumental assessments confirmed that the DC cream was able to rapidly improve the condition of the half-face previously having received the USC while continuing in parallel to improve the half-face previously treated with the DC cream.
In rosacea, the natural skin barrier is damaged and neurogenic inflammation is activated.20 Dermocosmetics combined with a topical treatment or applied as a maintenance regimen are useful for subjects with rosacea, as they help to improve clinical signs and symptoms.21
The present study did not only show that the tested DC cream improved clinical signs and symptoms of rosacea compared to routine skin care, but also significantly (p < 0.05) improved physical skin barrier parameters, objective redness measured by chromametry, and reduced the density of Demodex after 28 days of daily use, thus helping to restore skin homeostasis. These improvements contributed to a significantly improved QoL and perception of social stigmatization, as shown after 84 days, thus confirming the added value of the tested DC cream compared to routine skin care in the management of very mild to mild rosacea.
The small sample size may be considered a limitation. However, due to the intra-individual study design, the sample size of 42 hemi-faces may be considered as sufficient to assess the benefits of the DC cream in rosacea compared to the subjects’ usual skin care regimen.
In conclusion, in the present study in subjects with rosacea associated with erythema and skin sensitivity, DC cream containing S. xenophaga extract and Neurosensine® significantly improved signs and symptoms, regulated skin hydration and reduced the Demodex density compared to standard skin care. Moreover, the DC cream improved the subjects’ QoL and perception of social stigmatization and was very well tolerated.
ACKNOWLEDGEMENTS
The authors acknowledge the editorial assistance of Karl Patrick Göritz, Scientific and Medical Writing Services, France.
CONFLICT OF INTEREST STATEMENT
Margot Nioré, Ludivine Canchy and Delphine Kerob are employees of La Roche-Posay Laboratoire Dermatologique, Jerry Tan receives consultancy honoraria from La Roche-Posay Laboratoire Dermatologique. The other authors have no conflict of interest to disclose.
ETHICS STATEMENT
This single centre, randomized study was conducted between January 2023 and May 2023 and adhered to the principles of Good Clinical Practices and the declaration of Helsinki. According to Italian regulatory guidelines, this type of trial testing marketed cosmetics does not require approval from local ethics committees. Even so, the local ethics committee of Milan/Italy was informed about this study and subjects provided written informed consent prior to participation including for the use of images.
Open Research
DATA AVAILABILITY STATEMENT
Enzo Berardesca, the corresponding author, will share the study protocol and all data collected and statistically analysed and in relationship with this study, except de-identified participant data, upon reasonable request for one year after publication of this manuscript.




