The expression and significance of PD-L1 in condyloma acuminatum
Abstract
Background
It has been reported that programmed death-ligand 1 (PD-L1) is highly expressed in cells during viral infection, which helps the virus escape host immunity. However, the relationship between human papillomavirus (HPV) and PD-L1 in condyloma acuminatum and whether they participate in immunosuppression have not been reported. In this paper, we aimed to explore the expression and significance of PD-L1 in condyloma acuminatum.
Methods
The expression of PD-L1 in the wart of condyloma acuminatum patients and the foreskin of healthy individuals was evaluated. Lentivirus transfection was used to introduce the HPV11-E7 gene into HaCaT cells to investigate whether HPV infection could affect the expression of PD-L1. The successfully constructed HPV11-E7 HaCaT cells were cocultured with Jurkat cells, and Jurkat cell apoptosis and proliferation as well as the Jurkat cell cycle were evaluated by flow cytometry and cell counting kit-8 (CCK-8) assays.
Results
PD-L1 was highly expressed in keratinocytes of genital warts. Through the construction of a cell model, we found that HPV11-E7 could upregulate the expression of PD-L1 in HaCaT cells. Furthermore, HPV11-E7 HaCaT cells can promote the apoptosis of Jurkat cells, inhibit the proliferation of Jurkat cells and mediate the cell cycle arrest of Jurkat cells through the PD-1/PD-L1 signalling pathway.
Conclusions
HPV infection may upregulate PD-L1 expression in the keratinocytes of genital warts and participate in the inhibition of local T-cell function.
1 INTRODUCTION
Condyloma acuminatum is one of the most common sexually transmitted diseases.1 Most cases of condyloma acuminatum are caused by human papillomavirus (HPV) 6/11 infection, while some infections are caused by HPV16/18 infection. At present, the main treatment methods for condyloma acuminatum include surgical resection, local drug administration and laser and freezing treatment. Most of these traditional treatments are invasive and are associated with high rate of recurrence. Patients often need repeated treatment, which causes great physical and mental stress. To solve the problem of high recurrence associated with the traditional methods of treating condyloma acuminatum, researchers have been exploring the mechanism underlying the disease. Previous studies have found decreases in antigen-presenting cell number and function and in antiviral cytokine levels, increase in Treg cell aggregation in the local area of HPV infection,2-5 indicating that condyloma acuminatum produces an immunosuppressive local environment. The traditional treatment improves the appearance of the warts, but it does not improve the local immunosuppression. The dormant virus is not destroyed by the immune system, which enables the growth of new warts. Therefore, effective treatment of the local immunosuppression induced by condyloma acuminatum is the fundamental solution to the high recurrence rate of condyloma acuminatum.
T cells play an important role in the antiviral immune response, and T cell dysfunction is a main reason why hosts cannot eliminate persistent pathogens.6 It is well known that the effective activation of effector T cells require two signals. The first signal is provided by the interaction of the antigen-specific peptide-MHC complex with the T-cell receptor (TCR), which ensures the specificity of the immune response. The second signal is an antigen-dependent costimulus signal that regulates the function of T cells.7 Costimulation signals include positive signals (such as B7/CD28) and negative signals (such as PD-1/PD-L1). Positive costimulation signals can promote the activation and proliferation of T cells, while negative costimulation signals have the opposite effect.8 Under normal circumstances, there is a delicate balance between positive and negative stimulation signals to eliminate pathogens without causing tissue damage. However, in chronic viral infection, this balance is disrupted, and the body's immune responses to infection, such as T-cell-mediated cytotoxicity and/or the ability to produce proinflammatory cytokines, are diminished, indicating the dominance of negative costimulation signals.9 Condyloma acuminatum, a common viral infection, induces T-cell immunosuppression; the secretion of antiviral cytokines such as IL-2 and IFN-γ in the body of patients is decreased.4, 5 The ability of CD8+T cells to react to antigens is significantly decreased in patients with condyloma acuminatum compared with healthy people.10 The suppression of these T cells is most likely caused by the interaction between the virus and the host cell, with the virus evading the host's immune response. We hypothesized that the negative costimulation signalling pathway plays an important role in the evasion of HPV from the antiviral T-cell-mediated immune response. This speculation needs further study and confirmation. PD-1 and PD-L1 are important negative costimulatory signals and are currently considered to play an important role in the immunosuppression associated with chronic viral infections.11-14
PD-L1 (B7-H1, CD274), a member of the B7 family, is an immune checkpoint protein. PD-1 is the receptor of PD-L1, which is mainly expressed in activated T cells. PD-L1 binds with PD-1 on T cells to transmit negative costimulation signals, inhibit the function of T cells, and mediate immunosuppression. Previous studies have shown that in viral infectious diseases, the virus can upregulate the expression of PD-L1 in local tissue cells and transmit negative stimulation signals through the binding of PD-L1 to PD-1 on activated T cells to promote the immune escape of the virus.15-17 PD-1 blockers can block the PD-1/PD-L1 signalling pathway and restore the function of T cells. In addition, in some studies on viral infectious diseases, it has been found that the application of PD-1 blockers can effectively improve the antiviral ability of the body.18, 19 Is the PD-1/PD-L1 signal involved in the occurrence and development of HPV infectious diseases by inhibiting T-cell immunity?
HPV has two unique characteristics, strict host restriction and tissue tropism,20 and HPV infection and replication only occur in human keratinocytes. Keratinocytes are not only the main cell composition of the epidermis but also part of the innate immune defence system. They can act as nonspecialized antigen-presenting cells, inducing the expression of Th1 and Th2 cytokines and cytotoxic reactions in CD4+ and CD8+ T cells.21, 22 In relevant studies on high-risk HPV, it has been found that E7 protein expression can upregulate PD-L1 expression in keratinocytes, enhance the negative signals during the activation of T cells and inhibit T-cell function so that the virus can escape the host's immune response.23, 24 The immune escape patterns of different types of HPV are likely to be similar. The vast majority of condyloma acuminatum is caused by low-risk HPV infection, and we speculate that the local immunosuppression induced by HPV is related to the virus-mediated upregulation of PD-L1 expression on keratinocytes.
Based on the above analysis, this study first evaluates the expression of PD-L1 in keratinocytes from the wart tissue of condyloma acuminatum patients. Second, a cell model of HPV infection was constructed and used to investigate whether HPV infection can upregulate the expression of PD-L1 on keratinocytes. We further explored whether the upregulated expression of PD-L1 in keratinocytes infected with HPV inhibited the function of T cells.
2 MATERIALS AND METHODS
2.1 Ethics statement
The experiment was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Written informed consent documents were obtained from all participants prior to the experiment.
2.2 Study subjects
The wart tissues of 20 patients with condyloma acuminatum and the foreskin tissues of 20 healthy people were collected. All patients with condyloma acuminatum met the following inclusion and exclusion criteria. Inclusion criteria: 1. The clinical manifestations meet the diagnostic criteria for condyloma acuminatum, and the PCR test on the diseased tissues is positive for the HPV type 6 or type 11 virus; 2. Patients with condyloma acuminatum have a disease course of 3 months or more; 3. Patients with genital warts are between 18 and 60 years old. Exclusion criteria: 1. HPV-infected areas show evidence other infections or diseases, such as gonococcus, mycoplasma, chlamydia, dermatitis or eczema; 2. Patients with other chronic infectious diseases, such as HIV, HBV or HCV; 3. Patients received immunosuppressive or glucocorticoid treatment within the past month; 4. Patients with autoimmune diseases, such as dermatomyositis, pemphigus, or systemic lupus erythematosus; 5. Serious internal organ diseases; 6. HPV-infected skin was treated with topical medicine.
All healthy people undergoing circumcision met the following inclusion and exclusion criteria. Inclusion criteria: All healthy people were between 18 and 60 years old. Exclusion criteria: 1. Individuals with sexually transmitted diseases such as condyloma acuminatum, gonorrhoea, mycoplasma and chlamydia infection; 2. Foreskin with dermatitis or eczema; 3. Individuals with other chronic infectious diseases, such as HIV, HBV or HCV; 4. Individuals with autoimmune diseases, such as dermatomyositis, pemphigus, or systemic lupus erythematosus; 5. Serious other internal organ diseases; 6. Foreskin was treated with topical medicine.
2.3 Cell culture
Both HaCaT and Jurkat cells were purchased from the Chinese Type Culture Collection of Wuhan University. Initially, HaCaT cells were cultured with DMEM (C11995500BT, Gibco Company, USA) containing 10% foetal bovine serum (FBS) (10091148, Gibco Company, USA) and 1% penicillin‒streptomycin. Moreover, the Jurkat cells were cultured with Roswell Park Memorial Institute (RPMI) 1640 medium (C11875500BT, Gibco Company, USA) supplemented with 10% FBS and 1% penicillin‒streptomycin.
2.4 Immunohistochemical staining
The prepared paraffin sections were deparaffinized. At 80°C, the deparaffinized sections were placed into pH 6.0 0.01 M citrate buffer, soaked for 20 min, and removed when heated to the proper temperature (30°C). After the sections were rinsed with phosphate-buffered saline (PBS) and dried, they were incubated with 3% H2O2 for 30 min. After the sections were rinsed with PBS again, they were incubated with 10% goat serum for 20 min. The serum was gently washed off, and an appropriate amount of PD-L1 antibody working solution (66248-1-Ig, Mouse, Proteintech, USA) was added dropwise to the sections, which were placed at 4°C overnight. The next day, after reheating the sections, they were rinsed with PBS solution. Then, 50 μL of secondary antibody was added dropwise to each section and incubated at room temperature for 25 min. After rinsing with PBS solution, 50 μL of freshly prepared DAB was added to each section for colour development, and the sections were observed under a microscope. Finally, the sections were washed with tap water, and haematoxylin dye was added dropwise for counterstaining for 1 min. After counterstaining, the sections were washed with tap water, hydrochloric acid and alcohol were added for differentiation for 1–2 s, and the sections were quickly rinsed with tap water and finally stained with eosin dye. The sections were dehydrated and dried with gradient alcohol, and then the sections were cleared with xylene, mounted onto slides and coverslipped with neutral gum, and finally observed under a microscope.
2.5 Immunofluorescence
The prepared paraffin sections were deparaffinized. At 80°C, the deparaffinized sections were placed into pH 6.0 0.01 M citrate buffer, soaked for 20 min, and removed when heated to the proper temperature (30°C). After rinsing the sections with PBS, they were incubated with 10% goat serum for 20 min. The serum was washed off, and the appropriate amount of PD-L1 antibody working solution and K10 antibody working solution (E-AB-31137, Rabbit, Elabscience, China) was added dropwise to the sections and placed at 4°C overnight. On the next day, the sections were rewarmed and rinsed with PBS solution. Then, 50 μL of fluorescent secondary antibody was added dropwise to each section, incubated at room temperature in the dark for 30 min and rinsed with PBS solution. Next, 50 μL of freshly prepared DAPI working solution was added dropwise to the sections to stain the nuclei of the cells in the tissues, left at room temperature for 10 min, and then rinsed with PBS solution. The sections were mounted to slides and coverslipped with anti-fluorescence quenching mounting tablets.
2.6 Reverse transcription quantitative polymerase chain reaction (RT‒qPCR)
The total RNA of cells and tissues was extracted strictly according to the instructions of the TRIzol kit, and then the RNA concentration was determined. According to the instructions of the reverse transcription kit (11123ES, Yeasen, China), RNA was reverse transcribed into cDNA under the following conditions: 25°C for 5 min, 42°C for 30 min and 85°C for 5 s. The above cycle was performed once. An RT qPCR kit (11195ES, Yeasen, China) was used to obtain the final results. The reaction conditions were set as follows: predenaturation at 95°C for 30 s, denaturation at 95°C for 10 s, annealing at 57°C for 20 s and extension at 72°C for 20 s. The denaturation and annealing went through 40 cycles. The forwards and reverse primer sequences for human PD-L1 and β-actin were 5′-AGTACATGCTGGTGGTTCACA-3′ (forwards) and 5′-TCGAGGCGTGAGTATGACTTC-3′ (reverse) for PD-L1 and 5′-AGTCCTCTCCCAAGTCCAC-3′ (forwards) and 5′-ACCAAAAGCCTTCATACATCTC-3′ (reverse) for β-actin.
2.7 Western blot
Equal amounts of total protein isolated using radioimmunoprecipitation assay (RIPA) buffer were loaded onto sodium dodecyl sulphate‒polyacrylamide gels (SDS‒PAGE) and transferred to nitrocellulose membranes using an iBlot gel transfer device (Invitrogen, Grand Island, New York, USA). The following antibodies were used for immunoblotting: anti-PD-L1 (66248-1-Ig, Mouse, Proteintech, USA), anti-β-actin (AC026, Mouse, ABclonal, USA) and anti-DDDDK-Tag (AC005, Mouse, ABclonal, USA).
2.8 Building the cell model
Lentivirus infection and purine screening were used to construct a cell model with stable expression of HPV11-E7 protein (HPV11-E7 HaCaT). Lentivirus carrying HPV11-E7 was constructed by GeneChem (Shanghai, China). HaCaT cells were transfected with lentivirus (MOI = 10), and fresh DMEM (containing 10% FBS) was added 20 h later. After 72 h, 1 μg/mL of puromycin was added to the cells for screening and continued for 1 week. WB and RT‒qPCR were used to verify the transfection results.
2.9 Apoptosis analysis and cell cycle analysis
5 μg/mL PHA was added to Jurkat cells for 48 h to activate the Jurkat cells. A total of 2.5×105 control HaCaT cells and 2.5×105 HPV11-E7 HaCaT cells were cultured with 5×105 Jurkat cells for 24 h. The anti-PD-L1 antibody (329716, BioLegend) was incubated with HPV11-E7 HaCaT cells for 4 h, and the cells were washed with PBS to remove the unbound antibodies and then cocultured with Jurkat cells. To detect apoptotic Jurkat cells, the cells were stained with FITC-coupled Annexin V and propidium iodide (PI) according to the instructions of the apoptosis kit (556547, BD Pharmingen). Apoptosis staining was analysed with an Accuri C6 flow cytometer. To evaluate the cell cycle stages, Jurkat cells were collected and immobilized with 70% ethanol for 20 h. Jurkat cells were labelled according to the instructions of the cell cycle detection kit (KGA511, KeyGen Biotech). The DNA content of the cells was measured by Accuri C6 flow cytometry.
2.10 Cell counting kit-8 assay
5 μg/mL PHA was added to Jurkat cells for 48 h to activate the Jurkat cells. A total of 5×103 control HaCaT and 5×103 HPV11-E7 HaCaT cells were cocultured with 1×104 Jurkat cells in 96-well plates. The anti-PD-L1 antibody was incubated with HPV11-E7 HaCaT cells for 4 h, and the cells were washed with PBS to remove the unbound antibodies and cocultured with Jurkat cells in 96-well plates. The cell suspension containing Jurkat cells in each well was transferred to a new 96-well plate after 1 day, 2 days and 3 days in total culture. Then, 10 μL of CCK-8 reagent was added and incubated for 4 h. The optical density of the cells was measured at a wavelength of 450 nm.
2.11 Statistical analysis
All the data in this study were statistically analysed using GraphPad Prism 8.0 software. The comparison between the data were conducted using the two independent-sample t test, and p < 0.05 was considered to be significantly different.
3 RESULTS
3.1 PD-L1 is highly expressed in epidermal keratinocytes of genital warts
We used a variety of detection methods to investigate the expression of PD-L1 in condyloma acuminatum patients and healthy individuals. We conducted immunohistochemical staining on the wart from 20 condyloma acuminatum patients and the foreskin from 20 healthy individuals. The results showed that PD-L1 was expressed in the epidermis of 10 condyloma acuminatum, and PD-L1 was mainly expressed in the cytoplasm and membrane. PD-L1 expression was negative in the foreskin of all 20 healthy individuals (Figure 1A). In addition, we conducted qRT‒PCR on the wart from condyloma acuminatum patients and the foreskin from healthy individuals and found that the mRNA level of PD-L1 in warts was higher than that in healthy foreskins, and the difference was statistically significant (p < 0.05) (Figure 1B). Moreover, Western blot detection showed that the expression level of PD-L1 in warts was higher than that in healthy foreskins (Figure 1C). To further determine whether the cells with positive PD-L1 expression in warts were keratinocytes, we conducted two-colour immunofluorescence assay of K10 and PD-L1 on warts of condyloma acuminatum patients with positive PD-L1 expression and normal foreskin tissue sections. K10, as a marker of keratinocytes, fluoresced green, and PD-L1 fluoresced red. If PD-L1 was expressed in keratinocytes, it fluoresced orange. The results showed that some cells in warts showed orange fluorescence, while the foreskin of healthy individuals showed green fluorescence (Figure 1D). The results showed that PD-L1 was mainly expressed in the epidermal keratinocytes of warts.

3.2 HPV11-E7 upregulated the expression of PD-L1 in HaCaT cells
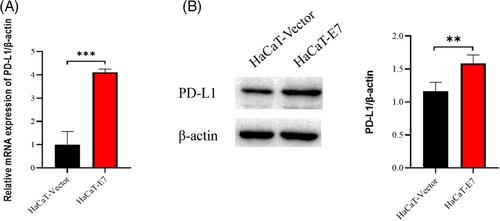
To further investigate whether the upregulated expression of PD-L1 on keratinocytes is induced by HPV infection and its relationship with the inhibition of T-cell function, we first constructed a cell model of HPV infection. The HPV11-E7 gene was selected as the target gene, which was introduced into HaCaT cells by lentivirus transfection technology, and a HaCaT cell model expressing HPV11-E7 was constructed. To evaluate the effect of HPV11-E7 on the expression of PD-L1, we extracted total RNA and total proteins from control lentivirus-infected HaCaT cells (HaCaT-vector) and lentivirus-transfected HaCaT cells carrying the HPV11-E7 gene (HaCaT-E7). The differences in PD-L1 expression between the two groups were determined by qRT‒PCR and Western blotting. The results showed that the RNA and protein levels of PD-L1 in HaCaT-E7 cells were significantly higher than those in HaCaT-vector cells (Figure 2).
3.3 HPV11-E7 HaCaT cells promote the apoptosis of Jurkat cells through the PD-1/PD-L1 signalling pathway
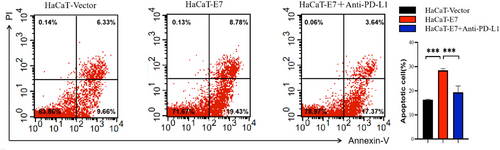
To explore whether HPV-infected keratinocytes can affect the apoptosis of T cells through the PD-1/PD-L1 signalling pathway, HPV11-E7 HaCaT cells and control cells were cocultured with Jurkat cells, and flow cytometry was used to detect the apoptosis of Jurkat cells. We activated Jurcat cells with PHA. PD-L1 antibodies and IgG2b, a PD-L1 blocker isotype control, were added to HaCaT-E7 HaCaT cells. IgG2b was added to HaCaT-vector cells. And the drug was removed after 4 h of incubation. The above three types of cells were cocultured with activated Jurkat cells and were divided into the HaCaT-vector group, HaCaT-E7 group and HaCaT-E7+anti-PD-L1 group. The number of apoptotic Jurkat cells in the HaCaT-E7 group was significantly higher than that in the HaCaT-vector group. In the HaCaT-E7+anti-PD-L1 group, after blocking the PD-1/PD-L1 signal in advance, the number of apoptotic Jurkat cells decreased significantly compared with that in the HaCaT-E7 group. HPV11-E7 HaCaT was confirmed to promote the apoptosis of Jurkat cells through the PD-1/PD-L1 signalling pathway (Figure 3).
3.4 HPV11-E7 HaCaT cells inhibit the proliferation of Jurkat cells and induce cell cycle arrest through the PD-1/PD-L1 signalling pathway
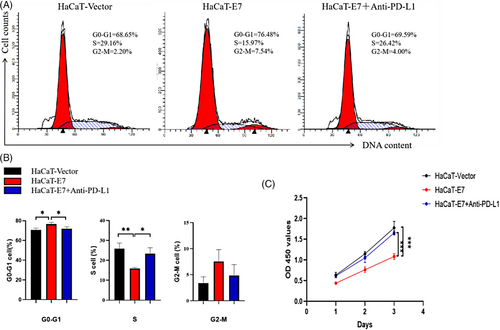
HPV11-E7 HaCaT cells and control cells were cocultured with Jurkat cells. The coculture method was the same as in the previous experiment. The Jurkat cell cycle was evaluated by flow cytometry. After coculture for 24 h, Jurkat cells were collected from each group, fixed and stained, and then tested. Compared with that in the HaCaT-vector group, the number of Jurkat cells in the G0-G1 phase in the HaCaT-E7 group was significantly increased, and the number of Jurkat cells in the S phase in the HaCaT-E7 group was significantly decreased, indicating that compared with the HaCaT-vector group, the HaCaT-E7 group Jurkat cells exhibited cell cycle arrest in the G0-G1 phase. After blocking the PD-L1 signal in the HaCaT-E7+anti-PD-L1 group, the number of Jurkat cells in the G0-G1 phase decreased compared with that in the HaCaT-E7 group, the number of cells in the S phase increased compared with that in the HaCaT-E7 group, and the cell cycle arrest was alleviated (Figure 4A, B). The proliferation of Jurkat cells was determined by CCK-8 assay. The optical density (OD) of Jurkat cells at 450 nm was measured after 24, 48 and 72 h of coculture. The higher the OD value, the higher the cell density. The results showed that the OD values of Jurkat cells in the HaCaT-E7 group were significantly lower than those in the HaCaT-vector group after 24, 48 and 72 h of coculture. After blocking the PD-1/PD-L1 signal in the HaCaT-E7+anti-PD-L1 group, the OD value of Jurkat cells after 24, 48 and 72 h of coculture increased significantly compared with that of the HaCaT-E7 group (Figure 4c).
4 DISCUSSION
Studies have shown that T cells with antiviral function generally have lower apoptosis rates and higher proliferation rates25, 26 However, the function of T cells is often impaired in persistent viral infections, and many previously conducted studies show that PD-1/PD-L1 signalling play an important role in this impairment.15, 27 Some viruses upregulate the expression of PD-L1 in infected cells, which promotes viral evasion from the host immune response.28 A large number of studies on condyloma acuminatum, a low-risk HPV infection disease, show T cell inhibition in warts,29 but the mechanism behind this functional inhibition is not completely clear.
In this study, it was found that PD-L1 expression was upregulated in the epidermis of approximately 50% of the collected specimens from condyloma acuminatum patients. Furthermore, the cells with high expression of PD-L1 were identified as keratinocytes, rather than other immune cells in the epidermis, by using a two-colour immunofluorescence technique. What factors lead to PD-L1 expression upregulation in the keratinocytes of genital warts? Based on the theory that many viruses upregulate the expression of PD-L1 in infected cells,28 the recognition that HPV, as a very successful pathogen, can evade host immunity in a variety of ways,5 and the evidence that the E7 protein can upregulate the expression of PD-L1 in keratinocytes during high-risk HPV infection,24 we speculated that the increase in PD-L1 expression levels in the keratinocytes of genital warts may be induced by HPV infection to mediate the immune escape of the virus. In this experiment, lentivirus transfection was used to introduce the HPV11-E7 gene into HaCaT cells, and the expression was stable. Compared with control cells, the HPV11-E7 HaCaT cells exhibited upregulated expression of PD-L1. It has been proven that HPV11-E7 can upregulate the expression of PD-L1 in HaCaT cells.
To answer the question of whether this HPV-induced expression of PD-L1 can mediate the functional inhibition of T cells, we conducted in vitro experiments. HPV11-E7 HaCaT and control HaCaT cells were cocultured with activated Jurkat cells. It was found that Jurkat cells cocultured with HaCaT cells expressing HPV11-E7 exhibited increased apoptosis and decreased proliferation. To prove that the inhibition of the function of Jurkat cells was related to the upregulation of PD-L1 in HaCaT cells, we added PD-L1 blockers to the HPV11-E7 HaCaT cell culture and found that the apoptosis of Jurkat cells was reduced and the proliferation of Jurkat cells was improved. It has been proven that HPV-11 E7 can promote the apoptosis of Jurkat cells and inhibit the proliferation of Jurkat cells by upregulating the expression of PD-L1 in HaCaT cells.
In diseases associated with high-risk HPV infection, the role of the PD-1/PD-L1 signalling pathway has been explored, and it is believed that high-risk HPV infection can mediate the expression of PD-L1 in cells and play an important role in virus-mediated immunosuppression through the PD-1/PD-L1 signalling pathway.30-33 Further studies have also found that the application of blocking agents of the PD-1/PD-L1 signalling pathway in cervical cancer patients with high-risk HPV infection is conducive to the recovery of the disease and has certain clinical efficacy.34, 35 However, in condyloma acuminatum, the expression of PD-L1 in epidermal cells of warts induced by viral infection and its effects have not been reported. The results of this experiment prove that HPV infection can upregulate PD-L1 expression in the keratinocytes of genital warts and participate in the inhibition of local T-cell function.
In our previous study, we found that the Treg cells were locally clustered in the wart of condyloma acuminatum patients and mediated the local immunosuppressive response.3, 36 Treg cells are a subset of T cells that can effectively inhibit the immune response of CD4+ and CD8+ T cells, thus producing negative immune regulation.37 Studies have found that the PD-1/PD-L1 signalling pathway plays a crucial role in regulating the development and function of Tregs and can transform Th1 cells into Treg cells.38 Endothelial cells, as nonimmune cells, can enhance the function of Treg cells through the PD-1/PD-L1 signalling pathway.39, 40 The PD-1/PD-L1 signalling pathway has been found to play a certain role in regulating the immunosuppression of Treg cells in patients with high-risk HPV infection-related cervical cancer.41, 42 Based on this finding, we speculated that the local aggregation of Treg cells in the wart from condyloma acuminatum patients may also have a certain correlation with the PD-1/PD-L1 signalling pathway.
Only 50% of the clinical specimens collected in this study exhibited high expression of PD-L1, which we speculated might be because PD-L1 is not continuously expressed in keratinocytes or because multiple factors regulate the expression of PD-L1 at the same time. The specific reasons need further study. Due to the limitations of time and conditions, this study only constructed the HPV11-E7 cell model and failed to demonstrate whether other viral proteins play a role. Due to the strict host limitation of HPV, which only infects humans, an animal model of low-risk HPV infection could not be constructed to further verify the findings of this study. In the follow-up of this experiment, it is necessary to further study the changes in keratinocyte expression of PD-L1 and the signalling pathway that mediates these changes after low-risk HPV infection.
5 CONCLUSION
In summary, HPV infection may upregulate PD-L1 expression in the keratinocytes of genital warts and participate in the inhibition of local T-cell function. The results of this study suggest that interference with PD-1/PD-L1 signalling in condyloma acuminatum may improve the function of local T cells, which has a certain clinical importance for the treatment of condyloma acuminatum and is expected to provide a new therapeutic strategy for condyloma acuminatum.
ACKNOWLEDGEMENTS
National Natural Science Foundation of China, Grant/Award Number: 81974308.
The authors would like to thank everyone who contributed to this study.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




