Effect of DNA aptamer through blocking of negative regulation of Wnt/β-catenin signaling in human hair follicle dermal papilla cells
Abstract
Background
When Wnt binds to the N-terminal of Frizzled, a conformational change occurs in the C-terminal of Frizzled, which binds to Dishevelled1 (Dvl1), a Wnt signaling component protein. When Dvl1 binds to the C-terminal of Frizzled, the concentration of β-catenin increases and it enters the nucleus to transmit cell proliferation signals. CXXC-type zinc finger protein 5 (CXXC5) binds to the Frizzled binding site of Dvl1 and interferes with Dvl1–Frizzled binding. Therefore, blocking CXXC5–Dvl1 binding may induce Wnt signal transduction.
Materials and methods
We used WD-aptamer, a DNA aptamer that specifically binds to Dvl1 and interferes with CXXC5–Dvl1 interaction. We confirmed the penetration of WD-aptamer into human hair follicle dermal papilla cells (HFDPCs) and measured β-catenin expression following treatment with WD-aptamer in HFDPCs, wherein Wnt signaling was activated by Wnt3a. In addition, MTT assay was performed to investigate the effect of WD-aptamer on cell proliferation.
Results
WD-aptamer penetrated the cell, affected Wnt signaling, and increased β-catenin expression, which plays an important role in signaling. Additionally, WD-aptamer induced HFDPC proliferation.
Conclusion
CXXC5-associated negative feedback of Wnt/β-catenin signaling can be regulated by interfering with CXXC5–Dvl1 interaction.
1 INTRODUCTION
Cells detect and respond to signals in an environment. Cells regulate their own responses by interpreting various signals from other cells.1 When signaling pathways interact with each other, they create networks and allow cellular responses.2 Among various signaling pathways, Wnt signaling is one of the basic mechanisms involved in embryonic development, tissue homeostasis, cell proliferation, cell polarity, and cell fate.3, 4 According to previous studies, Wnt signaling is known to be directly involved in bone formation, hair regrowth, and wound healing.5-14 It regulates the expression of intracellular β-catenin, which in turn regulates embryonic development and homeostasis.15-19 Wnt signaling is activated when extracellular Wnt binds to the membrane protein family, Frizzled.20
The dermal papilla (DP) of hair follicles plays a crucial role in generating instructive signals that regulate hair production and growth cycle.5, 21 Recent studies have demonstrated that a decrease in the number of DP cells (DPCs) is sufficient to cause thinning and loss of hair and that the successful maintenance of DP activity is an important factor in the prevention and treatment of hair loss.5-7, 12, 13, 21
Wnt signaling is regulated by the negative feedback regulator, CXXC5, which is localized in the cytosol. CXXC5 acts as a negative feedback regulator for Wnt/β-catenin signaling through direct interaction with Dvl1. Recent studies have confirmed that CXXC5 expression is higher in the DP of bald hair follicles than in that of normal hair follicles.11-14, 20, 22
An aptamer is a single-stranded DNA or RNA oligonucleotide with a three-dimensional structure that captures a specific target molecule with its unique binding ability.23 Aptamers have the ability to bind to various target molecules (cells, enzymes, viruses, proteins, and small molecules); therefore, they can be used in various fields, such as therapeutic agents, sensing reagents, therapeutic delivery systems, and clinical and environmental diagnostics.23-31 Aptamers are released out of the body with no risk of accumulation in the body; thus, they can be safely used as a cosmetic ingredient or therapeutic agent.
Previously, we reported the development of DNA aptamer (WD-aptamer), which specifically binds to Dvl1, and confirmed that WD-aptamer inhibits the negative regulation of Wnt signaling by CXXC5 using in vitro and in vivo tests.19, 32 In this study, through in vitro experiments using hair follicle dermal papilla cells (HFDPCs), we confirmed that WD-aptamer affects the activation of Wnt signaling.
2 MATERIALS AND METHODS
2.1 Materials
Dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Recombinant human Wnt-3a protein was purchased from R&D systems (Minneapolis, MN, USA). β-catenin, α-tubulin antibodies, horseradish peroxidase-conjugated mouse IgGκ binding protein (m-IgGκ BP-HRP), and CruzFluor 488-conjugated mouse IgGκ binding protein (m-IgGκ BP-CFL 488) were obtained from Santa Cruz Biotechnology (CA, USA). The bicinchoninic acid (BCA) assay kit and enhanced chemiluminescence (ECL) substrate were obtained from Thermo scientific (Rockford, IL, USA). Alexa Fluor 555 streptavidin was obtained from Invitrogen (CA, USA), and 4,6′-diamidino-2-phenylindole (DAPI) was purchased from Vector Laboratories (Burlingame, CA, USA).
2.2 Cell culture
HFDPCs were purchased from CEFO Co. Ltd (Seoul, Korea) and were cultured in an HFDPC-specific growth medium (CEFO Co. Ltd) according to the manufacturer's instructions. The cells at passages 3−7 were passaged every 3 days and incubated at 37°C in a 5% CO2 incubator.
2.3 Cell proliferation
HFDPCs were seeded in 96-well plates at a density of 1 × 104 cells per well and incubated at 37°C and 5% CO2 for 24 h. The cells were treated with various concentrations (0.01, 0.1, 1, and 5 μM) of WD-aptamer and 10 μM Minoxidil, the positive control. After 24 h of incubation, MTT solution (final concentration of 0.5 mg/mL) was added to each well, and the cells were incubated for 2 h in the dark. The supernatant was removed, and 100 μL DMSO was added to each well to completely dissolve the formazan crystals. Absorbance was measured at 540 nm using Synergy H1 Hybrid Multi Mode Reader (Bio-Tek Instruments, USA).
2.4 Cell penetration
HFDPCs were seeded in 12-well plates at a density of 1 × 105 cells per well and incubated at 37°C and 5% CO2 for 24 h. Biotinylated DNA aptamers (WD-aptamer or negative control) were added to the culture medium at a final concentration of 100 nM and incubated with live cells at 37°C for 30 min. After incubation, the cells were treated with 0.4 U/μL DNase I at 37°C for 10 min to remove extracellular residual DNA aptamers. They were fixed with 4% paraformaldehyde at room temperature for 10 min. The cells were permeabilized using 0.03% Triton X-100 and incubated with Alexa Fluor 555 streptavidin (Invitrogen) at room temperature for 30 min in the dark. Finally, the cells were counterstained with DAPI and imaged using a fluorescence microscope (Optinity, Korea).
2.5 Western blot analysis
HFDPCs were seeded in six-well plates at a density of 1 × 105 cells per well and incubated for 24 h. The cells were treated with 200 ng/mL recombinant Wnt3a and 10 μM WD-aptamer for 6 h. Next, they were lysed using RIPA buffer with a protease inhibitor. After centrifugation for 30 min at 15 000 rpm and 4°C, the supernatant was separated and used as a protein solution. The protein concentration was quantified using the BCA assay. The samples were separated on 4%−15% SDS polyacrylamide gels (BIO-RAD) and transferred onto a polyvinylidene fluoride membrane (BIO-RAD). After blocking with TBS containing 6% skim milk and 0.1% (v/v) Tween 20, the membranes were incubated overnight at 4°C with antibodies specific for β-catenin (1:1000) and α-tubulin (1:1000). The membranes were then incubated with m-IgGκ BP-HRP (1:5000) for 1 h at room temperature. The protein bands were detected using an ECL substrate and visualized via Chemi Doc (BIO-RAD, USA). The Western blot bands were analyzed using Image J (1.53k, National Institutes of Health, USA). The relative densitometry values are presented as the intensity ratio of each protein to that of the loading control protein (α-tubulin).
2.6 Immunofluorescence staining
HFDPCs were seeded in 12-well plates at a density of 7 × 104 cells per well and incubated for 24 h. The cells were treated with 200 ng/mL recombinant Wnt3a and 10 μM WD-aptamer for 6 h. Next, the cells were fixed with 4% paraformaldehyde for 10 min at room temperature. Permeabilization was performed using 0.2% Triton X-100 for 10 min, and the cells were blocked with 3% bovine serum albumin for 1 h at room temperature. The cells were then incubated overnight at 4°C with a β-catenin-specific antibody (1:100) and washed with phosphate-buffered saline (PBS) thrice, followed by incubation with m-IgGκ BP-CFL 488 (1:50) in the dark for 1 h at room temperature. The nuclei were counterstained with DAPI for 10 min, and images were captured under a fluorescence microscope.
2.7 Statistical analysis
All data were obtained from at least three independent experiments and were presented as mean ± standard deviation (SD). Statistical analysis was assessed by one-way analysis of variance (ANOVA), followed by Tukey's post hoc tests for multiple comparisons, via GraphPad Prism (San Diego, CA, USA). p-Values of less than 0.05 were considered to indicate statistical significance.
3 RESULTS
3.1 Penetration of WD-aptamer into HFDPCs
As WD-aptamer is known to exert its effect by interacting with the cytoplasmic protein Dvl1, we first confirmed its penetration into HFDPCs. We used fluorescence imaging and the biotin–streptavidin system to compare the penetration of WD-aptamer and a negative control into HFDPCs under identical conditions. We used biotinylated scrambled DNA as the negative control. HFDPCs were incubated with biotinylated DNA aptamer, and then treated with DNase I to remove the extracellular residual DNA aptamer. The fluorescent signals clearly showed that the WD-aptamer entered the HFDPCs compared to negative controls (Figure 1). These data demonstrated that WD-aptamer could be involved in Wnt/β-catenin signaling after entering HFDPCs.
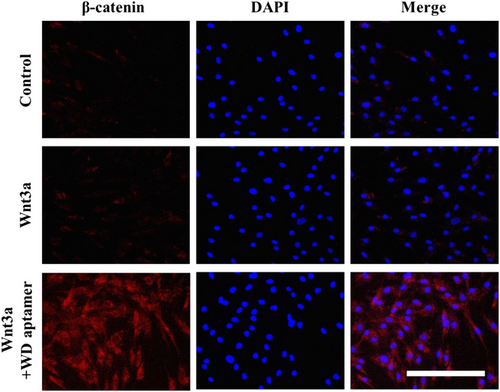
3.2 Effects of WD-aptamer on the Wnt/β-catenin signaling pathway
Wnt signaling reportedly regulates DP activity, and the presence of Wnt3a activates DP and enhances hair growth by maintaining an anagen state5, 8, 21; therefore, we activated Wnt signaling in HFDPCs using Wnt3a. We measured β-catenin expression in HFDPCs to prove the effect of WD-aptamer on Wnt/β-catenin signaling by co-treating HFDPCs with Wnt3a and WD-aptamer. Our Western blot and immunofluorescence staining results demonstrated that Wnt3a treatment alone induced β-catenin expression and that the co-treatment synergistically increased β-catenin expression in HFDPCs (Figure 2A,B). We used immunofluorescent staining to demonstrate the subcellular localization of β-catenin in HFDPCs co-treated with WD-aptamer and Wnt3a. Our results showed that β-catenin expression significantly increased in the nucleus and cytoplasm in the co-treatment group, demonstrating that WD-aptamer enhances β-catenin expression by competitively binding to Dvl1 with CXXC5 when Wnt signaling is activated.
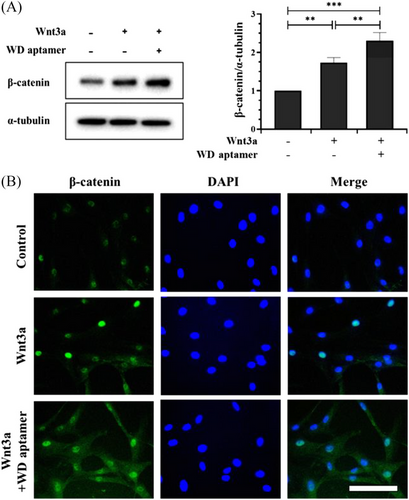
3.3 Effects of WD-aptamer on HFDPC proliferation
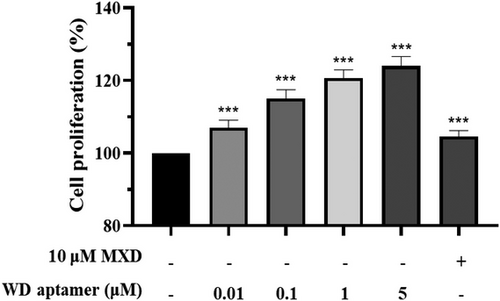
To investigate how the WD-aptamer affects cell proliferation, the HFDPCs were treated with the WD-aptamer or Minoxidil (MXD) at the concentration indicated in Figure 3. The WD-aptamer treatment significantly increased cell proliferation by 123.98% at 5 μM compared to the non-treated group, whereas the MXD treatment had no significant effect at 10 μM. These results suggest that the WD-aptamer regulates HFDPC proliferation.
4 DISCUSSION
Wnt/β-catenin pathway is one of the most important signaling pathways as it regulates DP activity, improves hair follicle development, and promotes hair regeneration in adults. Wnt signaling is regulated by the negative feedback regulator CXXC5, which shows higher expression in the DP of bald hair follicles than in that of normal hair follicles. Therefore, the current study suggests that interfering with the CXXC5-associated negative feedback of Wnt signals in DP is a potential solution for hair loss prevention.
We developed WD-aptamer to regulate the negative-feedback of Wnt/β-catenin signaling by interfering with CXXC5–Dvl1 interaction. Our study suggests that WD-aptamer interferes with CXXC5–Dvl1 interaction in Wnt signaling and inhibits the negative regulation of signal transduction, thereby enhancing β-catenin expression in HFDPCs. In addition, a previous study showed that WD-aptamer treatment plays a role in accelerating hair growth in vivo, resulting in faster hair regeneration in WD-aptamer group than in MXD group in telogen-matched C57BL6 mice.32 These observations suggest that WD-aptamer positively affects DP activity.
5 CONCLUSION
WD-aptamer blocks the negative regulation of Wnt/β-catenin signaling, induces HFDPC proliferation, and enhances β-catenin expression. The current study results and previous in vivo experimental results using mice indicate the molecular basis for the hair regrowth effect exerted by WD-aptamer on HFDPCs and suggest that WD-aptamer is an alternative therapeutic agent for hair regrowth.
Open Research
DATA AVAILABILITY STATEMENT
The author has provided the required Data Availability statement, and if applicable, included functional and accurate links to said data therein.


